Abstract
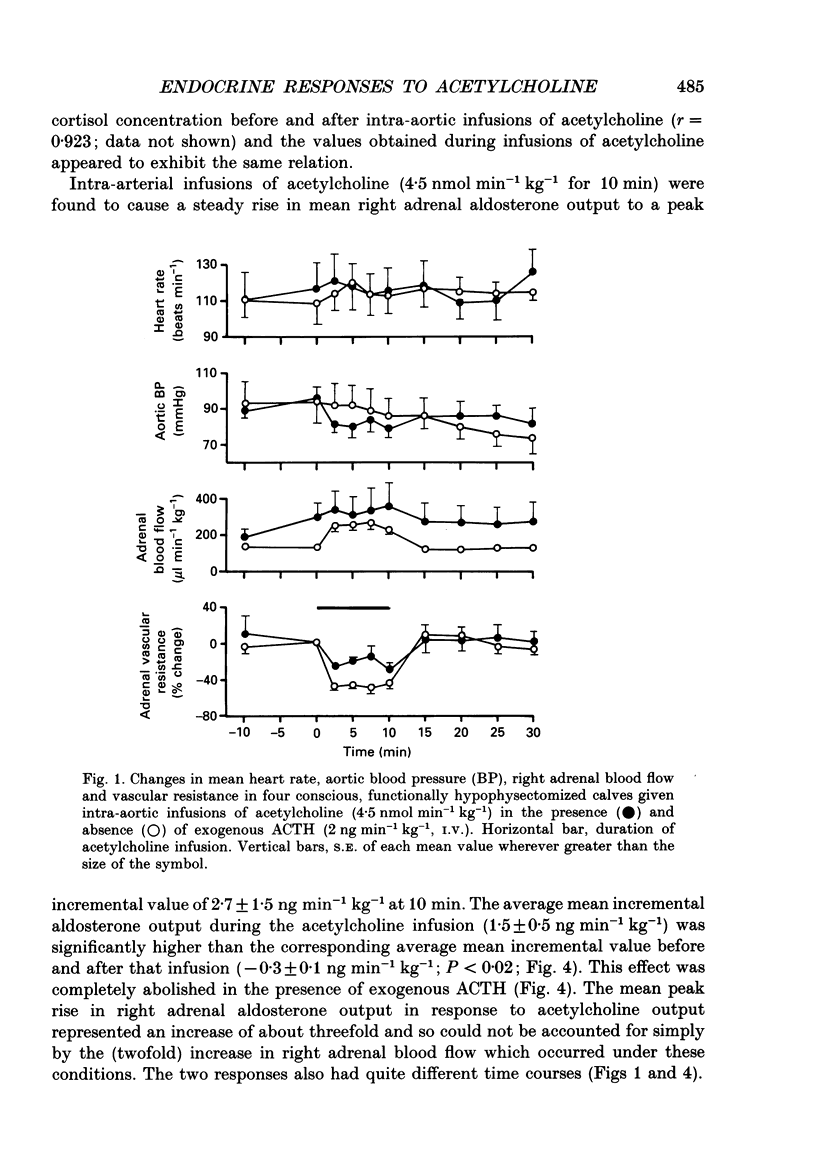
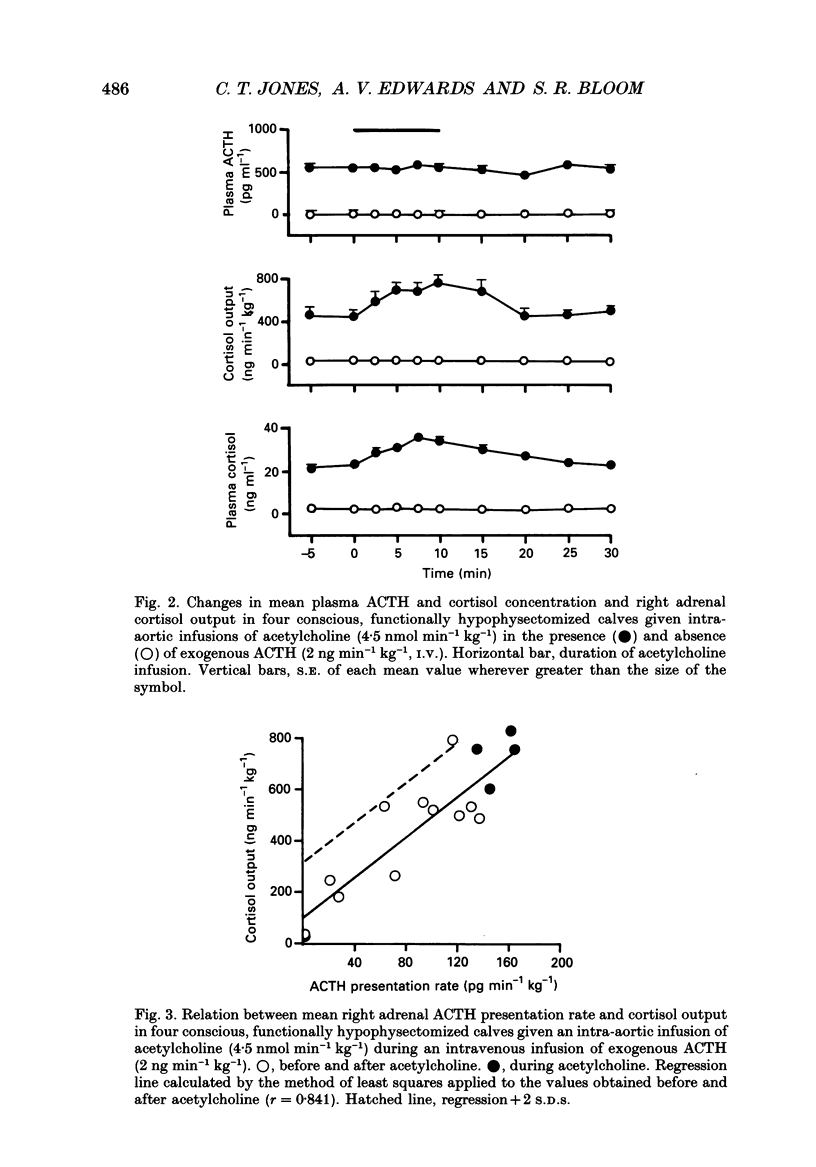
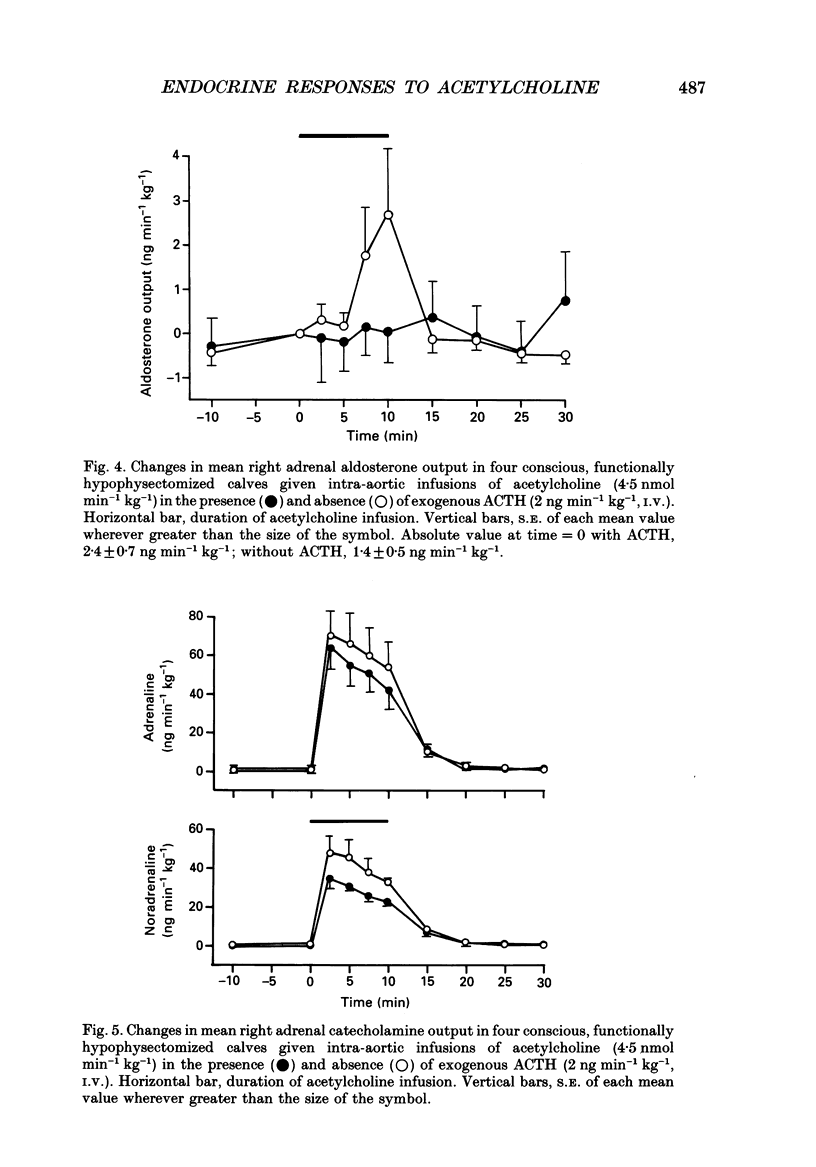
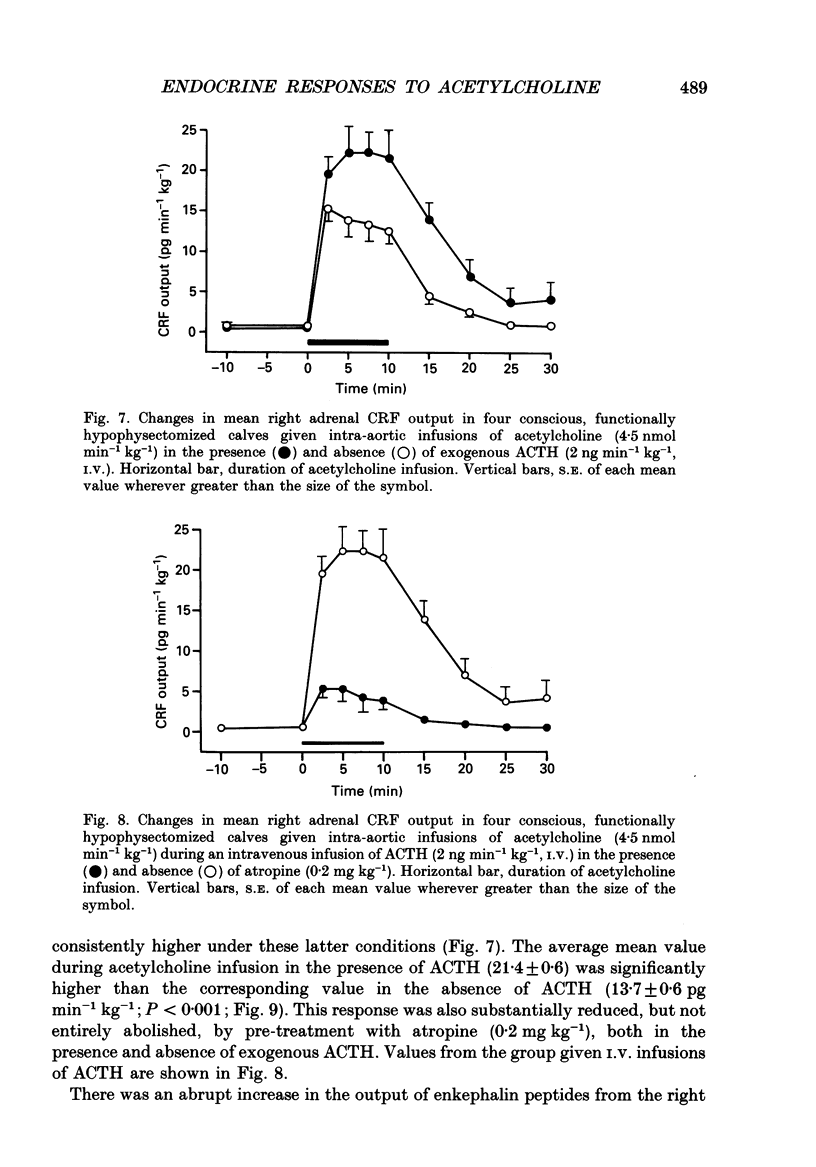
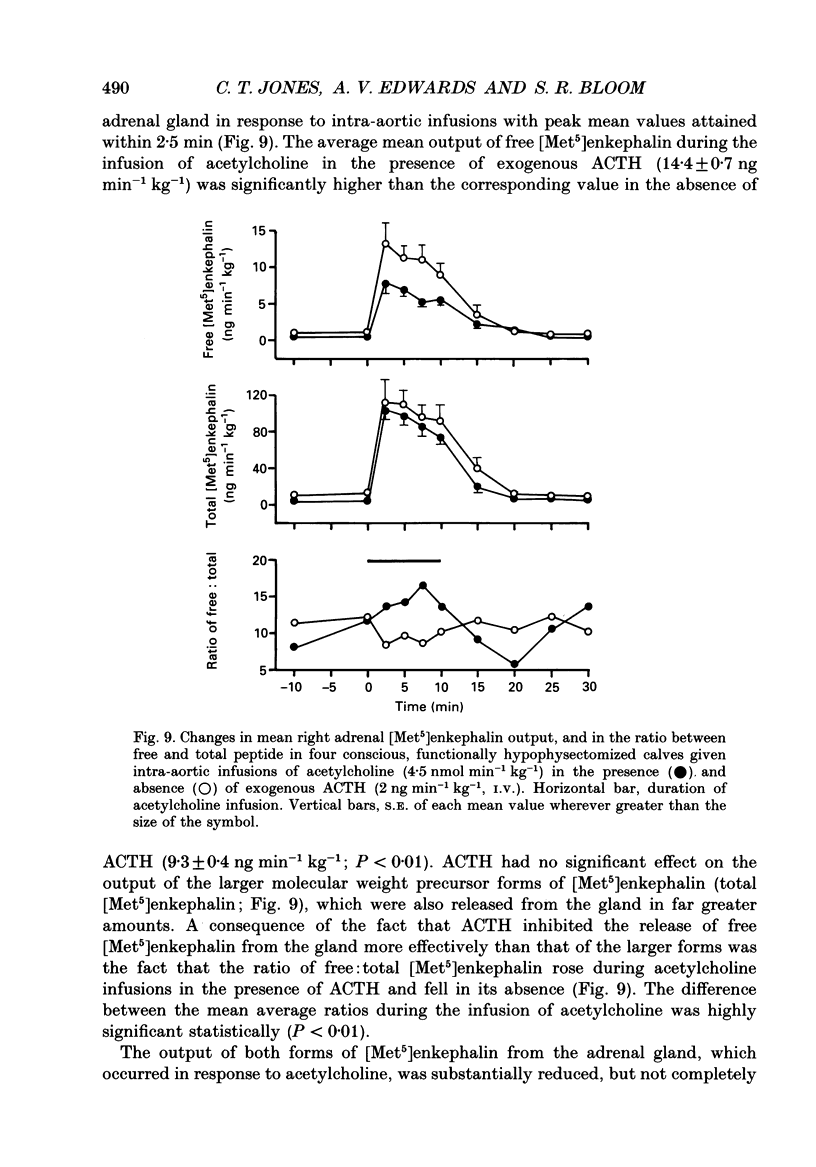
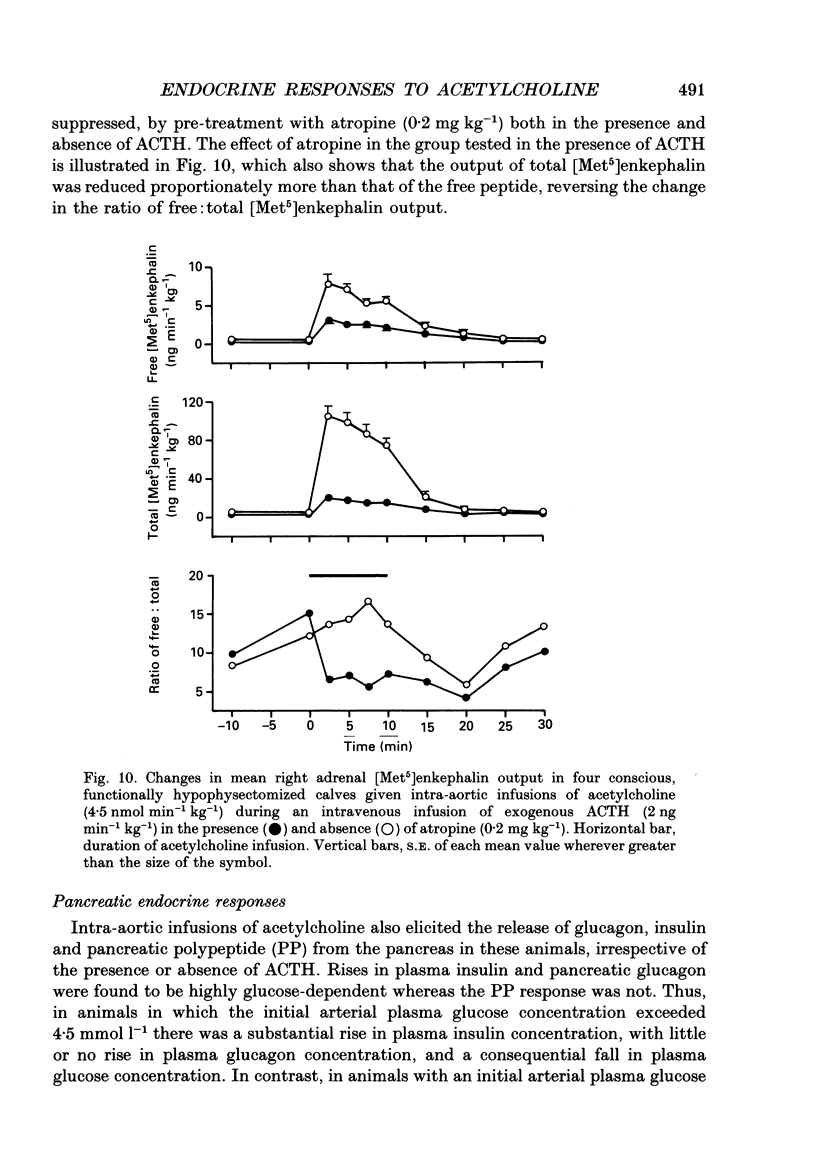
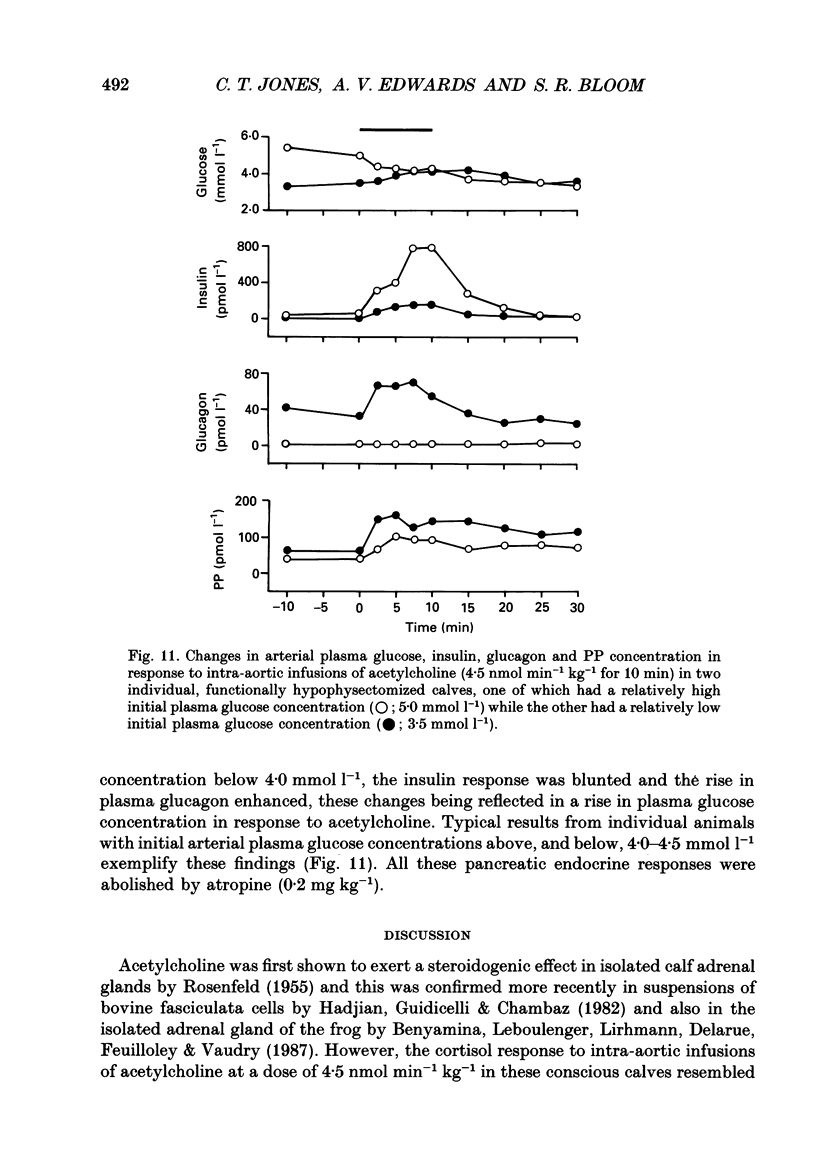
1. Adrenal responses to intra-aortic infusions of acetylcholine (4.5 nmol min-1 kg-1 for 10 min) have been investigated in conscious, functionally hypophysectomized, 3- to 6-week-old calves, in the presence and absence of exogenous ACTH (2 ng min-1 kg-1, I.V.). 2. Acetylcholine produced a substantial fall in adrenal vascular resistance, which was significantly reduced in the presence of exogenous ACTH, while producing minimal changes in aortic blood pressure and heart rate. 3. There was also a significant rise in right adrenal cortisol output which was sufficient to produce a measurable rise in plasma cortisol concentration. The effect could be accounted for by the increase in adrenal ACTH presentation. It was abolished by pre-treatment with atropine (0.2 mg kg-1). A small but significant rise in aldosterone output during acetylcholine infusions was also abolished in the presence of ACTH. 4. Both adrenaline and noradrenaline were released during intra-aortic acetylcholine infusions and these responses were substantially reduced, but not abolished, by pre-treatment with atropine. 5. Acetylcholine also stimulated the release of corticotrophin-releasing factor (CRF) and [Met5]enkephalins from the gland. The output of CRF was enhanced and that of free [Met5]enkephalin was significantly reduced in the presence of exogenous ACTH. All these responses were largely, but not completely, suppressed by atropine. 6. Acetylcholine also promoted the release of the pancreatic hormones glucagon, insulin and pancreatic polypeptide (PP). The amounts of pancreatic glucagon and insulin that were released were highly dependent on the concentration of glucose in the circulating plasma and all these responses were abolished by atropine. 7. It is concluded that acetylcholine is capable of stimulating the release of a wide variety of agonists from the adrenal gland when infused intra-aortically at a dose of 4.5 nmol min-1 kg-1. The increase in cortisol output appears to be secondary to an increase in blood flow whereas the adrenal medullary responses are not, and appear to be due largely, but not entirely, to activation of muscarinic receptors.
Full text
PDF



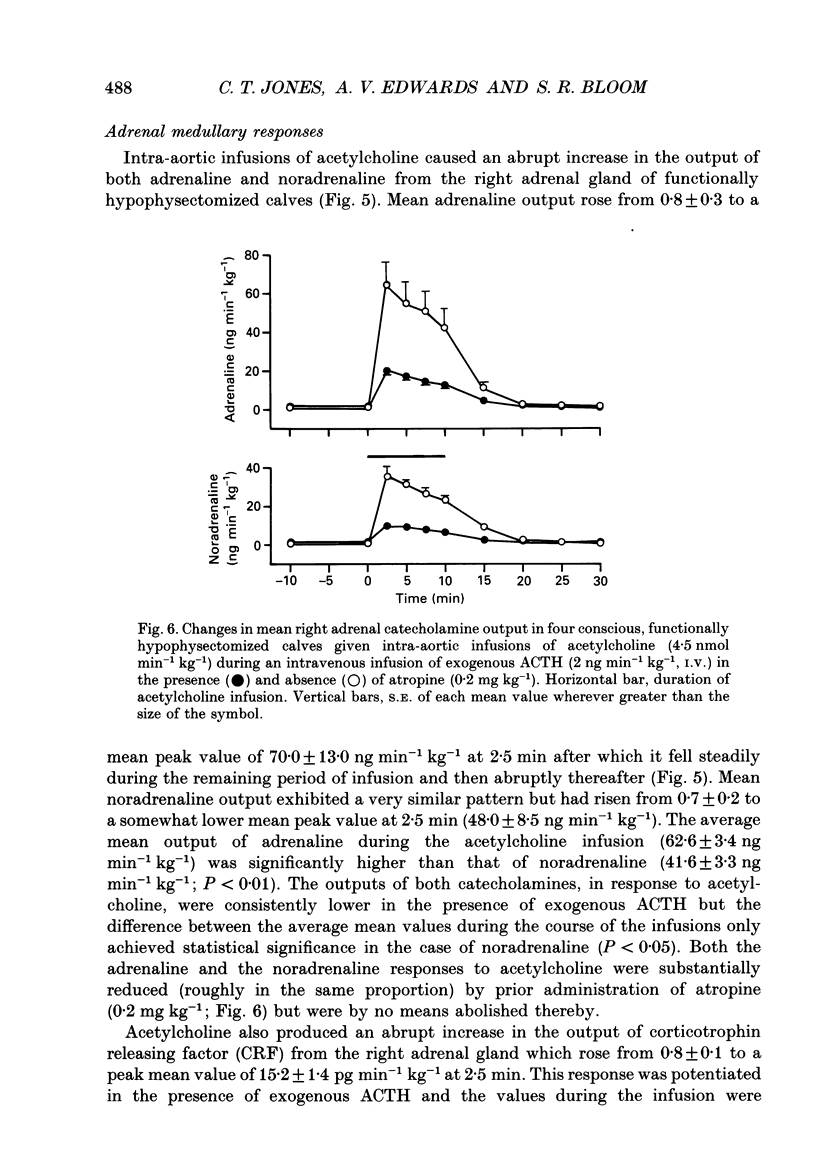







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abayasekara D. R., Vazir H., Whitehouse B. J., Price G. M., Hinson J. P., Vinson G. P. Studies on the mechanisms of ACTH-induced inhibition of aldosterone biosynthesis in the rat adrenal cortex. J Endocrinol. 1989 Sep;122(3):625–632. doi: 10.1677/joe.0.1220625. [DOI] [PubMed] [Google Scholar]
- Adamson A. R., Jamieson S. W. Urinary excretion of sodium and potassium in relation to plasma aldosterone concentration. J Endocrinol. 1972 Jun;53(3):425–431. doi: 10.1677/joe.0.0530425. [DOI] [PubMed] [Google Scholar]
- Adrian T. E., Bloom S. R., Bryant M. G., Polak J. M., Heitz P. H., Barnes A. J. Distribution and release of human pancreatic polypeptide. Gut. 1976 Dec;17(12):940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Arkinstall S. J., Jones C. T. Regional changes in catecholamine content of the pregnant uterus. J Reprod Fertil. 1985 Mar;73(2):547–557. doi: 10.1530/jrf.0.0730547. [DOI] [PubMed] [Google Scholar]
- Assan R., Slusher N. Structure-function and structure-immunoreactivity relationships of the glucagon molecule and related synthetic peptides. Diabetes. 1972 Aug;21(8):843–855. doi: 10.2337/diab.21.8.843. [DOI] [PubMed] [Google Scholar]
- Ballesta J. J., Borges R., García A. G., Hidalgo M. J. Secretory and radioligand binding studies on muscarinic receptors in bovine and feline chromaffin cells. J Physiol. 1989 Nov;418:411–426. doi: 10.1113/jphysiol.1989.sp017849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamina M., Leboulenger F., Lirhmann I., Delarue C., Feuilloley M., Vaudry H. Acetylcholine stimulates steroidogenesis in isolated frog adrenal gland through muscarinic receptors: evidence for a desensitization mechanism. J Endocrinol. 1987 Jun;113(3):339–348. doi: 10.1677/joe.0.1130339. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. Adrenal cortical responses to vasoactive intestinal peptide in conscious hypophysectomized calves. J Physiol. 1987 Oct;391:441–450. doi: 10.1113/jphysiol.1987.sp016748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. Adrenal responses to calcitonin gene-related peptide in conscious hypophysectomized calves. J Physiol. 1989 Feb;409:29–41. doi: 10.1113/jphysiol.1989.sp017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1988 Mar;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V. Pancreatic endocrine responses to stimulation of the peripheral ends of the vagus nerves in conscious calves. J Physiol. 1981 Jun;315:31–41. doi: 10.1113/jphysiol.1981.sp013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland R. E., Parker T. L., Kesse W. K., Mohamed A. A. The innervation of the adrenal gland. III. Vagal innervation. J Anat. 1989 Apr;163:173–181. [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Furness P. N., Helle K. B. Adrenal medullary responses to stimulation of the splanchnic nerve in the conscious calf. J Physiol. 1980 Nov;308:15–27. doi: 10.1113/jphysiol.1980.sp013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hansell D., Jones C. T. Effects of synthetic adrenocorticotrophin on adrenal medullary responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1986 Oct;379:1–16. doi: 10.1113/jphysiol.1986.sp016237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. Secretion of corticotrophin releasing factor from the adrenal during splanchnic nerve stimulation in conscious calves. J Physiol. 1988 Jun;400:89–100. doi: 10.1113/jphysiol.1988.sp017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. The effect of splanchnic nerve stimulation on adrenocortical activity in conscious calves. J Physiol. 1987 Jan;382:385–396. doi: 10.1113/jphysiol.1987.sp016373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Holz R. W., Agranoff B. W. Muscarinic receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981 Aug;37(2):491–497. doi: 10.1111/j.1471-4159.1981.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Forsberg E. J., Rojas E., Pollard H. B. Muscarinic receptor enhancement of nicotine-induced catecholamine secretion may be mediated by phosphoinositide metabolism in bovine adrenal chromaffin cells. J Biol Chem. 1986 Apr 15;261(11):4915–4920. [PubMed] [Google Scholar]
- Hadjian A. J., Guidicelli C., Chambaz E. M. Cholinergic muscarinic stimulation of steroidogenesis in bovine adrenal cortex fasciculata cell suspensions. Biochim Biophys Acta. 1982 Jan 12;714(1):157–163. doi: 10.1016/0304-4165(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Harish O. E., Kao L. S., Raffaniello R., Wakade A. R., Schneider A. S. Calcium dependence of muscarinic receptor-mediated catecholamine secretion from the perfused rat adrenal medulla. J Neurochem. 1987 Jun;48(6):1730–1735. doi: 10.1111/j.1471-4159.1987.tb05730.x. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Boddy K., Robinson J. S., Ratcliffe J. G. Developmental changes in the responses of the adrenal glands of foetal sheep to endogenous adrenocorticotrophin, as indicated by hormone responses to hypoxaemia. J Endocrinol. 1977 Mar;72(3):279–292. doi: 10.1677/joe.0.0720279. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Edwards A. V. Adrenal responses to corticotrophin-releasing factor in conscious hypophysectomized calves. J Physiol. 1990 Nov;430:25–36. doi: 10.1113/jphysiol.1990.sp018279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Schiavone M. T. Effect of muscarine on release of catecholamines from the perfused adrenal gland of the cat. Br J Pharmacol. 1982 Nov;77(3):455–460. doi: 10.1111/j.1476-5381.1982.tb09318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Observations on the muscarinic activation of catecholamine secretion in the chicken adrenal. Neuroscience. 1986 Sep;19(1):357–366. doi: 10.1016/0306-4522(86)90027-8. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Shibata H., Ogata E. Mechanism of cholinergic stimulation of aldosterone secretion in bovine adrenal glomerulosa cells. Endocrinology. 1986 Jul;119(1):284–291. doi: 10.1210/endo-119-1-284. [DOI] [PubMed] [Google Scholar]
- Ledbetter F. H., Kirshner N. Studies of chick adrenal medulla in organ culture. Biochem Pharmacol. 1975 May 1;24(9):967–974. doi: 10.1016/0006-2952(75)90429-3. [DOI] [PubMed] [Google Scholar]
- Lee F. L., Trendelenburg U. Muscarinic transmission of preganglionic impulses to the adrenal medulla of the cat. J Pharmacol Exp Ther. 1967 Oct;158(1):73–79. [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Vasoactive intestinal polypeptide and muscarine mobilize intracellular Ca2+ through breakdown of phosphoinositides to induce catecholamine secretion. Role of IP3 in exocytosis. J Biol Chem. 1988 Feb 15;263(5):2123–2126. [PubMed] [Google Scholar]
- ROSENFELD G. Stimulative effect of acetylcholine on the adrenocortical function of isolated perfused calf adrenals. Am J Physiol. 1955 Nov;183(2):272–278. doi: 10.1152/ajplegacy.1955.183.2.272. [DOI] [PubMed] [Google Scholar]
- Role L. W., Perlman R. L. Both nicotinic and muscarinic receptors mediate catecholamine secretion by isolated guinea-pig chromaffin cells. Neuroscience. 1983 Nov;10(3):979–985. doi: 10.1016/0306-4522(83)90236-1. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Cline H. T., Lemaire S. Rapid rise in cyclic GMP accompanies catecholamine secretion in suspensions of isolated adrenal chromaffin cells. Life Sci. 1979 Apr 9;24(15):1389–1394. doi: 10.1016/0024-3205(79)90009-2. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Lee R. W. Morphological characteristics and stimulus-secretion coupling in bovine adcrenal chromaffin cell cultures. Neuroscience. 1980;5(9):1533–1546. doi: 10.1016/0306-4522(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Urquhart J. Adrenal blood flow and the adrenocortical response to corticotropin. Am J Physiol. 1965 Dec;209(6):1162–1168. doi: 10.1152/ajplegacy.1965.209.6.1162. [DOI] [PubMed] [Google Scholar]
- Vale W., Vaughan J., Yamamoto G., Bruhn T., Douglas C., Dalton D., Rivier C., Rivier J. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–577. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Malhotra R. K., Wakade T. D. Phorbol ester facilitates 45Ca accumulation and catecholamine secretion by nicotine and excess K+ but not by muscarine in rat adrenal medulla. Nature. 1986 Jun 12;321(6071):698–700. doi: 10.1038/321698a0. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Noncholinergic transmitter(s) maintains secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons. J Neurochem. 1988 Apr;50(4):1302–1308. doi: 10.1111/j.1471-4159.1988.tb10608.x. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983 Nov;10(3):973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]
- Yanagihara N., Isosaki M., Ohuchi T., Oka M. Muscarinic receptor-mediated increase in cyclic GMP level in isolated bovine adrenal medullary cells. FEBS Lett. 1979 Sep 15;105(2):296–298. doi: 10.1016/0014-5793(79)80633-x. [DOI] [PubMed] [Google Scholar]
- Young D. B., Guyton A. C. Steady state aldosterone dose-response relationships. Circ Res. 1977 Feb;40(2):138–142. doi: 10.1161/01.res.40.2.138. [DOI] [PubMed] [Google Scholar]


