Abstract
The complete life cycle of Pneumocystis carinii has not been defined, but accumulating evidence suggests that the mammalian host may acquire this organism early in life. In the present study, the initial time of P. carinii acquisition was determined in rats by amplification of P. carinii DNA in oral swabs from seven sets of pups and dams and from fetal tissue obtained by cesarean section of three gravid female rats. DNA extracted from all samples was amplified by using PCR primers directed to the P. carinii mitochondrial large subunit rRNA. Amplicons were produced from 80% (28 of 35) of pups within 2 h after birth; from 97% (34 of 35) after 24 h, and in all of the serially sampled pups by 48 h. No P. carinii amplicons were produced from 48 fetuses or their placentae taken by cesarean section. Thus, P. carinii is acquired almost immediately after birth, and placental transmission occurs rarely, if ever, in rats.
Members of the genus Pneumocystis are fungal pathogens that cause an often lethal pneumonia in mammals with compromised immune systems, including humans. To date, the Pneumocystis life cycle is not fully defined and has been examined mostly within the confines of the mammalian lung. The lack of information about the life cycle of Pneumocystis organisms has complicated the study, diagnosis, and treatment of Pneumocystis disease and is primarily due to difficulties in long-term, in vitro cultivation of these organisms. Pneumocystis organisms are known to be transmitted from host to host via an airborne route, as shown by previous animal studies (9, 34); however, there has been no definitive microscopic identification of the infective airborne particle(s). Several investigators have amplified Pneumocystis-specific DNA from air samples (1, 11, 16, 17, 33), suggesting that a transmissive agent is present in the environment, but conclusive evidence linking this DNA to intact, viable Pneumocystis organisms remains elusive.
Pneumocystis pneumonia was first described as a pediatric disease (6), and exposure to the organisms was assumed to occur early in life. Later studies showed that Pneumocystis-specific antibodies could be detected in humans within the first 3 years of life (13, 21, 22), and Pneumocystis organisms have been identified in the lungs of neonates by histological examination (5, 6, 24, 32; E. Mortier, J. Pouchot, P. Bossi, and V. Moline, Letter, N. Engl. J. Med. 332:825, 1995), confirming this initial assumption. Case reports describing the presence of Pneumocystis in the lungs of stillborn infants suggested that placental transmission may also occur in humans (2, 5, 6, 20; Mortier et al., letter). Few animal studies addressing the early acquisition and/or vertical transmission of Pneumocystis have been conducted. Those that are available reported conflicting results, and many were performed prior to the advent of sensitive detection methods, such as PCR (8, 9, 23; M. Ito, T. Tsugane, K. Kabayashi, T. Kuramochi, K. Hioki, T. Furuta, and T. Nomura, abstract from the 2nd International Workshop on Opportunistic Protists 1991, J. Protozool. 38:218S-219S, 1991).
The initial time of acquisition has not been adequately addressed in human studies or in the animal models of Pneumocystis infection. Determination of the initial exposure to Pneumocystis is important for a number of reasons. For human beings, recent reports suggest that the primary infection may not be as cryptic as once thought (30, 32), and awareness of the potential for clinical manifestations in the neonate will be important for therapeutic decisions. In animal models, many experiments require the use of rodents not previously exposed to Pneumocystis, such as for inoculation of characterized Pneumocystis isolates to provide standardized models of infection or for the study of immunological responses to primary infection.
In the present study, Pneumocystis carinii-specific PCR amplification of DNA extracted from oral swabs was used to show that P. carinii DNA was present in the oral cavities of neonatal rats very shortly after birth. (Changes in the nomenclature of Pneumocystis were recommended at the Seventh International Workshop on Opportunistic Protists in Cincinnati, Ohio, in June 2001; thus, Pneumocystis carinii f. sp. carinii is referred to by its binomial, P. carinii, throughout this report [27].) Amplicons were produced from oral swabs up to 4 weeks after birth, the final time point of the study, suggesting that colonization may occur in neonatal rats and that these neonates could potentially serve as a reservoir of infection for the general rat population. In contrast, P. carinii amplicons were not produced from fetal homogenates of 48 rats, implying that vertical transmission is a mode rarely used by P. carinii. The data and techniques reported in these studies will contribute to better understanding of the life cycle and natural history of P. carinii and will allow investigators to compare the transmission of Pneumocystis in other mammalian systems to that in the rat.
MATERIALS AND METHODS
Rat groups.
A total of 7 rat dams and their pups (5 sets of Long Evans rats and 2 sets of Brown Norway rats), 3 gravid Long Evans dams, and 2 nongravid Long Evans rats were used in these studies, for a total of 122 rats. All rats were bred and housed at the Veterans Administration Veterinary Medical Unit, Cincinnati, Ohio (rooms 003 and 004). Breeder rats and neonates were housed in wire-topped shoebox cages in a room with other rats known to be infected with P. carinii. Rats were handled according to American Association for Accreditation of Laboratory Animal Care guidelines under an Institutional Animal Care and Use Committee-approved Veterans Administration Medical Center protocol.
Oral swabs.
Seven dams and their pups were studied to determine the time at which P. carinii is acquired by identifying P. carinii-specific DNA in the oral cavity, as described previously (10). Single oral swab samples were collected from 4 of the rat dams and their pups at 1, 2, or 4 weeks (9 pups at the age of 1 week, 8 pups at the age of 2 weeks, and 14 pups at the age of 4 weeks) after birth by using sterile cotton-tipped wooden applicators (catalog no. 14-959-91; Fisher, Pittsburgh, Pa.). Serial oral swab samples were collected from three other groups of rats at <2, 24, 48, and 72 h after birth by using sterile 6-in. tapered cotton-tipped miniapplicators (Hardwood Production Company LP, Guilford, Maine). All oral swab samples were collected by rubbing the dry cotton swab over the hard palate, surface of the tongue, and buccal surface and under the tongue of each rat. The intact tip of each cotton swab was broken off into a sterile, UV-irradiated 1.5-ml microcentrifuge tube and was then vortexed in 200 μl of DNA extraction buffer (100 mM Tris, 100 mM EDTA, 200 mM NaCl, 1% Sarkosyl). DNA was isolated by using a phenol-chloroform extraction method and 1.5-ml Phase Lock Heavy Gel tubes (Eppendorf, Westbury, N.Y.). DNA samples were stored in TE (1 mM EDTA-10 mM Tris-Cl) at −20°C until PCR analysis.
A number of measures were employed to ensure that amplicon or genomic contamination did not occur during the processing of the swabs and that the reagents were not contaminated. Cotton swabs taken directly from unopened packages were subjected to the same extraction procedure as the swabs used for sampling of the rats' oral cavities. This control was performed for each extraction. New reagents and plasticware were used for each set of extractions and were exposed to UV irradiation prior to use. Processing was performed under a laminar flow hood (The Germfree Laboratories, Inc., Miami, Fla.) which had previously been wiped down with a solution of DNA AWAY (Molecular BioProducts, San Diego, Calif.), a commercial DNase preparation.
Cesarean sections.
Three gravid and two nongravid female Long Evans rats (controls) were chosen for cesarean section analysis to explore the route of transplacental transmission of Pneumocystis in rats. At 10 days to 4 weeks of gestation, oral swabs were collected from each gravid dam. Subsequently, each dam was sacrificed by intraperitoneal injection of approximately 500 mg of chloral hydrate (Major Pharmaceuticals, Livonia, Mich.) and immediately placed under a laminar flow hood (The Germfree Laboratories, Inc.) which had previously been wiped down with DNA AWAY. All instruments used for dissection were treated with a solution of DNA AWAY after standard cleaning regimens and were autoclaved in separate pouches. Prior to dissection, each rat was sprayed with 70% alcohol followed by a spray of DNA AWAY solution. One set of instruments (forceps and scissors) was used to open the animal's abdomen. A separate set was used for organ removal. The intact uterus was removed from the abdominal cavity and placed in a large, sterile plastic petri plate. The lungs, liver, and spleen were removed from each dam and placed in individual sterile tubes. For the gravid females, each pup was removed from the uterus, the placenta (including the deciduae) was removed from each pup, and then each pup, each placenta, and the uterus were placed in individual sterile tubes. Three sets of internal negative controls were performed by treating three sterile tubes like all other samples, minus tissue, controlling for reagent contamination. All samples, including the three internal negative controls, were digested in 5 to 10 ml of proteinase K solution (0.5 mg of proteinase K/ml of DNA extraction buffer; Roche, Indianapolis, Ind.) overnight at 55°C. DNA was extracted from 250 μl of each sample by a phenol-chloroform extraction method (10) and was stored in TE at −20°C until analyzed by PCR.
PCR analysis.
All PCRs were set up within a PCR Workstation ductless hood (AirClean 600 Workstation; AirClean Systems, Raleigh, N.C.) that was UV irradiated and wiped with DNA AWAY prior to use. All pipettors and tips, tubes, and racks used for the reactions were sanitized and irradiated. DNA from all samples was amplified by using the Rcc primers (19) and rat globin (15) primers with the GeneAmp PCR System 9700 (Perkin-Elmer Applied Biosystems, Norwalk, Conn.). The Rcc primers target a region of the mitochondrial large subunit rRNA (mtLSU rRNA) specific for P. carinii. The level of sensitivity of this primer pair was previously determined to be 17 template copies (10). Each reaction used 1× JumpStart REDTaq ReadyMix PCR Reaction Mix (10 mM Tris-HCl, 2 mM MgCl2, 50 mM KCl, 0.001% gelatin, 0.2 mM each deoxynucleoside triphosphate, 0.06 U of Taq DNA polymerase/μl, and TaqStart antibody) (Sigma, St. Louis, Mo.), to which 0.05 ng each of Rcc or globin primers, 1.0 μl of template DNA, and molecular grade water were added. PCR conditions were as follows: a hot start at 94°C for 2 min, denaturing at 94°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 60 s (for a total of 40 cycles), and a final extension at 72°C for 10 min.
Amplified products were visualized by staining with ethidium bromide in 2% agarose gels run at 90 V for 1 h in 0.5× TBE (44 mM Tris, 44 mM boric acid, 25 mM EDTA). Gel images were captured with NIH Image software, version 1.6. Samples were scored positive for P. carinii by the presence of a band migrating at 137 bp in the gel and positive for rat DNA by the presence of a band migrating at 400 bp.
Accurate measurement of P. carinii DNA in the samples was problematic due to the presence of rat DNA. In addition, the DNA in oral swab extractions could not be quantified by spectrophotometric methods due to the small amount contained in each sample. Precautions were taken to treat all samples in a similar manner in order to produce equivalent DNA yields. The handling and treatment of each oral swab were standardized by use of a consistent swabbing pattern within each rat oral cavity and addition of equal volumes of reagents during extraction procedures. Equal volumes of reagents were used during extraction of the tissue homogenates obtained from preterm fetuses. To ensure that DNA was present, primers directed to the rat globin gene were used to amplify all of the extracted samples. Samples were excluded from analysis if a rat globin amplicon was not present.
RESULTS
Oral swab results from rat pups from 1 to 4 weeks of age.
The presence of P. carinii in four groups of rat pups ranging in age from 1 to 4 weeks was evaluated by PCR amplification, using P. carinii-specific primers, of DNA extracted from oral swabs taken at a single time point. All rats sampled (31 of 31), including the 4 dams, were positive for P. carinii-specific DNA in the oral cavity, as shown by the presence of an amplicon of 137 bp (Table 1, last column). A 400-bp amplicon was produced after amplification of the same samples using primers directed to the globin gene of the rat (data not shown).
TABLE 1.
Time of P. carinii presence in rat neonates by PCR detection of P. carinii-specific DNA from oral swabs
| Group | % Oral swabs positive for Pneumocystis-specific DNA (no. positive/total no.) at the following pup age:
|
|||||
|---|---|---|---|---|---|---|
| 1-2 h | 24 h | 48 h | 72 h | 1 wk | 1-4 wks | |
| Pups | 80 (28/35) | 97 (34/35) | 100 (35/35) | 100 (35/35) | 100 (35/35) | 100 (27/27) |
| Dams | 100 (3/3) | 100 (3/3) | 100 (3/3) | 100 (3/3) | 100 (3/3) | 100 (4/4) |
Oral swab samples from rat neonates within 1 week of age.
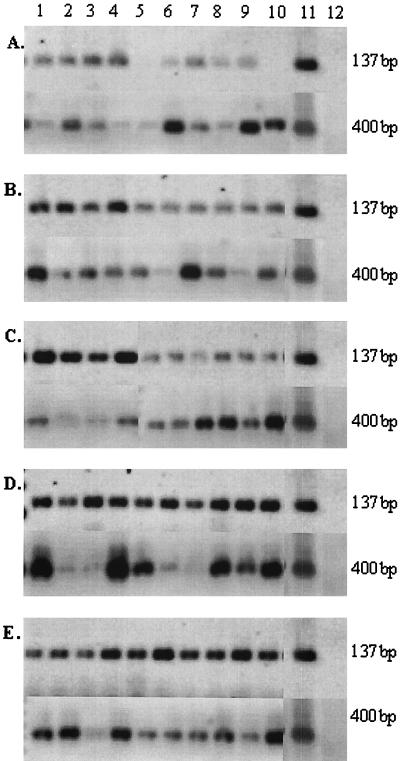
Since all rat pups were positive for P. carinii-specific DNA by the age of 1 week, a second experiment was conducted in which rat pups were sampled at multiple time points within the first week after birth. Sequential sets of oral swabs were collected from three rat dams and their pups at <2, 24, 48, and 72 h and 1 week after birth. Dam 1 had 8 pups, dam 2 had 11 pups, and dam 3 had 16 pups. Within the first 2 h of life, 80% (28 of 35) of these pups were positive for P. carinii-specific DNA by PCR amplification of their oral swabs (Table 1). The seven negative pups were from each of the three dams (three from the first dam, one from the second dam, and three from the last dam). By 24 h, 97% of the pups were positive for P. carinii-specific DNA. By 48 h after birth, all pups were positive for P. carinii-specific DNA and remained so at 72 h and 1 week of age (Table 1). The oral swabs from the three dams of these pups were also positive for P. carinii-specific DNA at all sample time points (Table 1). All samples were positive for rat globin DNA (Fig. 1), verifying the integrity of the extractions. Figure 1 displays representative samples from one group of pups and their dam at 1 to 2 h (Fig. 1A), 24 h (Fig. 1B), 48 h (Fig. 1C), 72 h (Fig. 1D), and 1 week (Fig. 1E).
FIG. 1.
Representative oral swab samples taken from one group of pups and their dam at time points ranging from 1 to 2 h to 1 week after pup birth. Oral swab samples were collected from individual rats within 2 h after pup birth (A) and at 24 h (B), 48 h (C), 72 h (D), and 1 week (E) after birth. All panels show amplicons for Rcc (137 bp) and rat globin (400 bp) primer sets. Lanes 1, oral swabs collected from the dam; lanes 2 to 10, oral swabs collected from individual rat pups; lanes 11, positive control (P. carinii DNA, contaminated with rat DNA); lanes 12, negative control (water).
Assessment of possible vertical transmission of P. carinii.
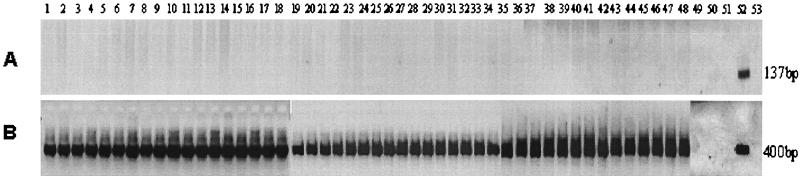
Because rat pups were positive for P. carinii-specific DNA within the first 2 h of life, the possibility that the organisms were transmitted via the placenta was investigated. Rat pups were collected by cesarean section from three gravid rats under aseptic conditions at time points ranging from 10 to 28 days of gestation. The fetuses were separated from maternal tissue as described in Materials and Methods, and DNA was extracted from digests of the intact fetuses. The results of the amplifications from the dams and fetuses and from the control female rats are summarized in Table 2. None of the 48 fetuses were positive for P. carinii-specific DNA amplicons. Likewise, the corresponding placentae from the fetuses were also negative for P. carinii-specific amplicons (but positive for rat globin products). Figure 2A illustrates the lack of amplification of the DNA extracted from the 48 fetuses with the Rcc primers. Figure 2B shows the successful amplification of the same samples with the primers directed to the rat globin gene, verifying the integrity of the extraction. All dams (three of three) were positive for P. carinii-specific DNA by PCR analysis of their oral swabs and also of their lung and spleen tissue homogenates (Table 2). One of the dams was positive for P. carinii-specific DNA in the uterine tissue homogenate. Two nongravid females were sampled as above. Both rats produced P. carinii-specific DNA amplicons from oral swabs and homogenates of lung, spleen, and uterus (Table 2). All samples collected in this study were positive for rat globin DNA.
TABLE 2.
PCR analysis of rat fetal tissues collected by cesarean section
| Tissue or group | % Tissues positive for P. carinii-specific DNA (no. positive/total no.) |
|---|---|
| Rat fetus | 0 (0/48) |
| Rat placenta | 0 (0/48) |
| Rat dams | |
| Oral swab, lung homogenate, spleen | 100 (3/3) |
| Uterus | 33 (1/3) |
| Control female rats (oral swab, lung homogenate, spleen, uterus) | 100 (2/2) |
FIG. 2.
Results of PCR, using P. carinii-specific and rat globin-specific primers, of fetal tissue homogenates collected by cesarean section. (A) Rcc primers; (B) primers directed to rat globin. Lanes 1 to 48, results from individual rat fetuses; lanes 49 to 51, internal negative controls (described in Materials and Methods); lanes 52, positive control (P. carinii or rat DNA); lanes 53, negative control (water). These data were generated from several gels that have been used to create this composite figure. All negative controls were negative on each gel, and all positive controls produced appropriately sized amplicons.
DISCUSSION
The studies presented here are the first systematic antemortem evaluation of the initial acquisition of Pneumocystis in a mammalian host. The presence of P. carinii was evaluated by PCR amplification of samples taken from the oral cavities of rats using primers directed to the mtLSU rRNA. These primers have been shown to be specific and sensitive for P. carinii, with a sensitivity threshold of 17 template copies (10). The presence of P. carinii amplicons in the oral cavities of rats has previously been shown to correlate with the development of pneumonia after chronic immunosuppression of rats under barrier conditions (10). This technique was used in the present study because it afforded the most sensitive method for detection of P. carinii; microscopic methods have a threshold of sensitivity of about 10,000 organisms.
Within 2 h after birth, P. carinii DNA was already present in the oral cavities of newborn rat pups. Attempts to detect P. carinii DNA in digests of whole cesarean section-derived fetuses were unsuccessful, suggesting that the organisms were not transmitted to the pups in utero but were acquired early after birth.
The presence of P. carinii-specific DNA in the oral cavities of the pups examined in our study does not confirm that intact, viable organisms were present in the oral cavities, but it does suggest that the pups were exposed to P. carinii prior to the time when the oral swabs were collected. This observation has important implications for the life cycle of P. carinii. The neonates likely acquired the infectious (but as yet unidentified) form of the organism through direct contact with the dam and/or close contact with their surroundings (i.e., bedding, feces, and air). Since P. carinii DNA was amplified from the oral cavities of the dams before and after the birth of the pups, grooming of the pups by the dam, which involves direct oral contact, could be a potential mechanism for transmission. We have also been able to amplify P. carinii DNA from the walls, air ducts, and floors of the animal room in which these infected rats are housed, as well as from the feces of infected rats (C. R. Icenhour and M. T. Cushion, unpublished data), which may be other potential sources of P. carinii.
Two additional sites of P. carinii transmission should be considered in future studies: the vaginal canal of the dam and her milk. Preliminary studies performed on DNA extracted from the milk of two pregnant dams from the same rat colony that was used in the present study failed to produce amplicons by use of the Rcc primers, suggesting that transmission through the milk to the neonate may not occur. In addition, at the time of oral swabbing of the neonates, we noted whether the pups had ingested milk by visual inspection of the stomach contents through the neonatal skin. P. carinii-positive amplicons were obtained from those pups that had suckled as well as from those that had not, indicating again that milk was not the source of organisms for the neonates. The vaginal cavities of the dams were not sampled during this experiment and cannot be ruled out as a source of infection at this point.
Our findings also raise the possibility that neonates may serve as reservoirs of infection for the general population. The early and efficient transmission of P. carinii to neonates and the sustained presence in this population help to explain our previous findings of the widespread prevalence of P. carinii in commercial rat colonies (10). A recent report from our laboratory showed that very few organisms were required to initiate P. carinii infection in immunosuppressed rats (M. T. Cushion, M. J. Linke, M. S. Collins, S. P. Keely, and J. R. Stringer, abstract from the 6th International Workshop on Opportunistic Protists 1999, J. Eukaryot. Microbiol. 46:111S, 1999), supporting the contention that these organisms are highly infective and very efficient in their transmission.
Another important aspect of these studies was the presence of P. carinii in adult rats that did not receive artificial immunosuppression. Besides the possibility that the neonates may serve as reservoirs of infection, the presence of the organism in the adult female rats (both gravid and nongravid) and in nonimmunosuppressed male rats, as reported in our previous study (10), raises the questions of the length of carriage and latency of P. carinii and the ability of the host animals to transmit the infection. It is plausible that low levels of P. carinii present in normal, healthy adult rats could serve as sources of infection. These low-level infections may be sustained by long-term carriage or by a continuous cycle of organism acquisition and elimination. In our animal facilities, all stages of the rat life cycle are represented, including breeder pairs, pregnant and nursing dams, juveniles, and nonimmunosuppressed and immunosuppressed adult rats. Transmission of P. carinii from infectious adults to the immunologically immature neonates could result in amplification of organisms in the pups; these organisms would then be circulated throughout the entire rat population. These questions must be addressed by a systematic evaluation, similar to the studies reported here.
Our results with rats differ from those of an earlier study which reported the presence of P. carinii in utero. In 1984, Pifer et al. (23) identified Pneumocystis “trophozoites” (presently called trophic forms), but not cysts, by microscopic analysis of lung homogenates from cesarean-section-derived rat fetuses. They proposed that the trophic form was the developmental stage responsible for placental transmission. This study was controversial at the time because microscopic detection of trophic forms in tissue homogenates was (and still is) problematic due to the lack of sufficient morphological criteria permitting differentiation from tissue components, such as platelets. Subsequent studies could not confirm these findings.
More recently, Hong et al. (8) explored the ability of immunosuppressed and nonimmunosuppressed rat dams to transmit the organisms in utero and the ability of their offspring to become infected after birth. Microscopic detection and in situ hybridization techniques were used for detection of the organisms in neonates born of the two maternal types. Only the pups administered steroids and born of the infected and immunosuppressed dam became infected with P. carinii after 1 week (three rats) and 5 weeks (two rats) postpartum as detected by histological staining. Vertical transmission was excluded as the source of the organisms in the rat pup lungs, since the in situ hybridization data were negative at the 1-week time point, indicating to the authors that the organisms had not yet begun to proliferate and were newly acquired after birth. Although our data are in agreement with the lack of vertical transmission in rats, the rat pups included in the study by Hong et al. were not collected by cesarean section, and thus, in utero infection cannot be definitively ruled out by their experimental design.
Animal inoculation studies and genetic analyses have provided sufficient evidence that Pneumocystis organisms harbored by different mammalian hosts are distinct species (27). To date, few biological differences have been reported among the various Pneumocystis populations, but this is likely due to difficulties in conducting such studies rather than to lack of biological diversity. Since our studies were conducted with rats, it seems prudent to consider our results in the context of transmission experiments using other mammalian hosts.
No evidence for Pneumocystis organisms in fetal SCID mouse lung tissue was found by Ito et al. (J. Protozool. 38:218S-219S, 1991) during their evaluations for placental transmission using histological methods. Although the rats used in our studies did not suffer from congenital immunodeficiency as do SCID mice, the lack of Pneumocystis organisms in the fetuses from both rodent species suggests that vertical transmission is not a routine mode used by mouse- and rat-derived Pneumocystis.
The most convincing case for vertical transmission of Pneumocystis in animal models has been reported for rabbits. Nonimmunosuppressed young rabbits routinely develop microscopically detectable infections with Pneumocystis early after birth that can reach burdens in the millions of organisms (26, 28). The infection is gradually resolved as the immune system matures. Cere et al. (3) investigated the possibility of placental transmission and early acquisition in this model using microscopic and PCR-based techniques. Within 2 to 4 h after birth, cysts and Pneumocystis-specific amplicons were detected in the lung homogenates of rabbit neonates. These findings are in agreement with our PCR-oral swab data collected from neonatal rats. However, unlike our results, Pneumocystis-specific DNA was also amplified from rabbit fetuses and their placentae, and organisms could be detected in the fetal lungs and the fetal sides of placentae by toluidine blue O staining of cysts and immunofluorescence.
The discrepancy between rats and rabbits may be due to the lower number of pregnant rats evaluated in our studies, biological differences among the different Pneumocystis species, or perhaps differences in the placental anatomy of rats and rabbits. We evaluated 3 dams and their 48 fetuses, while the studies of the rabbit model used 10 pregnant rabbits and 60 fetuses. It is possible that in utero transmission could rarely occur in rats, and with additional studies this mode of transmission might be revealed. However, all fetuses of all the pregnant rabbits showed the presence of Pneumocystis, suggesting that this may be a frequent mode of transmission for this Pneumocystis species. The placental mode of transmission used by Pneumocystis in rabbits could be due to differences in the biology of the rat- and rabbit-derived organisms. This possibility is difficult to address at this time because of problems associated with the continuous culture of Pneumocystis.
Differences in placental anatomy among the different mammalian hosts offer an alternative explanation. Mice, rats, rabbits, and humans are similar in the type of placenta they have, which is discoid in shape and deciduate in maternal relation, with a hemochorial placental membrane (25). Hemochorial placentae lack any of the maternal tissue present in early stages of gestation, resulting in direct contact of the chorionic villi with maternal blood (25). However, one significant difference among human, murine, and rabbit placentae has been noted by Cere et al. and proposed as a potential reason for the placental transmission of Pneumocystis in rabbits (3). The rabbit placenta is hemochorial until the 17th day of gestation, after which it becomes hemoendothelial. The barrier between fetal and maternal blood vessels in the hemoendothelial type of placenta is minimal, consisting only of the endothelial lining of the blood vessels in the villi (25). This reduced barrier might facilitate the transfer of organisms from the maternal tissue to the fetus, resulting in the in utero transmission observed in the rabbit model. The novel infection pattern of Pneumocystis in rabbits, specifically the relatively large organism burden in the neonates soon after birth, is consistent with an ongoing infection in the pups and supports in utero transmission. In contrast, neonatal rats do not harbor large organism burdens and differ in their placental anatomy from rabbits; thus, placental transmission would not be expected to be a major mode of transmission used by the rat-derived organism.
In human beings, morphological detection of Pneumocystis organisms in the lungs of stillborn infants and neonates suggested that Pneumocystis was acquired early in life, and at times through transmission in utero (2, 5, 6, 20, 24, 30; Mortier et al., letter). Recently, Vargas et al. (30) used nasopharyngeal swabs combined with PCR (directed to the same mtLSU region targeted in the present study) to identify Pneumocystis-specific DNA in healthy human neonates as early as 1 month of age. The presence of Pneumocystis in stillborn infants is suggestive of vertical transmission and is in contrast to our findings in the rat, while detection of the organism in the lungs and nasopharyngeal samples from human infants is in agreement with our findings. The evidence for vertical transmission of Pneumocystis in human beings has been sporadic, relying primarily on case reports of stillborn infants. The human placenta, like the murine, does not change from hemochorial to hemoendothelial; however, the length of gestation of humans might facilitate breaches in permeability, resulting in the rare placental transmission.
A somewhat unexpected finding was the presence of P. carinii amplicons in the spleen and uterine tissues of one out of three gravid dams and both nongravid female rats. There are numerous reports of extrapulmonary pneumocystosis in frankly immunosuppressed humans, involving a multitude of organs and body sites (14, 29). Dissemination of the organism outside the lungs has also been reported for animals, including simian immunodeficiency virus-infected macaques (35) and immunosuppressed rats (4). However, our rats were not frankly immunosuppressed, and attempts made by other investigators to amplify Pneumocystis DNA from the lungs and nasopharyngeal aspirates (washes of the upper respiratory tract with saline) of nonimmunosuppressed rats (18) or from organs other than the lung in 28-day-old rabbits with pneumocystosis (28) have been unsuccessful. Reasons for the detection of P. carinii in our female rats, which were either nonimmunosuppressed or slightly immunosuppressed due to pregnancy, may involve either sampling and sensitivity differences among the techniques used or the nature of the organism, in the case of rabbit versus rat Pneumocystis. Further investigation of the trafficking of P. carinii throughout its mammalian host, beginning with the initial contact, will be necessary to resolve the reasons for these apparent differences.
The ability to detect P. carinii DNA in oral swab samples represents a significant technical breakthrough that can be used to increase our understanding of the life cycle of P. carinii in an antemortem setting. Sampling of the oral cavity and PCR detection of Pneumocystis have now been authenticated as a means to determine Pneumocystis exposure in two mammalian hosts, rats and humans, in both immunosuppressed and nonimmunosuppressed states (7, 10, 12, 18, 28, 31). Besides the obvious diagnostic application, this technique provides an experimental tool that can be used for selection of rats exposed to P. carinii prior to induction into an experiment, so that investigators need not wait to discover the presence of the organism in control animals after 8 to 12 weeks of immunosuppression. This sampling method will also be useful for establishment of P. carinii-free rat colonies, by permitting identification of potential mating pairs that have not been exposed to the organism previously.
Acknowledgments
This research was supported by NIH grant RO1 AI32436.
REFERENCES
- 1.Bartlett, M. S., S. H. Vermund, R. Jacobs, P. J. Durant, M. M. Shaw, J. W. Smith, X. Tang, J. Lu, B. Li, S. Jin, and C. Lee. 1997. Detection of Pneumocystis carinii DNA in air samples: likely environmental risk to susceptible persons. J. Clin. Microbiol. 35:2511-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazaz, G. R., O. L. Manfredi, R. G. Howard, and A. A. Claps. 1970. Pneumocystis carinii pneumonia in three full-term siblings. J. Pediatr. 76:767-769. [DOI] [PubMed] [Google Scholar]
- 3.Cere, N., F. Drouet-Viard, E. Dei-Cas, N. K. Chanteloup, and P. Coudert. 1997. In utero transmission of Pneumocystis carinii f. sp. oryctolagi. Parasite 4:325-330. [DOI] [PubMed] [Google Scholar]
- 4.Chary-Reddy, S., and D. C. Graves. 1996. Identification of extrapulmonary Pneumocystis carinii in immunocompromised rats by PCR. J. Clin. Microbiol. 34:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutz, W. 1970. Pneumocystis carinii pneumonia. Pathol. Annu. 5:309-340. [PubMed] [Google Scholar]
- 6.Gajdusek, D. C. 1957. Pneumocystis carinii—etiologic agent of interstitial plasma cell pneumonia of premature and young infants. Pediatrics 19:543-564. [PubMed] [Google Scholar]
- 7.Helweg-Larsen, J., A. G. Tsolaki, R. F. Miller, B. Lundgren, and A. E. Wakefield. 1998. Clusters of Pneumocystis carinii pneumonia: analysis of person-to-person transmission by genotyping. Q. J. Med. 91:813-820. [DOI] [PubMed] [Google Scholar]
- 8.Hong, S., Y. Park, J. Kim, D. Kim, and C. Yun. 1999. Is Pneumocystis carinii vertically transmitted to neonatal rats? Korean J. Parasitol. 37:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes, W. T. 1982. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J. Infect. Dis. 145:842-848. [DOI] [PubMed] [Google Scholar]
- 10.Icenhour, C. R., S. L. Rebholz, M. S. Collins, and M. T. Cushion. 2001. Widespread detection of Pneumocystis carinii in commercial rat colonies using targeted PCR and oral swabs. J. Clin. Microbiol. 39:3437-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundgren, B., and A. E. Wakefield. 1998. PCR for detecting Pneumocystis carinii in clinical or environmental samples. FEMS Immunol. Med. Microbiol. 22:97-101. [DOI] [PubMed] [Google Scholar]
- 12.Martino, A. M., E. Visconti, M. Zolfo, O. Genovese, C. Rendeli, P. Mencarini, and E. Tamburrini. 1999. Noninvasive diagnosis of P. carinii pneumonia on oral washes in an HIV-infected child. Pediatr. Pulmonol. 28:352-355. [DOI] [PubMed] [Google Scholar]
- 13.Meuwissen, J. H. E., I. Tauber, A. D. E. M. Leeuwenberg, P. J. A. Beckers, and M. Sieben. 1977. Parasitologic and serologic observations of infection with Pneumocystis in humans. J. Infect. Dis. 136:43-49. [DOI] [PubMed] [Google Scholar]
- 14.Ng, V. L., D. M. Yajko, and W. K. Hadley. 1997. Extrapulmonary pneumocystosis. Clin. Microbiol. Rev. 10:401-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Leary, T. J., M. M. Tsai, C. F. Wright, and M. T. Cushion. 1995. Use of semiquantitative PCR to assess onset and treatment of Pneumocystis carinii infection in a rat model. J. Clin. Microbiol. 33:718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson, M., C. Lindman, S. Latouche, A. Bjorkman, P. Roux, E. Linder, and M. Wahlgren. 1998. Identification of Pneumocystis carinii f. sp. hominis gene sequences in filtered air in hospital environments. J. Clin. Microbiol. 36:1737-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsson, M., A. Sukara, L. Lindberg, and E. Linder. 1996. Detection of Pneumocystis carinii DNA by filtration of air. Scand. J. Infect. Dis. 28:279-282. [DOI] [PubMed] [Google Scholar]
- 18.Oz, H. S., and W. T. Hughes. 1999. DNA amplification of nasopharyngeal aspirates in rats: a procedure to detect Pneumocystis carinii. Microb. Pathog. 27:119-121. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, R. J., M. T. Cushion, and A. E. Wakefield. 1999. Discrimination of rat-derived Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. ratti using the polymerase chain reaction. Mol. Cell. Probes 13:147-155. [DOI] [PubMed] [Google Scholar]
- 20.Pavlica, F. 1962. The first observation of congenital pneumocystic pneumonia in a fully developed stillborn child. Ann. Paediatr. 198:177-184. [PubMed] [Google Scholar]
- 21.Peglow, S. L., A. G. Smulian, M. J. Linke, C. L. Pogue, S. Nurre, J. Crisler, J. Phair, J. W. Gold, D. Armstrong, and P. Walzer. 1990. Serologic responses to Pneumocystis carinii antigens in health and disease. J. Infect. Dis. 161:296-306. [DOI] [PubMed] [Google Scholar]
- 22.Pifer, L. L., W. T. Hughes, S. Stagno, and D. Woods. 1978. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics 61:35-41. [PubMed] [Google Scholar]
- 23.Pifer, L. L., C. P. Lattuada, C. C. Edwards, L. R. Woods, and D. R. Owens. 1984. Pneumocystis carinii infection in germ-free rats: implications for human patients. Diagn. Microbiol. Infect. Dis. 2:23-36. [DOI] [PubMed] [Google Scholar]
- 24.Post, C., W. Dutz, and I. Nasarian. 1964. Endemic Pneumocystis carinii pneumonia in south Iran. Arch. Dis. Child. 39:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey, E. M. 1982. The placenta: human and animal. Praeger, New York, N.Y.
- 26.Soulez, B., E. Dei-Cas, P. Charet, G. Mougeot, M. Caillaux, and D. Camus. 1989. The young rabbit: a nonimmunosuppressed model for Pneumocystis carinii pneumonia. J. Infect. Dis. 160:355-356. [DOI] [PubMed] [Google Scholar]
- 27.Stringer, J. R., M. T. Cushion, and A. E. Wakefield. 2001. New nomenclature for the genus Pneumocystis. J. Eukaryot. Microbiol. (Suppl.):184S-189S. [DOI] [PubMed]
- 28.Tamburrini, E., E. Ortona, E. Visconti, P. Mencarini, P. Martgutti, M. Zolfo, S. Barca, S. E. Peters, A. E. Wakefield, and A. Siracusano. 1999. Pneumocystis carinii infection in young non-immunosuppressed rabbits. Kinetics of infection and of the primary specific immune response. Med. Microbiol. Immunol. (Berlin) 188:1-7. [DOI] [PubMed] [Google Scholar]
- 29.Telzak, E. E., R. J. Cote, J. W. M. Gold, S. W. Campbell, and D. Armstrong. 1990. Extrapulmonary Pneumocystis carinii infections. Rev. Infect. Dis. 12:380-386. [DOI] [PubMed] [Google Scholar]
- 30.Vargas, S., W. T. Hughes, M. E. Santolaya, A. V. Ulloa, C. A. Ponce, C. E. Cabrera, F. Cumsille, and F. Gigliotti. 2001. Primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 32:855-861. [DOI] [PubMed] [Google Scholar]
- 31.Vargas, S., C. A. Ponce, F. Gigliotti, A. V. Ulloa, S. Prieto, M. P. Munoz, and W. T. Hughes. 2000. Transmission of Pneumocystis carinii DNA from a patient with P. carinii pneumonia to immunocompetent contact health care workers. J. Clin. Microbiol. 38:1536-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vargas, S., C. A. Ponce, W. T. Hughes, A. E. Wakefield, J. C. Weitz, S. Donoso, A. V. Ulloa, P. Madrid, S. Gould, J. J. Latorre, R. Avilla, S. Benveniste, M. Gallo, J. Belletti, and R. Lopez. 1999. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin. Infect. Dis. 29:1489-1493. [DOI] [PubMed] [Google Scholar]
- 33.Wakefield, A. E. 1996. DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J. Clin. Microbiol. 34:1754-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walzer, P. D., V. Schnelle, D. Armstrong, and P. P. Rosen. 1977. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science 197:177-179. [DOI] [PubMed] [Google Scholar]
- 35.Yanai, T., M. A. Simon, F. D. Doddy, K. G. Mansfield, D. Pauley, and A. A. Lackner. 1999. Nodular Pneumocystis carinii pneumonia in SIV-infected macaques. Vet. Pathol. 36:471-474. [DOI] [PubMed] [Google Scholar]