Abstract
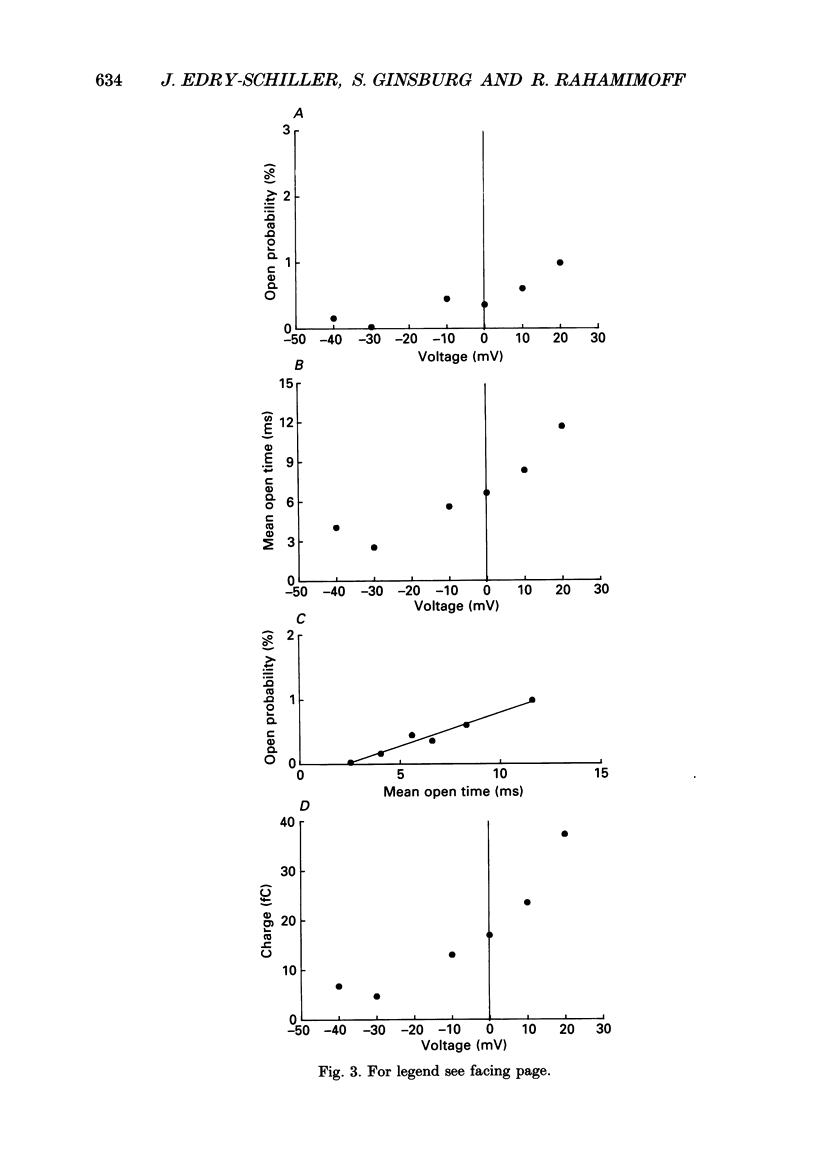
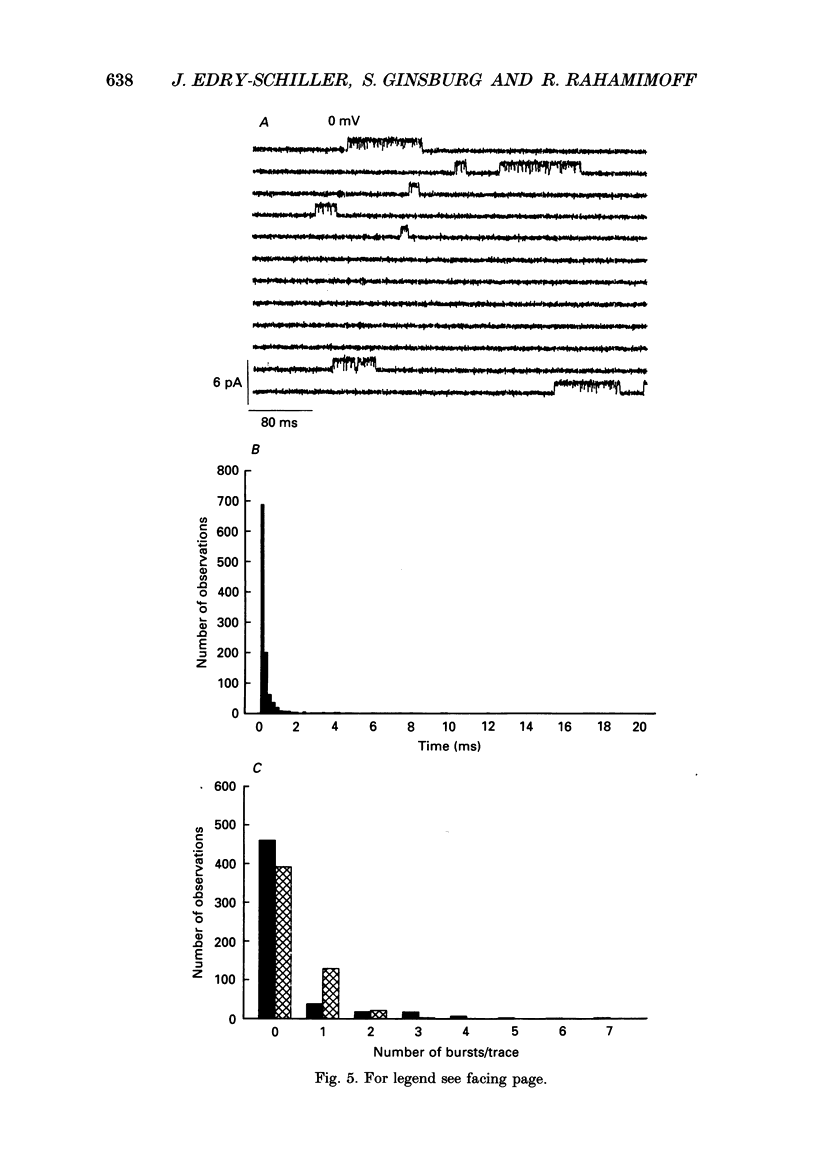
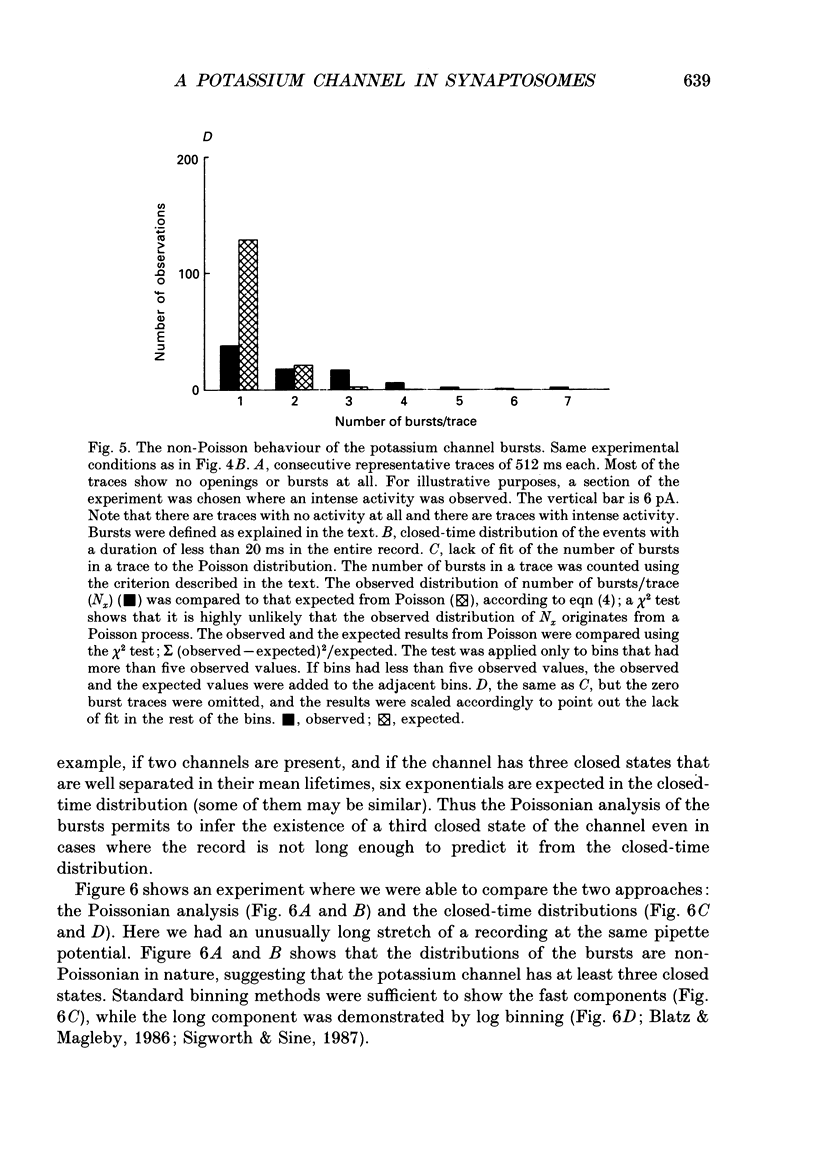
1. Pinched-off cholinergic nerve terminals (synaptosomes) prepared from the electric organ of Torpedo ocelata were fused into large structures (greater than 20 microns) using dimethyl sulphoxide and polyethylene glycol 1500, as previously described for synaptic vesicles from the same organ. 2. The giant fused synaptosomes were easily amenable to the patch clamp technique and 293 seals with a resistance greater than 4 G omega were obtained in the 'cell-attached' configuration. In a large fraction of the experiments, an 'inside-out' patch configuration was achieved. 3. Several types of unitary ionic currents were observed. This study describes the most frequently observed single-channel activity which was found in 247 out of the 293 membrane patches (84.3%). 4. The single-channel current-voltage relation was linear between -60 and 20 mV and showed a slope conductance of 23.8 +/- 1.3 pS when the pipette contained 350-390 mM-Na+ and the bath facing the inside of the synaptosomal membrane contained 390 mM-K+. 5. From extrapolated reversal potential measurements, it was concluded that this channel has a large selectivity for K+ over Na+ (70.4 +/- 11.5, mean +/- S.E.M.). Chloride ions are not transported significantly through this potassium channel. 6. This potassium channel has a low probability of opening. The probability of being in the open state increases upon depolarization and reaches about 1% when the inside of the patch is 20 mV positive compared to the pipette side. 7. The mean channel open time increases with depolarization; thus the product current x time (= charge) also increases upon depolarization, showing properties of an outward rectifier. 8. The potassium channel in the giant synaptosome membrane has a bursting behaviour. Open-time distribution, closed-time distribution and a Poisson analysis indicate that the minimal kinetic scheme requires one open state and three closed states.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Morita K., Scappaticci K. A. Effects of tetraethylammonium on the depolarizing after-potential and passive properties of lizard myelinated axons. J Physiol. 1988 Aug;402:65–78. doi: 10.1113/jphysiol.1988.sp017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartschat D. K., Blaustein M. P. Potassium channels in isolated presynaptic nerve terminals from rat brain. J Physiol. 1985 Apr;361:419–440. doi: 10.1113/jphysiol.1985.sp015653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990 Feb;421:247–262. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton F. L., Hutter O. F. Sensitivity to flow of intrinsic gating in inwardly rectifying potassium channel from mammalian skeletal muscle. J Physiol. 1990 May;424:253–261. doi: 10.1113/jphysiol.1990.sp018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Criado M., Keller B. U. A membrane fusion strategy for single-channel recordings of membranes usually non-accessible to patch-clamp pipette electrodes. FEBS Lett. 1987 Nov 16;224(1):172–176. doi: 10.1016/0014-5793(87)80442-8. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Penner R. The actions of presynaptic snake toxins on membrane currents of mouse motor nerve terminals. J Physiol. 1987 May;386:455–463. doi: 10.1113/jphysiol.1987.sp016544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Farley J., Rudy B. Multiple types of voltage-dependent Ca2+-activated K+ channels of large conductance in rat brain synaptosomal membranes. Biophys J. 1988 Jun;53(6):919–934. doi: 10.1016/S0006-3495(88)83173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Katz B., Miledi R. The antagonism between botulinum toxin and calcium in motor nerve terminals. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):369–376. doi: 10.1098/rspb.1982.0080. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hevron E., David G., Arnon A., Yaari Y. Acetylcholine modulates two types of presynaptic potassium channels in vertebrate motor nerve terminals. Neurosci Lett. 1986 Dec 3;72(1):87–92. doi: 10.1016/0304-3940(86)90624-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima N., Kirino Y. Potassium channels in synaptosomal membrane examined using patch-clamp techniques and reconstituted giant proteoliposomes. Biochim Biophys Acta. 1988 Dec 22;946(2):209–214. doi: 10.1016/0005-2736(88)90394-x. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. Electrical excitability of motor nerve terminals in the mouse. J Physiol. 1985 Sep;366:411–421. doi: 10.1113/jphysiol.1985.sp015805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J., Cooke I. M., Stuenkel E. L. Single channels and ionic currents in peptidergic nerve terminals. 1986 Jan 30-Feb 5Nature. 319(6052):410–412. doi: 10.1038/319410a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989 Jul;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. Electric current flow inside perineurial sheaths of mouse motor nerves. J Physiol. 1985 Nov;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. Presynaptic currents in frog motor endings. Pflugers Arch. 1984 Jan;400(1):8–13. doi: 10.1007/BF00670529. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Meunier F. M. Relationship between presynaptic membrane potential and acetylcholine release in synaptosomes from Torpedo electric organ. J Physiol. 1984 Sep;354:121–137. doi: 10.1113/jphysiol.1984.sp015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D. M., McDowall G., Sarne Y. Opiates inhibit acetylcholine release from Torpedo nerve terminals by blocking Ca2+ influx. J Neurochem. 1984 Sep;43(3):614–618. doi: 10.1111/j.1471-4159.1984.tb12779.x. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Sokolovsky M. Induced acetylcholine release from active purely cholinergic Torpedo synaptosomes. J Neurochem. 1978 Jan;30(1):217–230. doi: 10.1111/j.1471-4159.1978.tb07055.x. [DOI] [PubMed] [Google Scholar]
- Miyake M., Christie M. J., North R. A. Single potassium channels opened by opioids in rat locus ceruleus neurons. Proc Natl Acad Sci U S A. 1989 May;86(9):3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Barrett E. F. Evidence for two calcium-dependent potassium conductances in lizard motor nerve terminals. J Neurosci. 1990 Aug;10(8):2614–2625. doi: 10.1523/JNEUROSCI.10-08-02614.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Reinhardt R. Single ion-channel current measurements from rat brain synaptosomes in planar lipid bilayers. Biophys J. 1984 Jan;45(1):60–62. doi: 10.1016/S0006-3495(84)84108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Roudna M., Bamberg E. Single K+-channel current measurements from brain synaptosomes in lipid bilayers. Am J Physiol. 1983 Jul;245(1):C151–C156. doi: 10.1152/ajpcell.1983.245.1.C151. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., DeRiemer S. A., Ginsburg S., Kaiserman I., Sakmann B., Shapira R., Stadler H., Yakir N. Ionic channels in synaptic vesicles: are they involved in transmitter release? Q J Exp Physiol. 1989 Dec;74(7):1019–1031. doi: 10.1113/expphysiol.1989.sp003330. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., DeRiemer S. A., Sakmann B., Stadler H., Yakir N. Ion channels in synaptic vesicles from Torpedo electric organ. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5310–5314. doi: 10.1073/pnas.85.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., Rahamimoff R. Neuromuscular synapse: stochastic properties of spontaneous release of transmitter. Science. 1970 Nov 6;170(3958):648–649. doi: 10.1126/science.170.3958.648. [DOI] [PubMed] [Google Scholar]
- Saint D. A., Quastel D. M., Guan Y. Y. Multiple potassium conductances at the mammalian motor nerve terminal. Pflugers Arch. 1987 Nov;410(4-5):408–412. doi: 10.1007/BF00586518. [DOI] [PubMed] [Google Scholar]
- Saito Y., Hirashima N., Kirino Y. Giant proteoliposomes prepared by freezing-thawing without use of detergent: reconstitution of biomembranes usually inaccessible to patch-clamp pipette microelectrode. Biochem Biophys Res Commun. 1988 Jul 15;154(1):85–90. doi: 10.1016/0006-291x(88)90653-5. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]


