
Keywords: Chil1, hippocampus, learning and memory, M2 microglia, neuroinflammation, postoperative cognitive dysfunction (POCD), recombinant CHI3L1
Abstract
Postoperative cognitive dysfunction is a severe complication of the central nervous system that occurs after anesthesia and surgery, and has received attention for its high incidence and effect on the quality of life of patients. To date, there are no viable treatment options for postoperative cognitive dysfunction. The identification of postoperative cognitive dysfunction hub genes could provide new research directions and therapeutic targets for future research. To identify the signaling mechanisms contributing to postoperative cognitive dysfunction, we first conducted Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses of the Gene Expression Omnibus GSE95426 dataset, which consists of mRNAs and long non-coding RNAs differentially expressed in mouse hippocampus 3 days after tibial fracture. The dataset was enriched in genes associated with the biological process “regulation of immune cells,” of which Chil1 was identified as a hub gene. Therefore, we investigated the contribution of chitinase-3–like protein 1 protein expression changes to postoperative cognitive dysfunction in the mouse model of tibial fracture surgery. Mice were intraperitoneally injected with vehicle or recombinant chitinase-3–like protein 1 24 hours post-surgery, and the injection groups were compared with untreated control mice for learning and memory capacities using the Y-maze and fear conditioning tests. In addition, protein expression levels of proinflammatory factors (interleukin-1β and inducible nitric oxide synthase), M2-type macrophage markers (CD206 and arginase-1), and cognition-related proteins (brain-derived neurotropic factor and phosphorylated NMDA receptor subunit NR2B) were measured in hippocampus by western blotting. Treatment with recombinant chitinase-3–like protein 1 prevented surgery-induced cognitive impairment, downregulated interleukin-1β and nducible nitric oxide synthase expression, and upregulated CD206, arginase-1, pNR2B, and brain-derived neurotropic factor expression compared with vehicle treatment. Intraperitoneal administration of the specific ERK inhibitor PD98059 diminished the effects of recombinant chitinase-3–like protein 1. Collectively, our findings suggest that recombinant chitinase-3-like protein 1 ameliorates surgery-induced cognitive decline by attenuating neuroinflammation via M2 microglial polarization in the hippocampus. Therefore, recombinant chitinase-3–like protein 1 may have therapeutic potential for postoperative cognitive dysfunction.
Introduction
Cognitive impairment, disorientation, and personality changes are frequent complications following anesthesia and surgery, a condition termed postoperative cognitive dysfunction (POCD) (Liu et al., 2021a; Lang et al., 2023). Because of progressive population aging, greater numbers of older adult patients are receiving surgery under anesthesia, and this population is particularly prone to POCD. Furthermore, POCD can greatly impair quality of life and increases the risks of disability and mortality. Several mechanisms have been implicated in POCD, including neuroinflammation, oxidative stress, and dysregulated autophagy (Liu et al., 2022b). However, owing to the complexity of POCD pathogenesis, there are still no viable treatment options.
Second-generation gene sequencing, microarray technology, and bioinformatic analysis enable the identification of genes and gene networks associated with disease occurrence and development, including diagnostic biomarkers and therapeutic targets (Pan et al., 2023; Wang et al., 2024). We previously identified hub genes associated with cognitive impairment in Alzheimer’s disease (Xu et al., 2011; Liu et al., 2021c). Wei et al. (2017) used microarray analysis to identify long non-coding (lnc)RNAs and microRNAs in the hippocampus of POCD model mice. However, bioinformatics databases are frequently updated as new gene–gene and gene–function relationships are identified, warranting a reanalysis of potential hub genes in POCD pathogenesis.
Our reanalysis identified Chil1 as a hub gene in the network contributing to the occurrence and development of POCD. Chil1 encodes chitinase-3–like protein 1 (CHI3L1), a secretory glycoprotein with a molecular weight of 43 kDa. It is expressed by macrophages, neutrophils, endothelial cells, astrocytes, and dendritic cells (Sutherland, 2018), which strongly implicates CHI3L1 in the regulation of neuroinflammation. Indeed, CHI3L1 is regarded as a biomarker for several neurological diseases associated with neuroinflammation. At the cellular level, CHI3L1 may regulate the balance between proinflammatory and immunoregulatory microglia and astrocytes (Im et al., 2020). In addition to their role in neuroimmune modulation, microglia and astrocytes are critical regulators of neurotransmitter signaling, neuroplasticity, and brain metabolism, and can both promote and hinder neuronal repair and survival following various insults depending on immune phenotype.
Microglia differentiate into M1 and M2 subtypes and astrocytes differentiate into A1 and A2 types under specific signaling conditions (Liu et al., 2023; Flores and Tang, 2024). The M1 and A1 subtypes promote inflammation through the secretion of inflammatory cytokines, whereas M2 and A2 cells inhibit inflammation and promote neuronal survival and plasticity by secreting brain-derived neurotrophic factor (BDNF) (Kim and Son, 2021). CHI3L1 is secreted primarily by astrocytes and promotes microglial polarization to the M2 phenotype via the extracellular signal-regulated kinase (ERK) signaling pathway (Bonneh-Barkay et al., 2012). In addition, CHI3L1 modulates phosphoinositide 3-kinase (PI3K)–Akt and Wnt–β-catenin signaling pathways, both of which regulate neurotransmission and plasticity (He et al., 2013; Geng et al., 2018; Ma et al., 2021).
Therefore, changes in CHI3L1 may regulate neuroinflammation and the neural processing and plasticity mechanisms underlying learning and memory. In this study, we examined if recombinant CHI3L1 (rCHI3L1) treatment can alleviate neuroinflammation and cognitive decline in an animal model of POCD via ERK-regulated M2 microglia polarization.
Methods
Data Source
We downloaded the raw dataset of GSE95426 from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.gov/geo/). The platform of GSE95426 was GPL22782 (Agilent-074512 CBCmouselncRNAmRNA180k). GSE95426 consisted of hippocampal mRNA and lncRNA from POCD mice and control mice by microarray. Mice from the POCD group had experienced tibia fracture surgery, and mice from the control group did not receive any prior treatment or surgery (Wei et al., 2017).
Data preprocessing
Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml) is a tool to align sequence to reference sequence (Langmead and Salzberg, 2012). We used Bowtie2 to map the probe’s sequence to the specific gene. Then, we used R (http://cran.r-project.org/package) to conduct the remaining steps (R Core Team, 2021). The Aglip extension of R was used to process the microarray data of Agilent Platform (Chain, 2022).
Identification of differentially expressed genes
LIMMA (http://bioconductor.org/packages/release/bioc/html/limma.html) is a package for the comparison of gene expression in R (Ritchie et al., 2015). It was used to compare mRNA expression between POCD and control mice. Differentially expressed genes (DEGs) were screened out by the threshold of P < 0.05 and log2 |fold change| > 0.4 (Uddin et al., 2022), and were shown in a volcano plot and heatmap.
Enrichment analysis of differentially expressed genes
The Gene Ontology (GO) functional annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed by using the analysis package clusterProfiler in R (http://bioconductor.org/packages/release/bioc/html/clusterProfiler.html), which is an extension for functional and comparative study (Wu et al., 2021). GO analysis consists of cellular component, biological process and molecular function domains. A threshold of P < 0.05 and enrichment counts > 2 were set as thresholds.
Protein–protein interaction networks of differentially expressed genes
Search Tool for the Retrieval of Interacting Genes (STRING) database (https://string-db.org/) is an online tool to calculate protein–protein interaction (PPI) connections and identify potential relationships among DEGs (von Mering et al., 2003). Cytoscape software (https://apps.cytoscape.org/) was used to visualize the results (Shannon et al., 2003). The hub genes were screened out by the plugin cytoHubba of Cytoscape software, a tool that identifies hub genes through algorithms (Chin et al., 2014). The top 10 genes calculated from clustering coefficient algorithm by cytoHubba were constructed as the PPI network, among which ranked top were considered as hub genes (Parra-Medina et al., 2020).
Animals
All animal experiments were conducted in accordance with international laws and National Institutes of Health policies, including the Guide for the Care and Use of Laboratory Animals (8th ed., National Research Council, 2011). This study was reported in accordance with the ARRIVE 2.0 guidelines (Animal Research: Reporting of In Vivo Experiments; Percie du Sert et al., 2020). All procedures were approved by the Experimental Animals Welfare and Ethics Inspection of Nanjing Drum Tower Hospital Affiliated with the Medical College of Nanjing University (Nanjing, China) (approval No. 2019AE01077) on October 20, 2019. C57BL/6J specific-pathogen-free male mice (n = 200), weighing 25–28 g and aged 3 months, were purchased from Ziyuan Laboratory Animal Technology Co. Ltd. (Hangzhou, China) (license No. SCXK (Zhe) 2019-0004). All mice were maintained in separated cages with free access to food and water in a room with a 12-hour light/dark cycle, a temperature of 22 ± 2°C and a humidity of 50%–70%. The behavioral testing was initiated after the mice had adjusted to the environment for 2 weeks.
Animal model
Intramedullary fixation surgery for tibial fracture was conducted under isoflurane anesthesia, as described in a previous study (Xiong et al., 2018). The mice were first deeply anesthetized with isoflurane (H20020267, Lunan Better Pharmaceutical Co., Ltd., China) (3% isoflurane in oxygen for induction in an induction chamber and 1.5% isoflurane in oxygen for maintenance using an inhalation mask). The surgery was conducted once mice entered a deep anesthesia state. An incision was made at 1–2 mm lateral to the right knee, then an intramedullary fixation pin (0.38 mm) was inserted into the bone marrow cavity from the tibial plateau. Next, the distal third of the tibia was clipped. Finally, 2% lidocaine was injected at the incision site. For experiment 1, behavioral tests were conducted at 3 days after surgery (Figure 1). For experiment 2, rCHI3L1 (50929-M08H, Sino Biological, Beijing, China) was administered (0.25 µg/g, intraperitoneally) 24 hours after surgery, followed by behavioral testing at 3, 7, and 14 days after surgery (Figure 1), and the mice were divided into four groups: Control + Vehicle (C + Veh), Control + rCHI3L1 (C + rCLP), Surgery + Vehicle (S + Veh), and Surgery + rCHI3L1 (S + rCLP). For experiment 3, ERK inhibitor PD98059 (167869-21-8, MCE, Shanghai, China) was administered (0.3 mg/kg, intraperitoneally) 2 hours before rCHI3L1 treatment, followed by behavioral testing at 3 days after surgery (Figure 1), and the mice were divided into three groups: S + Veh, S + rCLP, and S + PD98059 + rCLP (S + PD + rCLP).
Figure 1.

Timeline of the experimental procedures.
FC: Fear conditioning; rCHI3L1: recombinant chitinase-3–like protein 1.
Y-maze test
The Y-maze test was performed as described in a previous study (Qiu et al., 2020a). The custom-made Y-maze comprised three equal arms at equivalent angles that were named as A, B, and C (material: polyvinyl chloride; length 35 cm, breadth 7 cm and height 15 cm). In each trial, mice were put in a predefined arm and allowed to explore the maze for 8 minutes, during which the sequence of entries to each arm was recorded. After each trial, 75% alcohol was used to clean the maze to remove odors. The spontaneous alternation percentage was calculated as: spontaneous alternation (%) = the number of correct entries set/(total entries – 2) × 100.
Fear conditioning test
The fear conditioning test (FCT) was performed using the Startle and Fear combined system (Panlab, Barcelona, Spain), which consisted of contextual and cued fear conditioning tests and were conducted as previously described to assess contextual memory and cued memory (Liu et al., 2022a). The FCT contained a training phase and a contextual test phase. During the training phase, mice were placed in the dark chamber and allowed to explore freely for 5 minutes. Then, a high frequency noise (4000 Hz, 80 dB) was presented for 30 seconds. During the last 2 seconds, a foot shock (0.8 mA) was administered. After the shock, mice were left in the chamber for 1 minute before the next noise–shock pair. After the second shock, mice remained in the chamber for 30 seconds. On the second day, the testing phase was conducted. Mice were placed into the same chamber for 5 minutes, and freezing time was recorded. Finally, freezing time percentage was calculated as: freezing time (%) = freezing time/total time × 100.
Protein extraction and western blotting
Hippocampi were sonicated in RIPA lysis buffer (Beyotime, Shanghai, China), and protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA) were used to prevent degradation. Protein concentrations were measured using the Bicinchoninic Acid Protein Assay Kit (Thermo). The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Biofroxx, Germany) and then transferred to Immobilon polyvinylidene fluoride membranes (Merck, Darmstadt, Germany). The blots were blocked with 5% bovine serum albumin for 2 hours and incubated with primary antibodies against the following proteins: mouse anti-tubulin (1:7500, AT8191, Beyotime); rabbit anti-CHI3L1 (1:1000, 12036-1-AP), rabbit anti-CD206 (1:1000, 12036-1-AP), rabbit anti-interleukin-13 receptor subunit α2 (IL13Rα2; 1:1000, 11059-1-AP) and rabbit anti-Arg1 (1:1000, 16001-1-AP) (all from Proteintech, Wuhan, China); rabbit anti-phosphoERK1/2 (Thr202/Tyr204; #4370, 1:1000) and rabbit anti-inducible nitric oxide synthase (iNOS; 1:1000, 13120S) (all from Cell Signaling Technology, Danvers, MA, USA); rabbit anti-interleukin-1 beta (IL-1β; 1:1000, ab9722), rabbit anti-brain-derived neurotrophic factor (BDNF; 1:3000, ab108319), and rabbit anti-phosphorylated N-methyl-D-aspartic acid receptor 2B subunit (p-NR2B; 1:1000, ab3856) (all from Abcam, Cambridge, MA, USA) at 4°C overnight. After washes with Tris-buffered saline-Tween 20, membranes were incubated with the corresponding horseradish peroxidase-conjugated goat anti-rabbit (1:5000, AT0208, Beyotime) or goat anti-mouse (1:5000, AT0216, Beyotime) secondary antibody for 2 hours at room temperature. Tubulin was used as a control. Finally, the protein bands were detected by using an enhanced chemiluminescence detection kit (Pierce Biotechnology, Rockford, IL, USA) and their relative intensity was quantified with ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA; Schneider et al., 2012). Relative protein expression was normlized to Tubulin.
Statistical analysis
All data were expressed as the mean ± standard deviation. Data were analyzed using Student’s t-test and one-way ANOVA followed by Tukey’s post hoc test. All data were analyzed with SPSS 26.0 software (IBM Corp., Armonk, NY, USA), and P < 0.05 was considered statistically significant.
Results
Chil1 plays a role in the hippocampus of POCD mice via bioinformatic analysis and verification
To identify the hub genes involved in POCD, we conducted bioinformatics analysis using the content of the GEO database. The dataset GSE95426 contained the expression profiles of genes in the hippocampus of six POCD and six control mice. In total, 101 DEGs were identified between the two groups, with 62 upregulated (e.g., Chil1 and Nusap1) and 39 downregulated (e.g., Lrg1 and Ctla2a) (Figure 2A and Additional Figure 1 (4.1MB, tif) ). All DEGs were visualized in a volcano plot and heatmap. The 101 DEGs were then submitted to GO and KEGG pathway enrichment analyses. In the biological process domain, the DEGs were significantly enriched in the regulation of leukocyte differentiation and regulation of lymphocyte differentiation (Figure 2B). In the molecular function domain, the DEGs were enriched in protease binding and peptide binding (Figure 2C). There were no statistically significant differences in the cellular component domain or KEGG analysis (Additional Figure 2 (891.7KB, tif) A and B). PPI network analysis was conducted using STRING. After removing isolated proteins from the PPI network, 36 proteins were identified from the STRING database (Figure 2D). PPI was visualized in Cytoscape software. Using a clustering coefficient algorithm via cytoHubba tools, the score of each gene in the PPI was calculated (Figure 2E). As a result, Chil1 and Lrg1 were identified to be the hub genes in the whole PPI. To validate the reliability of the surgery model and bioinformatic analysis, behavioral testing was conducted to assess contextual memory after surgery (Figure 2F and G). Western blot was performed to measure the relative hippocampal tissue expression of Chil1-encoded protein CHI3L1 (Figure 2H). Compared with that in the control group, the expression level of CHI3L1 was increased in the POCD group (P < 0.05). These results indicate that Chil1 and Lrg1 are differentially expressed hub genes involved in POCD, with CHI3L1 showing increased expression in the hippocampal tissue of POCD mice.
Figure 2.
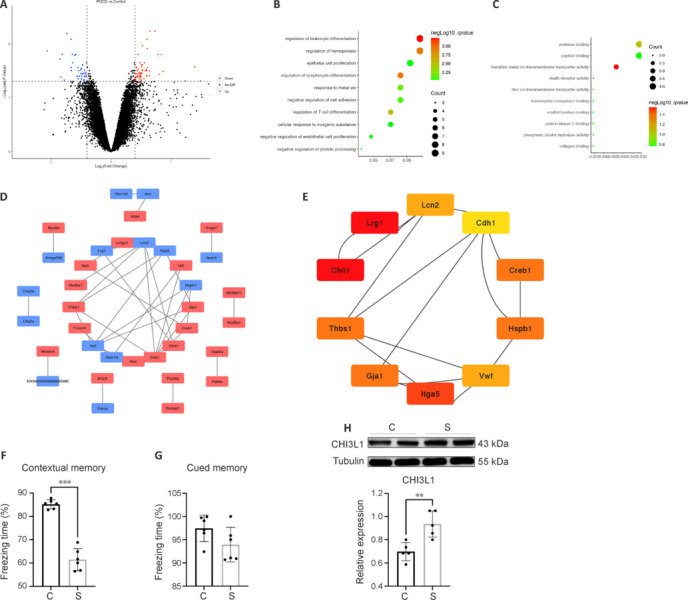
Differential gene expression profile.
(A) A volcano plot of the differentially expressed genes (DEGs) in the chip data. Upregulated genes are shown in red, downregulated genes in blue. (B, C) Gene Ontology (GO) analysis in biological process (B) and moelucar function (C) of the DEGs. (D, E) Protein–protein interaction (PPI) networks (D) and the top 10 genes of the clustering coefficient algorithms (E). (F, G) Freezing time (%) in the control (C) and surgery (S) groups of contextual memory (F) and cued memory (G) were conducted by contextual and cued fear conditioning tests on day 3 after surgery. (H) Representative western blots and densitometric analysis showing the expression of chitinase-3–like protein 1 (CHI3L1) on day 3 after surgery in the hippocampus. **P < 0.01, ***P < 0.001 (Student’s t-test). The experiments were repeated three times.
Recombinant CHI3L1 relieves neuroinflammation and promotes M2 microglial polarization in the hippocampus of postoperative cognitive dysfunction mice
To explore the potential role of CHI3L1 in POCD-induced neuroinflammation, we used rCHI3L1 for subsequent intervention. To ensure that intraperitoneal CHI3L1 crossed the blood–brain barrier (BBB), we measured the expression level of CHI3L1’s specific receptor, IL13Rα2, in the hippocampus using western blot in the POCD mice. The S + rCLP group showed a significant increase in IL13Rα2 levels (P < 0.05) compared with S + Veh group on the third day after surgery (Additional Figure 3 (1.5MB, tif) A). Additionally, the S + Veh group showed a significant increase of cytokines (iNOS, P = 0.043; IL-1β, P = 0.026) compared with the C + Veh group, and the S + rCLP group showed a significant decrease of these cytokines (iNOS, P = 0.002; IL-β, P = 0.026) compared with the S + Veh group 3 days after surgery (Figure 3A). On day 7, the S + rCLP group showed a significant decrease of IL-1β (P = 0.031) compared with the S + Veh group (Figure 3B). To explore the effect of rCHI3L1 on hippocampal microglial polarization, we measured the expression of M2 marker proteins Arg-1 and CD206, and the protein pERK. On day 3 after surgery, the S + rCLP group showed a significant upregulation of pERK, CD206 and Arg-1 (P < 0.001, P = 0.043, P = 0.029, respectively) compared with the S + Veh group (Figure 3C). On day 7 after surgery, the S + rCLP group showed a significant upregulation of CD206 (P = 0.002) compared with the S + Veh group (Figure 3D). These results suggest that CHI3L1 may mitigate neuroinflammation by promoting M2 microglial polarization.
Figure 3.

Representative western blots and densitometric analysis showing the protein expression of iNOS, IL-1β, CD206, Arg-1 and pERK in the hippocampus on days 3 and 7 after surgery and CHI3L1 treatment.
*P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc test). The experiments were repeated three times. Arg-1: Arginase-1; C: control; CHI3L1: chitinase-3–like protein 1; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; pERK: phosphorylated extracellular signal-regulated kinase; rCLP: recombinant CHI3L1; S: surgery; Veh: vehicle.
Recombinant CHI3L1 ameliorates the impairment of learning and memory in postoperative cognitive dysfunction mice
To investigate the impact of rCHI3L1 in postoperative learning and memory in POCD mice, we conducted behavioral tests related to spatial memory, contextual memory, and fear memory, specifically the FCT and Y-maze test. In the Y-maze test, the S + Veh group showed a significant decrease of spontaneous alternation (P = 0.006) compared with the C + Veh group, and the S + rCLP group showed a significant increase compared with the S + Veh group (P = 0.005) on day 2 after surgery. On day 6, the S + Veh group showed a significant decrease of spontaneous alternation (P < 0.001) compared with the C + Veh group, and the S + rCLP group showed a significant increase compared with the S + Veh group (P = 0.014; Figure 4A). In the FCT, the S + Veh group showed a significant decrease of freezing time (P = 0.027) compared with the C + Veh group, and the S + rCLP group showed a significant increase compared with the S + Veh group (P = 0.006) on day 3 after surgery. The S + Veh group showed a significant decrease (P < 0.001) compared with the C + Veh group, and the S + rCLP group showed a significant increase compared with the S + Veh group (P < 0.001) on day 7 after surgery (Figure 4B). On days 13 and 14 after surgery, there were no statistically significant differences in spatial memory or contextual memory. On days 3, 7 and 14 after surgery, there were no statistically significant differences in cued memory (Additional Figure 3 (1.5MB, tif) B).
Figure 4.
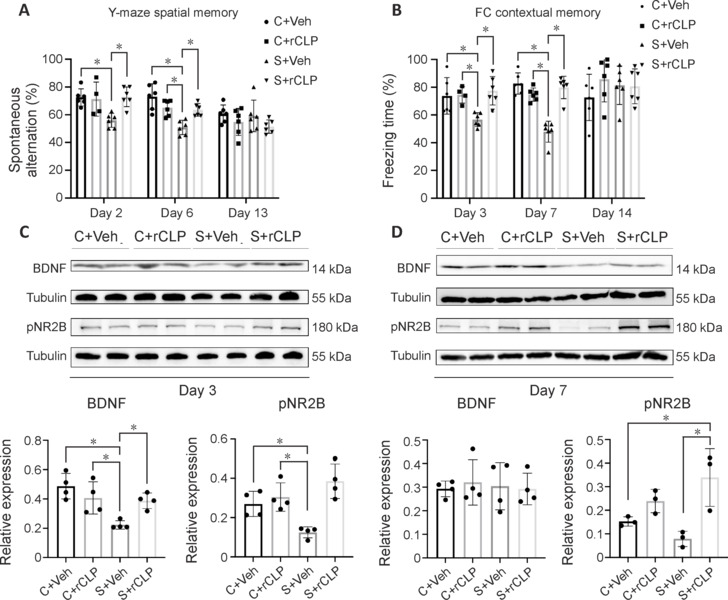
Changes in hippocampal BDNF, pNR2B protein expression and behavioral tests in all groups after CHI3L1 treatment.
(A) Spontaneous alternation (%) in Y-maze and (B) freezing time (%) in fear conditioning test of each group on days 2, 6, and 13 (A) and days 3, 7, and 14 (B) after surgery. (C, D) Representative western blots and densitometric analysis showing the expression of BDNF and pNR2B in the hippocampus on days 3 and 7 after surgery and CHI3L1 treatment. *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc test). The experiments were repeated three times. BDNF: Brain-derived neurotrophic factor; CHI3L1: chitinase-3–like protein 1; pNR2B: phosphorylated NMDA receptor subunit NR2B.
In the western blot analysis, the S + Veh group showed a significant decrease of BDNF and pNR2B levels (P = 0.002, P = 0.044, respectively) compared with the C + Veh group, and the S + rCLP group showed a significant increase of BDNF and pNR2B levels (P = 0.043, P < 0.001, respectively) compared with the S + Veh group 3 days after surgery (Figure 4C). On day 7 after surgery, the S + rCLP group showed a significant increase of pNR2B (P = 0.007) compared with the S + Veh group (Figure 4D). These results suggest that rCHI3L1 improved learning and memory in POCD mice.
ERK inhibitor blocks the effect of recombinant CHI3L1 in postoperative cognitive dysfunction mice
To investigate the signaling proteins involved in the effects of CHI3L1, we used the ERK-specific inhibitor PD98059. To confirm that intraperitoneal injection of PD98059 was effective in the hippocampus, we measured the hippocampal expression of pERK. The S + PD group showed a significant decrease of pERK (P = 0.004) compared with the S + Veh group, and the S + rCLP + PD group showed a significant decrease of pERK (P < 0.001) compared with the S + rCLP group (Additional Figure 4 (1.7MB, tif) A). We next measured the expression of M2 microglia marker protein CD206 and cytokines iNOS and IL-1β after combination treatment with PD98059 and rCHI3L1. The S + rCLP group showed a significant increase of CD206 (P < 0.001), and a significant decrease of iNOS and IL-1β (P = 0.009, P < 0.001, respectively) compared with the S + Veh group. The S + PD + rCLP group showed a significant decrease of CD206 (P < 0.001), and a significant increase of iNOS and IL-1β (P = 0.001, P = 0.007, respectively) compared with the S + rCLP group (Figure 5A).
Figure 5.
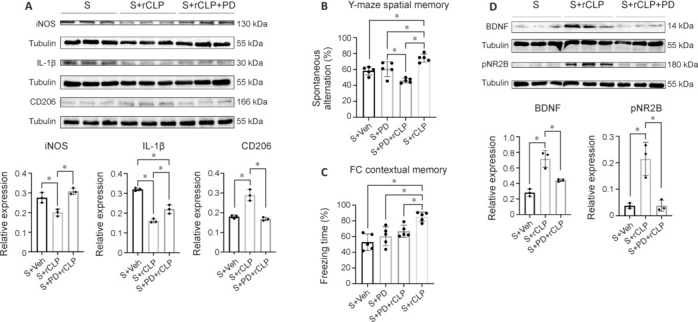
Changes in hippocampal iNOS, IL-1β, and CD206 protein expression and behavioral tests after ERK inhibitor treatment.
(A) Representative western blots and densitometric analysis showing the expression of iNOS, IL-1β, and CD206 in the hippocampus on day 3 after surgery and treatment. (B) Spontaneous alternation (%) and (C) freezing time (%) of each group on day 3 after surgery. (D) Representative western blots and densitometric analysis showing the expression of BDNF and pNR2B in the hippocampus on day 3 after surgery. The experiments were repeated three times. *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc test). CHI3L1: Chitinase-3–like protein 1; ERK: extracellular signal–regulated kinase; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; PD: ERK inhibitor PD98059; rCLP: recombinant CHI3L1; S: surgery; Veh: vehicle.
In the Y-maze test, the S + PD + rCLP group showed a significant decrease of spontaneous alternation (P < 0.001) compared with the S + rCLP group (Figure 5B). In the FCT, the S + PD + rCLP group showed a significant decrease of freezing time (P = 0.030) compared with the S + rCLP group (Figure 5C). However, there was no statistically significant difference in cued memory (Additional Figure 4 (1.7MB, tif) B). In the western blot assay, the S + rCLP group showed a significant increase of BDNF and pNR2B levels compared with the S + Veh group (P < 0.001, P = 0.003, respectively), and the S + PD + rCLP group showed a significant decrease of BDNF and pNR2B levels (P = 0.006, P = 0.003, respectively) compared with the S + rCLP group (Figure 5D). The results indicate that CHI3L1 may alleviate neuroinflammation in POCD mice and improve postoperative learning and memory through the ERK signaling protein.
Discussion
Cognitive dysfunction is a common complication following surgery, especially among elderly patients, and more intensive study is needed on its underlying pathomechanisms. Neuroinflammation, oxidative stress, and autophagy disorder are strongly implicated in POCD (Netto et al., 2018; Safavynia and Goldstein, 2018; Lin et al., 2020), so we used bioinformatics analysis to identify hub genes associated with both POCD and neuroinflammation. Screening for DEGs in the GEO GSE95426 dataset obtained from mouse hippocampus following experimental tibial fracture and subsequent GO and KEGG pathway enrichment analyses revealed that many of these DEGs are involved in the differentiation of immunocytes (such as leukocytes, lymphocytes, and T cells) (Zhang et al., 2016, 2019). Immune cells in the hippocampus, including mast cells, microglia, and T cells, may regulate neuroinflammation associated with POCD (Liu and Yin, 2018). Of the POCD-related DEGs, Chil1, and Lrg1 demonstrated the strongest clustering coefficients, suggesting that they act as hub genes in POCD-associated gene networks. CHI3L1 has been implicated in cognitive impairment (Muszynski et al., 2017; Connolly et al., 2023), but no such relationship has been established for LRG1 (Im et al., 2020; Lananna et al., 2020). Therefore, this study focused on Chil1. The plasma and cerebrospinal fluid concentrations of serum CHI3L1 were reported to increase during the early phase of Alzheimer’s disease, suggesting its potential use as a disease biomarker. Here, we found that reduced CHI3L1 expression in the hippocampus was associated with POCD, and that augmentation of CHI3L1 mitigated both molecular and behavioral features of POCD.
Intramedullary fixation surgery for tibial fracture is a well-validated model of POCD associated with neuroinflammation (Liu et al., 2021b), and CHI3L1 has been demonstrated to suppress neuroinflammation in a proinflammatory environment (Wang et al., 2020). This model induced impaired learning and memory in mice on the 3rd and 7th days, with restoration to normalcy observed by the fourteenth day in our study. We found that rCHI3L1 injection reversed post-surgical impairments in hippocampus-dependent learning (O’Shea et al., 2016). Furthermore, these effects appeared to be centrally mediated because intraperitoneal rCHI3L1 crossed the BBB, as evidenced by the change in hippocampal expression of the CHI3L1 receptor IL13Rα2. In addition, intraperitoneal rCHI3L1 administration downregulated proinflammatory mediators (iNOS and IL-1β) and upregulated plasticity-related proteins (BDNF and pNR2B) in the hippocampus. The changes in inflammatory mediators followed distinct kinetics—both were downregulated on day 3 post-surgery, but only IL-1β expression was downregulated on day 7, possibly because of the delayed downregulation of iNOS transcription by the IL-1β-activated NF-κB pathway (Yamanishi et al., 2014).
CHI3L1 is involved in many signaling pathways (He et al., 2013; Geng et al., 2018; Ma et al., 2021), including the ERK pathway linked to the polarization of M2 microglia (Xu et al., 2019). The cognitive impairments, upregulation of neuroinflammatory markers, and downregulated expression levels of pERK and M2 marker proteins (CD206 and Arg-1) following surgery were reversed by rCHI3L1. It is worth noting that CD206 upregulated while Arg-1 not changed on the seventh day. This could be related to the inactivation of rCHI3L1 between the third and seventh days. Currently, there is a lack of research on the protein pharmacokinetics of CHI3L1, warranting further research into the serum half-life of this protein. All of these effects induced by rCHI3L1 were mitigated by cotreatment with an ERK inhibitor. Thus, the findings suggest that suppression of neuroinflammation via ERK-mediated M2 polarization mitigated POCD. In a previous study, Im et al. (2020) found that neuroinflammation following cerebral ischemia was aggravated in CHI3L1-knockout mice compared with the findings in wildtype mice, and that this effect was associated with M1 microglia upregulation and M2 microglia downregulation, which is consistent with our findings. However, Choi et al. (2018) reported that the CHI3L1 inhibitor K284-6111 relieved Aβ-induced BV2 cell inflammation and cognitive impairment. These discrepancies may be explained by the effects of infiltrating immune cells, as Zhu et al. (2018) suggested that CD4+ T cells (T helper 2 cells or Th2 cells) infiltrate the hippocampus via BBB disruption during POCD and promote M2 polarization (Wu and Watabe, 2017). It is unclear, however, if CHI3L1 promotes M2 microglia polarization via effects on hippocampal Th2 infiltration.
Neuroinflammation may be a major contributor to cognitive impairment following surgery (Safavynia and Goldstein, 2018), and many experimental therapeutic strategies for POCD target neuroinflammation (Suo and Wang, 2021; Li et al., 2022). CHI3L1 was shown to ameliorate neuroinflammation and improve learning (Muszynski et al., 2017; Connolly et al., 2023), and CHI3L1 KO mice demonstrated poorer cognitive performance compared with wildtype littermates following experimental surgery. In accordance with these results, rCHI3L1 improved spatial and contextual memory in the Y-maze and fear memory tests in the present study, and these effects were associated with reversal of post-surgical BDNF and NR2B downregulation. It is possible that the increases in BDNF and NR2B rescue hippocampal long-term potentiation and other forms of synaptic plasticity implicated in hippocampus-dependent cognitive function (Miwa et al., 2008; Zhuo, 2009), but further study is required to determine if rCHI3L1 restores long-term potentiation in POCD model mice.
The ERK inhibitor PD98059 inhibited ERK phosphorylation and reversed the beneficial effects of rCHI3L1 on neuroinflammation, M2 polarization, spatial memory, and contextual fear memory, but had no effects when administered alone. These findings strongly suggest that CHI3L1 suppresses POCD symptoms in mice by promoting M2 microglia polarization via ERK phosphorylation and activation of downstream targets. These findings are consistent with a previous study reporting that ERK signaling contributes to social and emotional behavior and memory formation (Albert-Gasco et al., 2020). Moreover, Chen et al. (2023) reported that reduced ERK signaling was associated with POCD. Qiu et al. (2020b) found that the sedative dexmedetomidine relieved microglial inflammation via activation of the ERK pathway. Collectively, these results suggest that CHI3L1 alleviates postoperative cognitive dysfunction via ERK-mediated M2 microglia polarization.
Our study had several limitations worth noting. First, the intraperitoneal injection of rCHI3L1 may have some nonspecific effects. In the future, the drug targeting should be enhanced and the indicators for evaluating the effect should be increased to improve the therapeutic efficacy. Second, we focused on the role of CHI3L1 in microglia, but future work should also focus on its role in other cell types of the central nervous system, such as astrocytes. Finally, we investigated whether CHI3L1 ameliorates postoperative cognitive impairment by attenuating neuroinflammation; however, it is possible that CHI3L1 may also improve cognition through other mechanisms, such as sleep-wake rhythms, which is an intriguing direction for further research. In the future, we will continue to explore the mechanisms by which CHI3L1 improves POCD, with the goal of noninvasive treatment with potential for clinical translation.
We conclude that downregulation of CHI3L1 in the hippocampus contributes to neuroinflammation, synaptic dysfunction, and cognitive deficits following surgery, and that postoperative augmentation of hippocampal CHI3L1 suppresses neuroinflammation via ERK-mediated M2 polarization, resulting in improved synaptic function and rescue of learning and memory impairments.
Additional files:
Additional Figure 1 (4.1MB, tif) : Heatmap of the differentially expressed genes.
Heatmap of the differentially expressed genes.
Upregulated genes are shown in red, downregulated genes are shown in blue.
Additional Figure 2 (891.7KB, tif) : Gene Ontology (GO) analysis in cellular component (A) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (B).
Gene Ontology (GO) analysis in cellular component (A) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (B).
Additional Figure 3 (1.5MB, tif) : Changes in hippocampal IL13Rα2 and cued memory after rCLP treatment.
Changes in hippocampal IL13Rα2 and cued memory after rCLP treatment.
(A) Representative western blots and densitometric analysis showing the expression of interleukin-13 receptor subunit α2 (IL13Rα2) in the hippocampus on day 3 after surgery. (B) Freezing time (%) of cued memory of each group on days 3, 7, 14 after surgery. *P < 0.05, vs. C + Veh group; &P < 0.05, vs. S + Veh group (one-way analysis of variance followed by Tukey’s post hoc test). The experiments were repeated three times. C: control; CHI3L1: Chitinase-3-like protein 1; IL: interleukin; rCLP: recombinant CHI3L1; S: surgery; Veh: vehicle.
Additional Figure 4 (1.7MB, tif) : Changes in hippocampal pERK and cued memory after ERK inhibitor treatment.
Changes in hippocampal pERK and cued memory after ERK inhibitor treatment.
(A) Representative western blots and densitometric analysis showing the expression of phosphorylated extracellular signal-regulated kinase (pERK) in the hippocampus after combined treatment. (B) Freezing time (%) of cued memory of each group on day 3 after surgery. *P < 0.05, vs. S + Veh group; #P < 0.05, vs. S + PD group; &P < 0.05, vs. S + rCLP group (one-way analysis of variance followed by Tukey’s post hoc test). The experiments were repeated three times.CHI3L1: Chitinase-3-like protein 1; PD: ERK inhibitor PD98059; pERK: phosphorylated extracellular signal-regulated kinase; rCLP: recombinant CHI3L1; S: surgery; Veh: vehicle.
Acknowledgments:
We are very grateful to Dr. Lizhi Xu, Dr. Huan Dou and Dr. Jie Ding (Medical School, Nanjing University) for their technical support.
Funding Statement
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81730033, 82171193 (to XG); the Key Talent Project for Strengthening Health during the 13th Five-Year Plan Period, No. ZDRCA2016069 (to XG); the National Key R&D Program of China, No. 2018YFC2001901 (to XG); and Jiangsu Provincial Medical Key Discipline, No. ZDXK202232 (to XG).
Footnotes
Conflicts of interest: The authors have no relevant financial or non-financial interests to disclose.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
Data availability statement:
All relevant data are within the paper and its Additional files.
References
- Albert-Gascó H, Ros-Bernal F, Castillo-Gómez E, Olucha-Bordonau FE. MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes. Int J Mol Sci. 2020;21:4471. doi: 10.3390/ijms21124471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain B. agilp: Agilent expression array processing package. R package version 3.28.0 2022 [Google Scholar]
- Chen Y, Dong J, Gong L, Hong Y, Hu C, Bao Y, Chen H, Liu L, Huang L, Zhao Y, Zhang J, He S, Yan X, Wu X, Cui W. Fucoxanthin, a marine derived carotenoid, attenuates surgery-induced cognitive impairments via activating Akt and ERK pathways in aged mice. Phytomedicine. 2023;120:155043. doi: 10.1016/j.phymed.2023.155043. [DOI] [PubMed] [Google Scholar]
- Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Yeo IJ, Kim KC, Choi WR, Jung JK, Han SB, Hong JT. K284-6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-kappaB-mediated CHI3L1 expression. J Neuroinflammation. 2018;15:224. doi: 10.1186/s12974-018-1269-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Connolly K, Lehoux M, O’Rourke R, Assetta B, Erdemir GA, Elias JA, Lee CG, Huang YA. Potential role of chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and Alzheimer’s disease. Alzheimers Dement. 2023;19:9–24. doi: 10.1002/alz.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J, Tang J. Role of N-formyl peptide receptor 2 in germinal matrix hemorrhage: an intrinsic review of a hematoma resolving pathway. Neural Regen Res. 2024;19:350–354. doi: 10.4103/1673-5374.379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng B, Pan J, Zhao T, Ji J, Zhang C, Che Y, Yang J, Shi H, Li J, Zhou H, Mu X, Xu C, Wang C, Xu Y, Liu Z, Wen H, You Q. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through beta-catenin/Erk/Akt signaling in gastric cancer. J Exp Clin Cancer Res. 2018;37:208. doi: 10.1186/s13046-018-0876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, Nour AM, Parikh CR, Schmidt I, Modis Y, Cantley L, Elias JA. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep. 2013;4:830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JH, Yeo IJ, Park PH, Choi DY, Han SB, Yun J, Hong JT. Deletion of Chitinase-3-like 1 accelerates stroke development through enhancement of Neuroinflammation by STAT6-dependent M2 microglial inactivation in Chitinase-3-like 1 knockout mice. Exp Neurol. 2020;323:113082. doi: 10.1016/j.expneurol.2019.113082. [DOI] [PubMed] [Google Scholar]
- Kim S, Son Y. Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia. Int J Mol Sci. 2021;22:8800. doi: 10.3390/ijms22168800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lananna BV, McKee CA, King MW, Del-Aguila JL, Dimitry JM, Farias FHG, Nadarajah CJ, Xiong DD, Guo C, Cammack AJ, Elias JA, Zhang J, Cruchaga C, Musiek ES. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci Transl Med. 2020;12:eaax3519. doi: 10.1126/scitranslmed.aax3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang HL, Zhao YZ, Xiao RJ, Sun J, Chen Y, Hu GW, Xu GH. Small extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells improve postoperative cognitive dysfunction in mice with diabetes. Neural Regen Res. 2023;18:609–617. doi: 10.4103/1673-5374.350205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhu Y, Kang Y, Qin S, Chai J. Neuroinflammation as the underlying mechanism of postoperative cognitive dysfunction and therapeutic strategies. Front Cell Neurosci. 2022;16:843069. doi: 10.3389/fncel.2022.843069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol. 2020;130:110791. doi: 10.1016/j.exger.2019.110791. [DOI] [PubMed] [Google Scholar]
- Liu J, Huang K, Zhu B, Zhou B, Ahmad Harb AK, Liu L, Wu X. Neuropsychological tests in post-operative cognitive dysfunction: methods and applications. Front Psychol. 2021;12:684307. doi: 10.3389/fpsyg.2021.684307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sun YM, Huang H, Chen C, Wan J, Ma LH, Sun YY, Miao HH, Wu QY. Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J Neuroinflammation. 2021;18:41. doi: 10.1186/s12974-021-02089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Song J, Zhou Q, Chu S, Liu Y, Zhao X, Ma Z, Xia T, Gu X. The role of 5-HT7R in the memory impairment of mice induced by long-term isoflurane anesthesia. Neurobiol Learn Mem. 2022;188:107584. doi: 10.1016/j.nlm.2022.107584. [DOI] [PubMed] [Google Scholar]
- Liu XL, Sun DD, Zheng MT, Li XT, Niu HH, Zhang L, Zhou ZW, Rong HT, Wang Y, Wang JW, Yang GL, Liu X, Chen FL, Zhou Y, Zhang S, Zhang JN. Maraviroc promotes recovery from traumatic brain injury in mice by suppression of neuroinflammation and activation of neurotoxic reactive astrocytes. Neural Regen Res. 2023;18:141–149. doi: 10.4103/1673-5374.344829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yin Y. Emerging roles of immune cells in postoperative cognitive dysfunction. Mediators Inflamm. 2018;2018:6215350. doi: 10.1155/2018/6215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fu H, Wang T. Neuroinflammation in perioperative neurocognitive disorders: From bench to the bedside. CNS Neurosci Ther. 2022;28:484–496. doi: 10.1111/cns.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Liu TT, Jiang LH, Liu Q, Ma ZL, Xia TJ, Gu XP. Identification of hub genes associated with cognition in the hippocampus of Alzheimer’s disease. Bioengineered. 2021;12:9598–9609. doi: 10.1080/21655979.2021.1999549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Akosman B, Kamle S, Lee CM, He CH, Koo JS, Lee CG, Elias JA. CHI3L1 regulates PD-L1 and anti-CHI3L1-PD-1 antibody elicits synergistic antitumor responses. J Clin Invest. 2021;131:e137750. doi: 10.1172/JCI137750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Fukaya M, Watabe AM, Watanabe M, Manabe T. Functional contributions of synaptically localized NR2B subunits of the NMDA receptor to synaptic transmission and long-term potentiation in the adult mouse CNS. J Physiol. 2008;586:2539–2550. doi: 10.1113/jphysiol.2007.147652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszynski P, Groblewska M, Kulczynska-Przybik A, Kulakowska A, Mroczko B. YKL-40 as a potential biomarker and a possible target in therapeutic strategies of Alzheimer’s disease. Curr Neuropharmacol. 2017;15:906–917. doi: 10.2174/1570159X15666170208124324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 8th. Washington, DC: The National Academies Press; 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Netto MB, de Oliveira AN, Junior, Goldim M, Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa AB, Rezin GT, Fortunato JJ, Giustina AD, Barichello T, Dal-Pizzol F, Petronilho F. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun. 2018;73:661–669. doi: 10.1016/j.bbi.2018.07.016. [DOI] [PubMed] [Google Scholar]
- O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 2016;8:298. doi: 10.3389/fnagi.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Guo D, Jing L, Li K, Li X, Li G, Gao X, Li ZW, Zhao W, Feng H, Cao MH. Long noncoding RNA Pvt1 promotes the proliferation and migration of Schwann cells by sponging microRNA-214 and targeting c-Jun following peripheral nerve injury. Neural Regen Res. 2023;18:1147–1153. doi: 10.4103/1673-5374.353497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Medina R, Lopez-Kleine L, Ramirez-Clavijo S, Payan-Gomez C. Identification of candidate miRNAs in early-onset and late-onset prostate cancer by network analysis. Sci Rep. 2020;10:12345. doi: 10.1038/s41598-020-69290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu LL, Pan W, Luo D, Zhang GF, Zhou ZQ, Sun XY, Yang JJ, Ji MH. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca(2+)/calpain might contribute to postoperative cognitive dysfunction in aging mice. J Neuroinflammation. 2020;17:23. doi: 10.1186/s12974-019-1695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Lu P, Wang K, Zhao X, Li Q, Wen J, Zhang H, Li R, Wei H, Lv Y, Zhang S, Zhang P. Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem Res. 2020;45:345–353. doi: 10.1007/s11064-019-02922-1. [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Goldstein PA. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psychiatry. 2018;9:752. doi: 10.3389/fpsyt.2018.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo L, Wang M. Dexmedetomidine facilitates the expression of nNOS in the hippocampus to alleviate surgery-induced neuroinflammation and cognitive dysfunction in aged rats. Exp Ther Med. 2021;22:1038. doi: 10.3892/etm.2021.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland TE. Chitinase-like proteins as regulators of innate immunity and tissue repair: helpful lessons for asthma? Biochem Soc Trans. 2018;46:141–151. doi: 10.1042/BST20170108. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing. Vienna: 2021. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Uddin MJ, Leslie JL, Burgess SL, Oakland N, Thompson B, Abhyankar M, Revilla J, Frisbee A, Donlan AN, Kumar P, Petri WA., Jr The IL-33-ILC2 pathway protects from amebic colitis. Mucosal Immunol. 2022;15:165–175. doi: 10.1038/s41385-021-00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu C, Zhong H, Hu B, Wei L, Liu N, Zhang Y, Shi Q, Wang C, Qi M, Gu Y, Shen X, Tian Y, Liu Y, Cao P, Chen H, Yuan W. Inflammatory-sensitive CHI3L1 protects nucleus pulposus via AKT3 signaling during intervertebral disc degeneration. FASEB J. 2020;34:3554–3569. doi: 10.1096/fj.201902096R. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cai Z, Zhan G, Li X, Li S, Wang X, Li S, Luo A. Caffeic acid phenethyl ester suppresses oxidative stress and regulates M1/M2 microglia polarization via Sirt6/Nrf2 pathway to mitigate cognitive impairment in aged mice following anesthesia and surgery. Antioxidants (Basel) 2023;12:714. doi: 10.3390/antiox12030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhou W, Zhang Z, Zhang L, Li M. Metformin alleviates spinal cord injury by inhibiting nerve cell ferroptosis through upregulation of heme oxygenase-1 expression. Neural Regen Res. 2024;19:2041–2049. doi: 10.4103/1673-5374.390960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Luo T, Zou S, Zhou X, Shen W, Ji X, Li Q, Wu A. Differentially expressed lncRNAs and miRNAs with associated ceRNA networks in aged mice with postoperative cognitive dysfunction. Oncotarget. 2017;8:55901–55914. doi: 10.18632/oncotarget.18362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Watabe K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front Biosci (Landmark Ed) 2017;22:1805–1829. doi: 10.2741/4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (N Y) 2021;2:100141. doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C, Zhang Z, Baht GS, Terrando N. A mouse model of orthopedic surgery to study postoperative cognitive dysfunction and tissue regeneration. J Vis Exp. 2018:56701. doi: 10.3791/56701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Bo Q, Shao R, Liang J, Zhai Y, Yang S, Wang F, Sun X. Chitinase-3-like-1 promotes M2 macrophage differentiation and induces choroidal neovascularization in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60:4596–4605. doi: 10.1167/iovs.19-27493. [DOI] [PubMed] [Google Scholar]
- Xu W, Seok J, Mindrinos MN, Schweitzer AC, Jiang H, Wilhelmy J, Clark TA, Kapur K, Xing Y, Faham M, Storey JD, Moldawer LL, Maier RV, Tompkins RG, Wong WH, Davis RW, Xiao W; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program Human transcriptome array for high-throughput clinical studies. Proc Natl Acad Sci U S A. 2011;108:3707–3712. doi: 10.1073/pnas.1019753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi R, Yoshigai E, Okuyama T, Mori M, Murase H, Machida T, Okumura T, Nishizawa M. The anti-inflammatory effects of flavanol-rich lychee fruit extract in rat hepatocytes. PLoS One. 2014;9:e93818. doi: 10.1371/journal.pone.0093818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li N, Wang Y, Lu W, Zhang Y, Chen Y, Deng X, Yu X. Methane ameliorates post-operative cognitive dysfunction by inhibiting microglia NF-kappaB/MAPKs pathway and promoting IL-10 expression in aged mice. Int Immunopharmacol. 2019;71:52–60. doi: 10.1016/j.intimp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong H, Li N, Zhang S, Sun J, Zhang S, Qian Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13:127. doi: 10.1186/s12974-016-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Liu W, Fang H. Inflammation caused by peripheral immune cells across into injured mouse blood brain barrier can worsen postoperative cognitive dysfunction induced by isoflurane. BMC Cell Biol. 2018;19:23. doi: 10.1186/s12860-018-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M. Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain. 2009;2:4. doi: 10.1186/1756-6606-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heatmap of the differentially expressed genes.
Upregulated genes are shown in red, downregulated genes are shown in blue.
Gene Ontology (GO) analysis in cellular component (A) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (B).
Changes in hippocampal IL13Rα2 and cued memory after rCLP treatment.
(A) Representative western blots and densitometric analysis showing the expression of interleukin-13 receptor subunit α2 (IL13Rα2) in the hippocampus on day 3 after surgery. (B) Freezing time (%) of cued memory of each group on days 3, 7, 14 after surgery. *P < 0.05, vs. C + Veh group; &P < 0.05, vs. S + Veh group (one-way analysis of variance followed by Tukey’s post hoc test). The experiments were repeated three times. C: control; CHI3L1: Chitinase-3-like protein 1; IL: interleukin; rCLP: recombinant CHI3L1; S: surgery; Veh: vehicle.
Changes in hippocampal pERK and cued memory after ERK inhibitor treatment.
(A) Representative western blots and densitometric analysis showing the expression of phosphorylated extracellular signal-regulated kinase (pERK) in the hippocampus after combined treatment. (B) Freezing time (%) of cued memory of each group on day 3 after surgery. *P < 0.05, vs. S + Veh group; #P < 0.05, vs. S + PD group; &P < 0.05, vs. S + rCLP group (one-way analysis of variance followed by Tukey’s post hoc test). The experiments were repeated three times.CHI3L1: Chitinase-3-like protein 1; PD: ERK inhibitor PD98059; pERK: phosphorylated extracellular signal-regulated kinase; rCLP: recombinant CHI3L1; S: surgery; Veh: vehicle.
Data Availability Statement
All relevant data are within the paper and its Additional files.


