Abstract
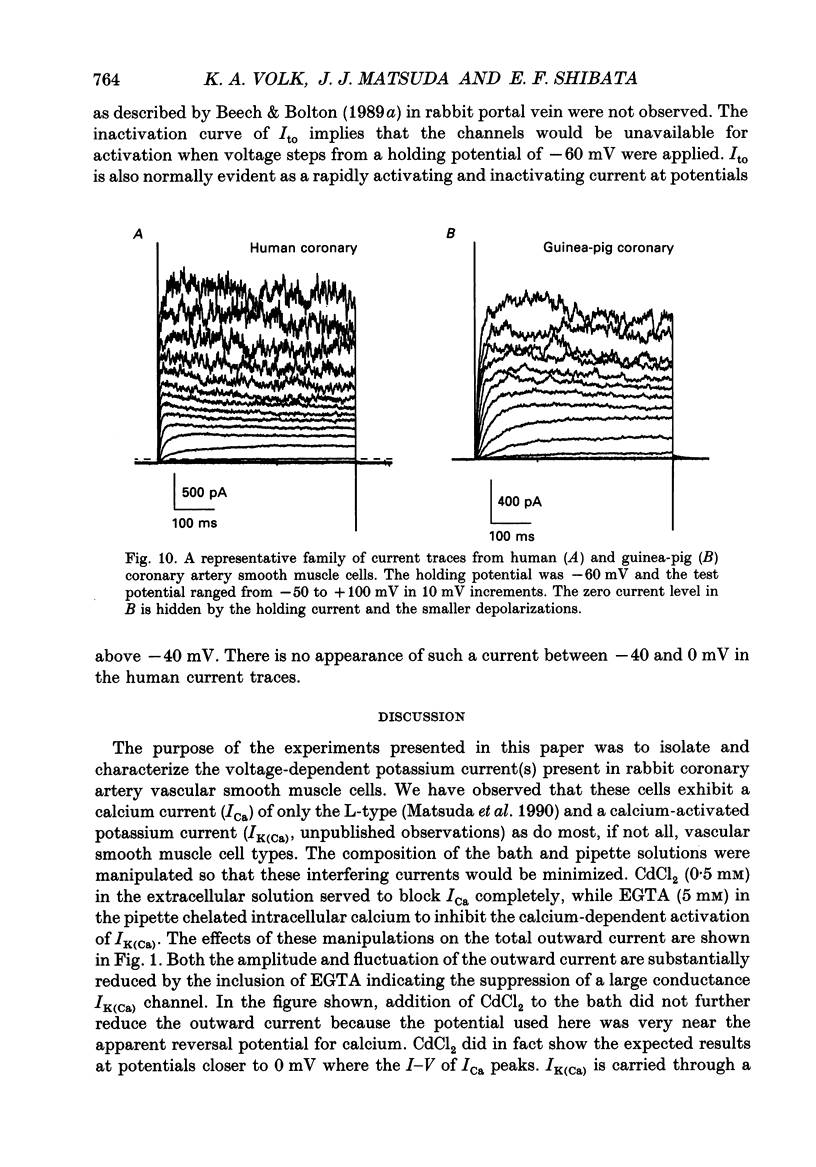
1. Voltage- and time-dependent outward currents were recorded from relaxed enzymatically isolated smooth muscle cells from the rabbit left descending coronary artery using a single pipette voltage clamp technique. The calcium-activated potassium current was blocked by inclusion of EGTA in the pipette solution and CdCl2 in the extracellular bath. 2. Outward currents were elicited with depolarizing voltage steps to potentials positive to -20 mV. Long (5 s) voltage steps revealed slow inactivation of the current with a time constant of nearly 3 s at +60 mV. Potassium was identified as the predominant charge carrier by reversal potential measurements in potassium substitution experiments. 3. The results of kinetic analyses compared favourably with the Hodgkin-Huxley model for a delayed rectifier with some deviations. The sigmoid current onset was best fitted by raising the activation variable (n) to the second power. Deactivation tail currents were consistently found to be comprised of two exponential components. The kinetics of activation and deactivation were strongly voltage-dependent from -80 to +60 mV. 4. Envelope of tails experiments showed that the scaled tail current amplitudes followed the kinetic behaviour of current activation. The contribution of each of the two exponential tail components was also measured in these experiments. They did not reveal kinetically separable currents, nor were they differentially altered by 4-aminopyridine (4-AP), tetraethylammonium (TEA), or elevated [K+]o. 5. The steady-state voltage-dependence curves for both activation and inactivation were well fitted by a Boltzmann distribution with V1/2 = -5.60 mV and k = -8.66 mV for n infinity act and V1/2 = -24.20 mV and k = 5.16 mV for n infinity act. Super-imposition of the two curves revealed a 'window' of voltage where channels are available for activation without completely inactivating. 6. Neither of the commonly used potassium channel blockers, TEA or 4-AP, were particularly effective blockers of IK, reducing current by only 50-70% at an extracellular concentration of 10 mM. TEA block was mildly voltage-dependent and was more effective in reducing current towards the end of a 500 ms depolarization. 4-AP, on the other hand, demonstrated considerable voltage-dependence and preferentially reduced early currents. 7. Outward currents recorded from guinea-pig and human coronary artery myocytes under the same conditions as in the rabbit cell experiments displayed similar characteristics.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
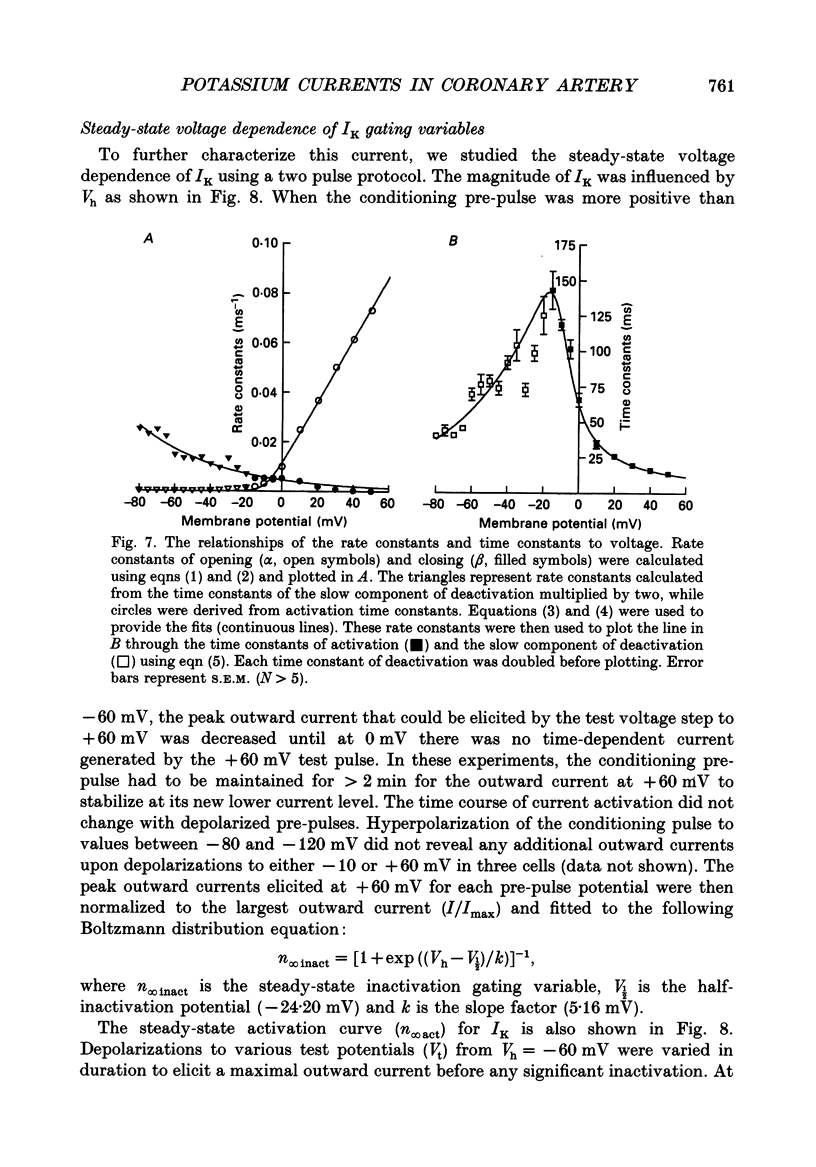
PDF




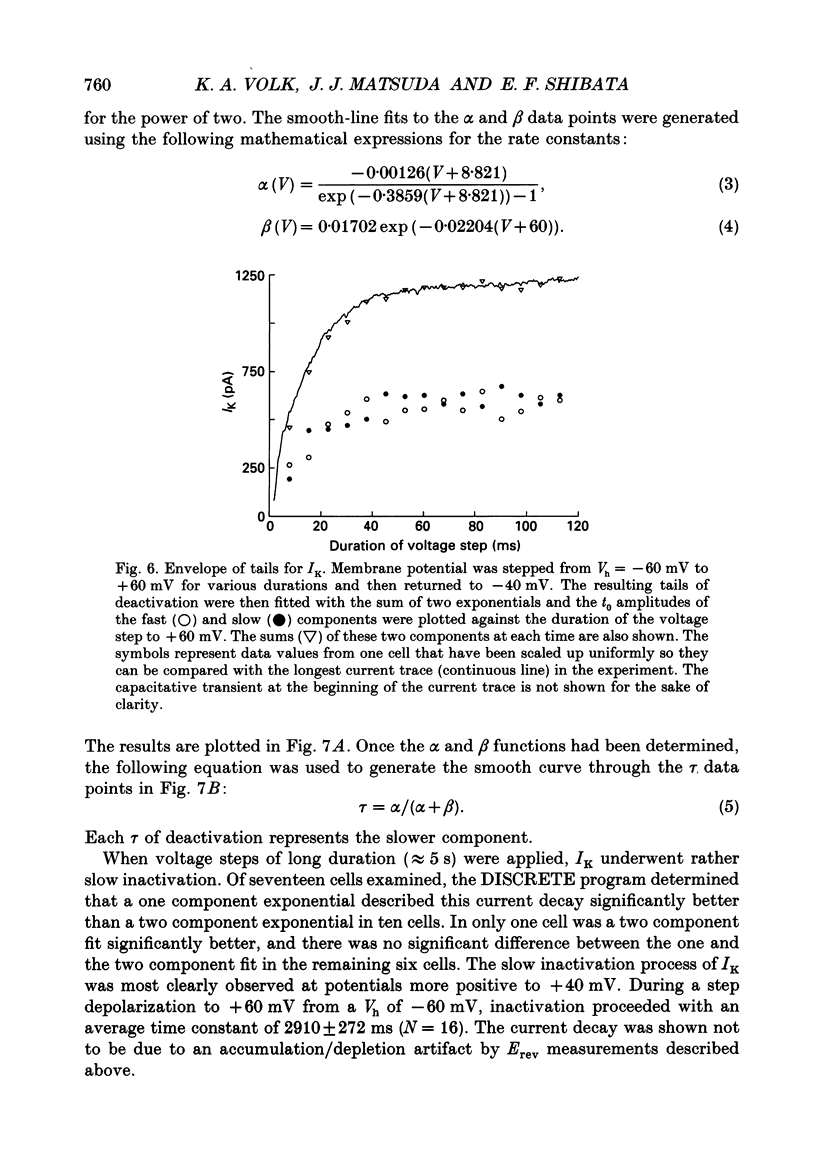




Selected References
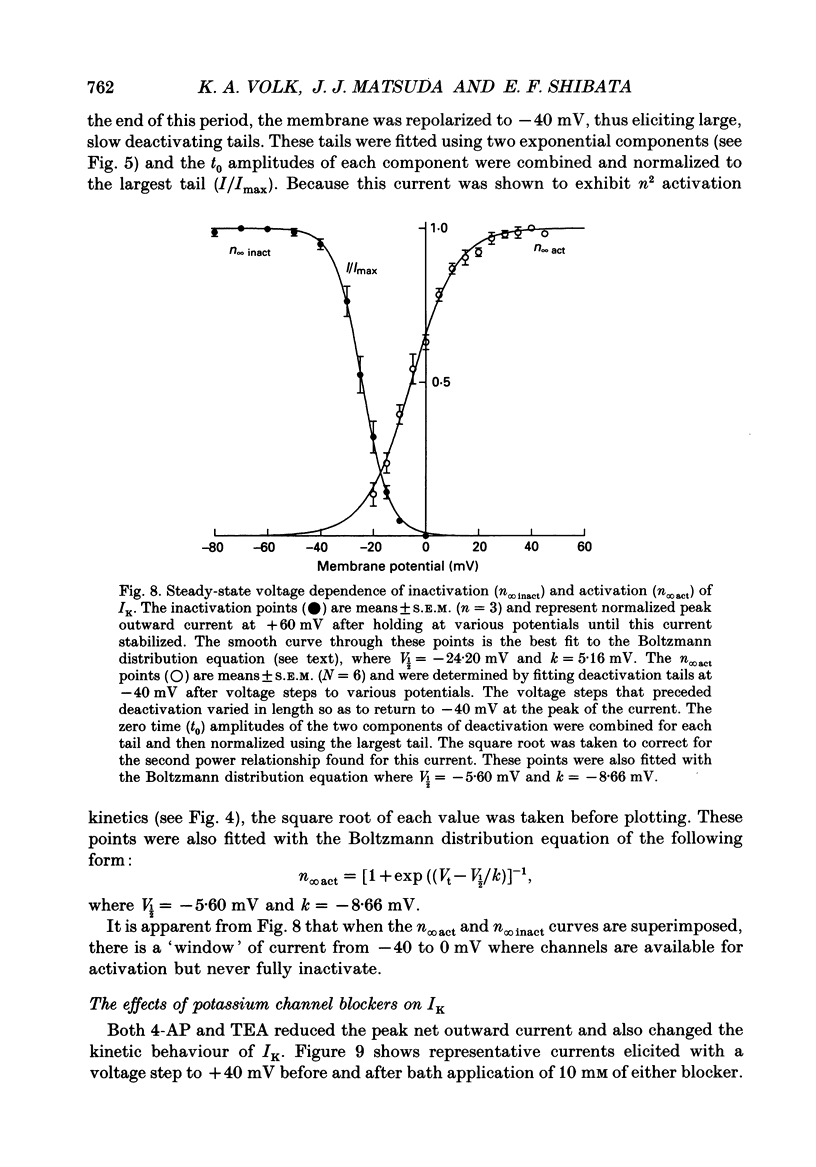
These references are in PubMed. This may not be the complete list of references from this article.
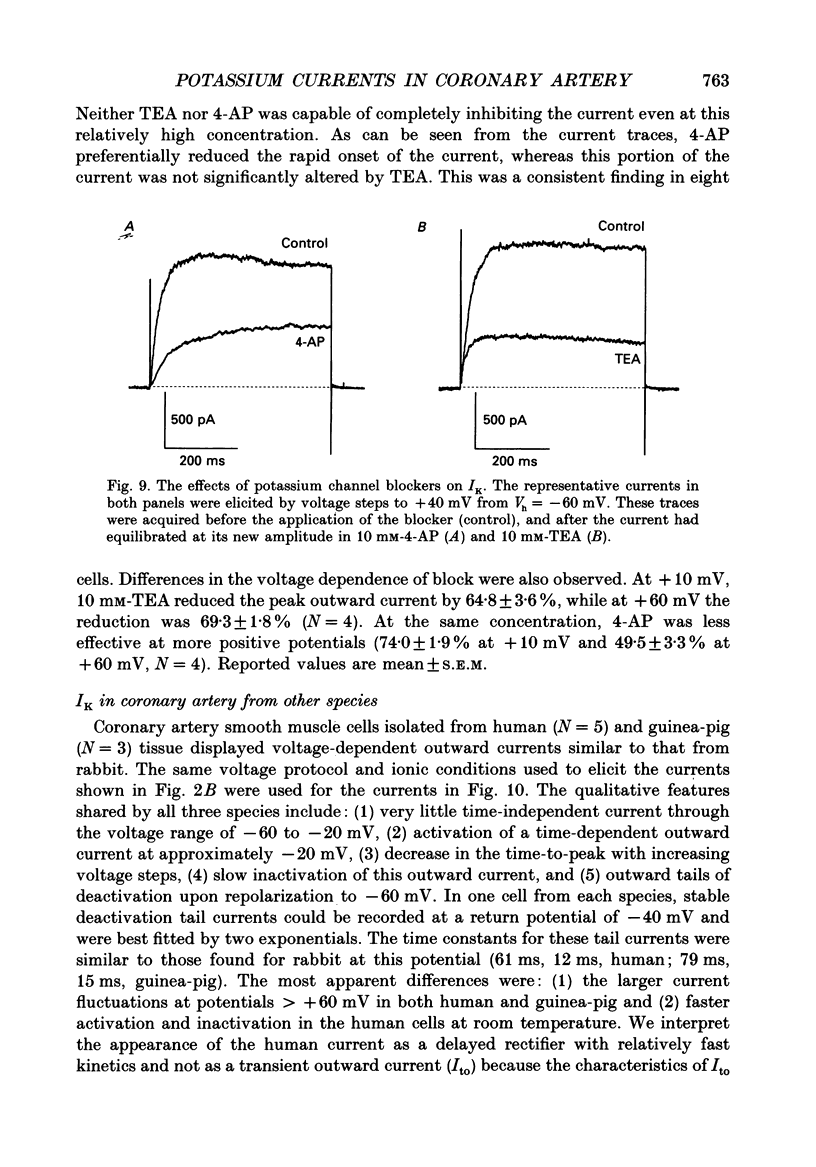
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. The effects of tetraethylammonium ions, 4-aminopyridine or quinidine on K+-currents in single smooth muscle cells of the rabbit portal vein. Biomed Biochim Acta. 1987;46(8-9):S673–S676. [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. B., McKinney L. C., Kass R. S., Begenisich T. Delayed rectification in the calf cardiac Purkinje fiber. Evidence for multiple state kinetics. Biophys J. 1985 Oct;48(4):553–567. doi: 10.1016/S0006-3495(85)83813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G., Caillé J. P., Payet M. D., Peyrow M., Sauvé R., Renaud J. F., Sperelakis N. Bethanidine increases one type of potassium current and relaxes aortic muscle. Can J Physiol Pharmacol. 1988 Jun;66(6):731–736. doi: 10.1139/y88-116. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. R., Nicholson C. D. Comparison of the effects of cromakalim, a potassium conductance enhancer, and nimodipine, a calcium antagonist, on 5-hydroxytryptamine responses in a variety of vascular smooth muscle preparations. Naunyn Schmiedebergs Arch Pharmacol. 1989 Sep;340(3):293–299. doi: 10.1007/BF00168513. [DOI] [PubMed] [Google Scholar]
- Gintant G. A., Datyner N. B., Cohen I. S. Gating of delayed rectification in acutely isolated canine cardiac Purkinje myocytes. Evidence for a single voltage-gated conductance. Biophys J. 1985 Dec;48(6):1059–1064. doi: 10.1016/S0006-3495(85)83869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki M., Mizobuchi S., Nakaya Y., Kawano K., Niki T., Mori H. Tetraethylammonium induced coronary spasm in isolated perfused rabbit heart: a hypothesis for the mechanism of coronary spasm. Cardiovasc Res. 1987 Feb;21(2):130–139. doi: 10.1093/cvr/21.2.130. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lynch C., 3rd Ionic conductances in frog short skeletal muscle fibres with slow delayed rectifier currents. J Physiol. 1985 Nov;368:359–378. doi: 10.1113/jphysiol.1985.sp015862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J. J., Volk K. A., Shibata E. F. Calcium currents in isolated rabbit coronary arterial smooth muscle myocytes. J Physiol. 1990 Aug;427:657–680. doi: 10.1113/jphysiol.1990.sp018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Simurda J., Simurdová M., Christé G. Use-dependent effects of 4-aminopyridine on transient outward current in dog ventricular muscle. Pflugers Arch. 1989 Nov;415(2):244–246. doi: 10.1007/BF00370600. [DOI] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y. Decreasing potassium conductance--a possible mechanism of phasic coronary vasospasm. Jpn Circ J. 1985 Jan;49(1):128–139. doi: 10.1253/jcj.49.128. [DOI] [PubMed] [Google Scholar]
- Wilde D. W., Lee K. S. Outward potassium currents in freshly isolated smooth muscle cell of dog coronary arteries. Circ Res. 1989 Dec;65(6):1718–1734. doi: 10.1161/01.res.65.6.1718. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


