Abstract
Background:
Traumatic rib fractures are associated with pain lasting weeks to months and a decreased ability to inspire deeply or cough to clear secretions. Ultrasound-guided percutaneous cryoneurolysis involves reversibly ablating peripheral nerve(s) using exceptionally low temperature with a transdermal probe, resulting in a prolonged nerve block with a duration measured in months. The authors hypothesized that cryoneurolysis would improve analgesia and inspired volume after rib fracture.
Methods:
Adults with one to six traumatic rib fractures were randomized to either active cryoneurolysis and sham peripheral nerve block or sham cryoneurolysis and active peripheral nerve block in a participant/observer-masked fashion. The primary endpoint was the maximum inspired volume the day after the procedure as measured with an incentive spirometer.
Results:
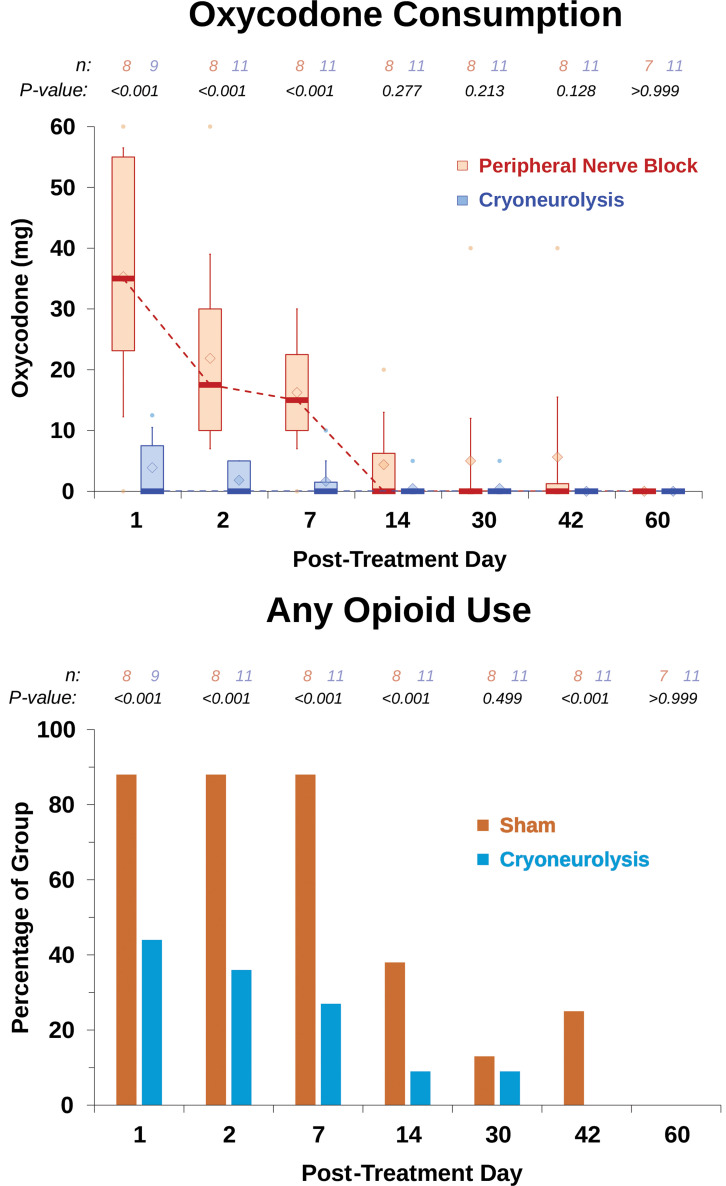
The day after the procedure, the unadjusted median [interquartile range] maximum inspired volume for participants who received cryoneurolysis (n = 11) was 2,250 ml [1,500, 2,500 ml] versus 1,300 ml [750, 2,500 ml] for peripheral nerve block (n = 9, mean difference, 496; 95% CI, –428 to 1,420; t test P = 0.269). When adjusted for covariates (e.g., baseline lung volume), the cryoneurolysis group had an estimated 793 ml greater mean volume than peripheral nerve block (95% CI, 273 to 1,312 ml; analysis of covariance P = 0.005). Improvement from baseline in maximum inspired volume for cryoneurolysis was 1,000 ml [1,000, 1,375 ml] versus 300 ml [0, 1,000 ml] for peripheral nerve block (t test P = 0.002). This was equivalent to an improvement over baseline of 100% [90%, 188%] for cryoneurolysis versus 30% [0%, 50%] for peripheral nerve block (t test P = 0.003). Average daily pain scores were generally lower for the cryoneurolysis group throughout the first month. Total cumulative oxycodone equivalents were 5 mg [0, 13 mg] for cryoneurolysis versus 45 mg [43, 135 mg] for peripheral nerve block (t test P = 0.013).
Conclusions:
Ultrasound-guided percutaneous cryoneurolysis improves maximum inspired lung volume while concurrently decreasing pain and opioid consumption after traumatic rib fracture. These results should be considered preliminary, requiring confirmation with a trial including a larger sample size.
This small, randomized, single-blinded clinical study in 20 patients with rib fractures compared treatment with an ultrasound-guided peripheral nerve block with 3 ml 0.5% ropivacaine (n = 9) versus ultrasound-guided percutaneous cryoneurolysis (n = 11). Cryoneurolysis was associated with greater improvement in lung volumes at 24 h after treatment. Both immediate pain and pain at several timepoints out to 3 months after the block were also lower in the cryoneurolysis group, as was opioid consumption.
Editor’s Perspective.
What We Already Know about This Topic
Intercostal nerve blocks can reduce pain, opioid consumption, and pulmonary dysfunction after rib fracture
Percutaneous cryoneurolysis, which has been used as an alternative to perineural deposition of local anesthetics, can provide longer-lasting blockade through localized nerve structure disruption
What This Article Tells Us That Is New
This small, randomized, single-blinded clinical study in 20 patients with rib fractures compared treatment with an ultrasound-guided peripheral nerve block with 3 ml 0.5% ropivacaine (n = 9) versus ultrasound-guided percutaneous cryoneurolysis (n = 11)
Cryoneurolysis was associated with greater improvement in lung volumes at 24 h after treatment
Both immediate pain and pain at several timepoints out to 3 months after the block were also lower in the cryoneurolysis group, as was opioid consumption
Within the United States each year, more than 240,000 individuals—10% of all trauma patients—experience thoracic trauma with one or more rib fractures.1–3 Fractures frequently result in severe pain that is exacerbated with every breath—approximately 20,000 daily—which may last more than 2 months.4,5 Importantly, pain from thoracic trauma is not just a symptom5 but a significant contributor to morbidity: pain is associated with decreased ability to cough, clear secretions, and inspire deeply,6 increasing the risk of pulmonary complications (33% incidence),2 mechanical ventilation, and mortality (incidence up to 12%).6,7 Reflecting the considerable health consequences of even uncomplicated fractures, the guidelines from the Western Trauma Association (Batavia, Illinois) recommend that older patients (older than 65 yr) with just three rib fractures be admitted to an intensive care unit for close monitoring.8 Furthermore, inadequately controlled acute pain in the weeks after fracture is the greatest risk factor for the development of chronic pain and disability at 6 months (incidence, 28%).9
Accordingly, multimodal analgesia in patients with traumatic rib fractures has received increasing attention, but few systemic options are effective.3,10 In contrast, local anesthetic–based regional anesthetics can provide potent analgesia and improve pulmonary function.11–13 For example, intercostal nerve blocks with local anesthetic have been shown to improve pain scores, peak expiratory flow rates, sustained maximal inspiration lung volumes, and arterial oxygen saturation on room air.14–16 Unfortunately, single-injection peripheral nerve blocks, even with ultralong-acting liposomal bupivacaine,17,18 provide thoracic analgesia for less than 24 h.19–21 Continuous paravertebral nerve blocks and epidural infusions extend these benefits and are also associated with decreased delirium, days of intubation, and mortality.10,12,13,22–25 Yet paravertebral infusions are still ordinarily limited to a few days,22,26–29 and epidurals12 are usually contraindicated in trauma patients receiving most forms of prophylactic anticoagulation due to the risk of an epidural hematoma.30 Furthermore, epidurals are limited to hospitalized patients due to the risks of hypotension and infection. Thus, local anesthetic nerve blocks are only temporizing measures for a small fraction of the multiple-month healing process after thoracic trauma, leaving most patients dependent upon opioids due simply to a lack of options.
One alternative is percutaneous cryoneurolysis, an analgesic modality consisting of the application of exceptionally low temperatures to reversibly ablate peripheral nerves.31 The procedure is essentially the same as placing an ultrasound-guided peripheral nerve block; however, instead of injecting local anesthetic, a gas circulates through the probe, inducing cold at its distal end and freezing the surrounding tissue, including the target nerve.31 Nothing remains within the patient, and there is no external equipment to prepare, manage, or malfunction—a single administration results in effects that lasts for months without any subsequent patient or healthcare provider interventions. A case report32 and short series33 suggest that cryoneurolysis may have substantial analgesic-based benefits after traumatic rib fractures.
We therefore conducted a randomized, active-controlled pilot study hypothesizing that cryoneurolysis would improve analgesia and inspired volume after rib fracture to a greater extent than a ropivacaine intercostal nerve block of the involved intercostal nerves.
Materials and Methods
This study followed Good Clinical Practice and was conducted within the ethical guidelines outlined in the Declaration of Helsinki. The protocol was approved by the institutional review board (University of California-San Diego, La Jolla, California) and prospectively registered (clinicaltrials.gov NCT04198662; principal investigator, Brian M. Ilfeld, M.D., M.S.; initial posting, December 13, 2019). Written, informed consent was obtained from all participants.
Participants
Enrollment was offered to adult patients of at least 18 yr of age with a total of one to six traumatic rib fractures at least 3 cm distal to the costo-transverse joint sustained within the previous 3 days (bilateral fractures acceptable, but the total number of fractured ribs could not exceed six). Patients were excluded for (1) chronic analgesic use including opioids (daily use within the 2 weeks before injury and duration of use more than 4 weeks); (2) pregnancy; (3) incarceration; (4) inability to communicate with the investigators; (5) morbid obesity (body mass index greater than 40 kg/m2); (6) possession of any contraindication specific to percutaneous cryoneurolysis such as a localized infection at the treatment site, cold urticaria, cryofibrinogenemia, cryoglobulinemia, paroxysmal cold hemoglobinuria, and Raynaud disease; (7) inability to correctly use an incentive spirometer; (8) any degree of decreased mental capacity as determined by the surgical service; (9) flail chest; (10) a chest tube; (11) fracture of the first rib on either side as there is no intercostal nerve cephalad to the first rib and thus the intercostal nerves above and below the fracture could not be treated; and (12) any moderate or severe pain (numeric rating scale greater than 3, unrelated to a rib fracture). No compensation was provided to subjects for study participation.
Treatment Group Assignment
Participants were randomly allocated to one of two treatments: (1) active cryoneurolysis and placebo peripheral nerve block or (2) sham cryoneurolysis and active peripheral nerve block (peripheral nerve block group). Computer-generated randomization lists were used by the University of California-San Diego Investigational Drug Service (San Diego, California) to create sealed, opaque randomization envelopes enclosing the treatment group assignment. Randomization was stratified by laterality (unilateral vs. bilateral) in a 1:1 ratio in blocks of four. The investigator administering the study intervention opened the randomization envelope. Therefore, investigators, subjects, and clinical staff were masked to treatment group assignment, with the only exception being the unmasked individual who performed the procedure (and did not have subsequent contact with the participant).
The maximum inspired volume was measured using a handheld incentive spirometer based on the American Association of Respiratory Care (Irving, Texas) clinical practice guidelines.34 The best of three measurements was recorded as the maximum inspired volume.
Participants were positioned either prone or seated; received oxygen via nasal canula or facemask; had standard American Society of Anesthesiologists (Schaumburg, Illinois) monitors applied; and administered intravenous midazolam and/or fentanyl, as needed, while remaining responsive to verbal cues. The treatment sites were cleansed with chlorhexidine gluconate and isopropyl alcohol. Using the optimal ultrasound transducer for the specific anatomic location and subject anatomy (linear vs. curvilinear array), the target nerves were identified in a transverse cross-sectional (short-axis) view. The intercostal nerve of each fractured rib as well as the level above and below were treated.
Intercostal Nerve Blocks
Local anesthetic (1% lidocaine) was used to infiltrate the skin and underlying muscle at each entry point. A 20-gauge Tuohy needle was introduced through the skin and along the anesthetized muscle tract. Subjects randomized to active cryoneurolysis received a sham nerve block with 3 ml normal saline injected into the muscle superficial to the nerve; for subjects randomized to sham cryoneurolysis, 3 ml ropivacaine 0.5% (with epinephrine) was injected perineurally to provide the intercostal nerve block.
Cryoneurolysis of Intercostal Nerves
Cryoneurolysis probes are available for a console neurolysis device (PainBlocker, Epimed, USA) that either (1) pass nitrous oxide to the tip, inducing freezing temperatures, or (2) vent the nitrous oxide at the base of the probe so that no gas reaches the probe tip, resulting in no temperature change. The latter is a sham procedure since without the temperature change, no ice ball forms, and therefore the target nerve is not affected. An angiocatheter/introducer was inserted beneath the ultrasound transducer and directed until the probe tip was immediately adjacent to the target nerve. The angiocatheter needle was removed, leaving the angiocatheter through which the appropriate cryoneurolysis probe was inserted until it was adjacent to the target nerve. The device was triggered using two cycles of 2-min gas activation (active or sham) separated by a 1-min defrost period. For active probes, the nitrous oxide was deployed to the tip, where a drop in temperature to approximately –70°C resulted in cryoneurolysis. For the sham probes, the nitrous oxide was vented before reaching the probe shaft, resulting in a lack of perineural temperature change.
Of note, it is impossible to safely mask the individual performing the cryoneurolysis procedure because the ice ball forming at the distal end of the probe during active treatment is clearly visible by ultrasound, and the lack of an ice ball for placebo subjects is equally clear (fig. 1).35,36 It is essential to continuously visualize the probe and target nerve throughout the two freeze/thaw cycles to ensure (1) the entire nerve diameter is adequately treated and (2) the ice ball remains relatively motionless to prevent it from tearing surrounding tissue. This cannot be achieved if the ultrasound is turned off during nitrous oxide administration to mask the provider, and we prioritized patient safety over provider masking.
Fig. 1.
Ultrasound images of percutaneous cryoneurolysis of an intercostal nerve (used with permission from Wolters Kluwer36).
For all participants, the process was repeated for each treated intercostal nerve. For bilateral fractures, the entire process was repeated on the contralateral side with the same probe. Acetaminophen (975 to 1,000 mg every 4 h up to four times each day), oxycodone (5-mg tablets, one to two every 4 h), and intravenous morphine (2 to 4 mg every 4 h) were permitted as needed. Nonsteroidal anti-inflammatory drugs, gabapentin, ketamine, and other analgesics were not permitted. Participants were discharged by the blinded surgical service when appropriate. Participants and their caretakers were provided with an incentive spirometer, the contact information of an investigator, and a prescription for oxycodone that did not differ from the hospital regimen.
Unmasking
Observers were masked to treatment group assignment. After the full year of data collection for the first eight participants, the treatment allocation of these individuals was unmasked exclusively for the principal investigator for inclusion as pilot data for a federal grant proposal (HT9425-23-2-0013). After the study conclusion, the remainder of the group allocations were unmasked for the principal investigator, who subsequently provided the dataset to the statistician for analysis in a masked fashion, with participants combined into unidentified “Treatment A” and “Treatment B” groups. After the completion of analysis, the study results were provided to each participant.
Outcome Measurements (Endpoints)
We selected outcome measures that have established reliability and validity, with minimal interrater discordance, and are recommended for pain-related clinical trials by the World Health Organization (Geneva, Switzerland) and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus statement.37 Outcomes were evaluated at baseline (before treatment) and on posttreatment days 1, 2, 7, and 14, as well as months 1, 1.5, 2, 3, 6, and 12. Baseline data were collected in person, while all subsequent outcomes were collected by telephone or database retrieval (hospital opioid consumption). Staff masked to treatment group assignment performed all measures and assessments.
Days 1 to 7
The maximum inspired volume was measured using a handheld incentive spirometer based on the American Association of Respiratory Care clinical practice guidelines,34 and the best of three measurements was recorded as the maximum inspired volume. The numeric rating scale is a highly sensitive measure of pain intensity with numbers ranging from 0 to 10, with 0 equivalent to no pain and 10 equivalent to the worst imaginable pain. Participants were asked to rate the worst (maximum), least, and average pain levels they had experienced in the previous 24 h, as well as the current level of pain and level while using the spirometer. If a patient responded with a range, the average of the range was recorded (e.g., “2 to 3” was recorded as 2.5). Participants were also asked the number of awakenings due to pain the previous night. Intravenous opioids were converted to oxycodone equivalents and presented combined with oral oxycodone consumption.
Week 2 to Month 12
The primary instrument was the Brief Pain Inventory (short form), which assesses pain and its interference with physical and emotional functioning (time frame: previous 24 h).38 The instrument includes three domains: (1) pain in the surgical site, with four questions using a numeric rating scale to evaluate four pain levels: “current,” “least,” “worst,” and “average”; (2) percentage of relief provided by pain treatments with one question (not used for this study); and (3) interference with physical and emotional functioning using a 0 to 10 scale (0, no interference; 10, complete interference). The seven interference questions involve general activity, mood, walking ability, normal work activities (both inside and outside of the home), relationships, sleep, and enjoyment of life.38 These seven functioning questions can be combined to produce an interference subscale (0 to 70). The use of both single items (e.g., mood) and the composite scores is supported by the IMMPACT consensus recommendations for assessing pain in clinical trials.37 In addition, maximum inspired volume and pain during use continued to be measured for as long as patients used the incentive spirometers.
Statistical Analysis
The primary endpoint was the maximum inspired volume the day after treatment as measured with an incentive spirometer. There is no generally accepted minimal clinically relevant change in incentive spirometry volume. However, the median [interquartile range] of inspired volume for patients with rib fracture(s) is 1,250 [750, 1,750] ml, and volume less than 1,000 ml measured with an incentive spirometer is associated with an increased risk of acute respiratory failure.39 We therefore used the difference between 1,250 and 1,000 (250 ml) as the minimal clinically relevant difference.
Assuming a normal distribution, the interquartile range is approximately 1.35 SDs. Therefore, an interquartile range of 250 – 50 = 200 ml corresponds to, approximately, an SD of 200/1.35 = 148 ml.40 Assuming this SD of 148 ml, a sample size of n = 7 per group provided 80% power to detect a group difference of d = 250 ml per group with a two-sided alpha = 5%. However, there is high variability in the reported increase in inspired volume with various regional analgesic interventions such as continuous intercostal nerve blocks41 and serratus plane blocks,40 and we consequently increased enrollment to account for an unpredicted increase in variability or nonnormal data distribution. We accordingly enrolled 10 subjects per group with an evaluable primary outcome measure (n = 20 for both groups combined). To account for dropouts, we requested a maximum enrollment of 30 subjects from the institutional review board.
Key baseline characteristics were tested between groups using two-sample t tests and summarized with Cohen’s d for continuous measures and the Fisher exact test for categorical variables. The Wilcoxon signed-rank test was used as a sensitivity analysis. The primary outcome was also submitted to an analysis of covariance with baseline lung volume, unilateral versus bilateral fractures, and the total number of fractures (as a continuous variable). A mixed model of repeated measures with fixed effects for the baseline value (where collected for the outcome), time, and time-by-group interaction was used to assess group differences over time. The model treated time as a categorical variable and assumed a compound symmetric correlation and heterogeneous variance with respect to time. No multiplicity adjustments were applied for secondary analyses, and thus results in secondary outcomes require confirmation with a subsequent, presumably larger, clinical trial. All tests were two-sided, and P < 0.05 was considered statistically significant.
R (R-project.org, https://www.r-project.org; accessed March 11, 2024) was used for sample size calculations and analysis.
Results
Between April 2020 and August 2023, a total of 45 patients with diagnosed rib fractures were evaluated, with 20 participants enrolled and subsequently randomized to either cryoneurolysis (n = 11) or peripheral nerve block (n = 9; table 1; fig. 2). Immediately after the intervention, participants who had received cryoneurolysis (and sham peripheral nerve block) increased maximum inspiratory volume from baseline a median [interquartile range] of 1,250 ml [1,000, 1,375 ml] versus 750 ml [250, 1,000 ml] for patients who received sham cryoneurolysis and ropivacaine peripheral nerve block (t test P = 0.012). This was equivalent to an improvement of an increase over baseline of 225% [200%, 283%] for cryoneurolysis versus 150% [133%, 188%] for peripheral nerve block (t test P = 0.005). Pain scores while using the incentive spirometer went from a median [interquartile range] of 10 for both groups at baseline before the intervention to 3 [0, 5] for cryoneurolysis and 5 [2, 6] for peripheral nerve block after the intervention (t test P = 0.165).
Table 1.
Population Data
| Cryoneurolysis (n = 11) |
Sham (Placebo) (n = 9) |
Absolute Standardized Difference | |
|---|---|---|---|
| Age, yr | 46 ± 19 | 57 ± 14 | 0.671 |
| Female (%) | 3 (27%) | 2 (22%) | 0.026 |
| Height, m | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.026 |
| Weight, kg | 90 ± 19 | 83 ± 22 | 0.331 |
| Body mass index, kg/m2 | 27 ± 7 | 30 ± 6 | 0.375 |
| Fractured ribs, n | 4.1 ± 1.4 | 3.9 ± 1.5 | 0.143 |
| Left | 1.7 ± 1.8 | 2.0 ± 2.5 | 0.125 |
| Right | 2.4 ± 2.3 | 1.9 ± 2.1 | 0.214 |
| Laterality | 0.667 | ||
| Unilateral | 9 (82%) | 9 (100%) | |
| Bilateral | 2 (18%) | 0 (0%) | |
| Inspiratory volume at baseline, ml | 1,222 ± 551 | 1,023 ± 564 | 0.358 |
Values are reported as mean ± SD or number of subjects (percentage). Any variable with an absolute standardized difference greater than 0.877 was considered imbalanced.
Fig. 2.
Consolidated Standards of Reporting Trials diagram.
Primary Outcome
The day after the procedure, the unadjusted median [interquartile range] maximum inspired volume for participants who received cryoneurolysis was 2,250 ml [1,500, 2,500 ml] versus 1,300 ml [750, 2,500 ml] for peripheral nerve block (mean difference, 496; 95% CI, –428 to 1,420; t test P = 0.269). When adjusted for baseline lung volume, unilateral versus bilateral fractures, and the total number of fractures, the cryoneurolysis group had an estimated 793 ml greater mean volume than peripheral nerve block (95% CI, 273 to 1,312; analysis of covariance P = 0.005).
Secondary Outcomes
Participants who received cryoneurolysis had a greater percentage increase in volume relative to their preintervention baseline compared with patients given peripheral nerve block on posttreatment days 1 and 2 (fig. 3). Pain scores were lower for the cryoneurolysis group at each time point from day 1 through months 1 to 3 (figs. 3 and 4). Post hoc analysis revealed that 18% of the treatment group experienced solely mild pain (numeric rating scale less than 4) throughout the entirety of the first year, compared with 0% of controls, while 36% of the treatment group reported severe pain (at least one pain score greater than 7) during the course of the first year after surgery, compared to 67% in the control group (fig. 5; Fisher exact test P < 0.001).
Fig. 3.
Effects of percutaneous cryoneurolysis on the increase in maximum inspiratory volume and pain after traumatic rib fracture. The maximum inspired volume was measured using a handheld incentive spirometer based on the American Association of Respiratory Care clinical practice guidelines, and the best of three measurements was recorded as the maximum inspired volume. For the panels involving improvement from baseline, data presented are the specific time point relative to baseline measured immediately before the intervention on day 0. Day 0 in the graphs was measured immediately after the intervention. Pain severity during incentive spirometer use was measured using a numeric rating scale with 0 equivalent to no pain and 10 being the worst imaginable pain. Data expressed as median (dark horizontal bars) with 25th to 75th percentiles (box), 10th to 90th percentiles (whiskers), mean (diamonds), and outliers below the 10th or above the 90th percentiles (circles). P values are derived from a mixed model for repeated measures with covariates for the baseline value (where collected for that outcome), time, and the group-by-time interaction.
Fig. 4.
Effects of percutaneous cryoneurolysis on pain and pain’s interference in functioning after traumatic rib fracture. Pain severity was measured using a numeric rating scale with 0 equivalent to no pain and 10 being the worst imaginable pain for the 24 h before data collection. Regarding the Brief Pain Inventory, pain interference indicated using a numeric rating scale of 0 to 70, with 0 and 70 equivalent to no and maximal interference, respectively. Data expressed as median (dark horizontal bars) with 25th to 75th percentiles (box), 10th to 90th percentiles (whiskers), mean (diamonds), and outliers below the 10th or above the 90th percentiles (circles). P values are derived from a mixed model for repeated measures with covariates for the baseline value (where collected for that outcome), time, and the group-by-time interaction.
Fig. 5.
Effects of percutaneous cryoneurolysis on the maximum pain level experienced in the entire year after traumatic rib fracture. Pain severity was measured using a numeric rating scale with 0 equivalent to no pain and 10 the worst imaginable pain. Post hoc analysis results (left) expressed as median (dark horizontal bars) with 25th to 75th percentiles (box), 10th to 90th percentiles (whiskers), mean (diamonds), and outliers below the 10th or above the 90th percentiles (circles); and (right) categorized as mild (numeric rating scale less than 4), moderate (numeric rating scale 4 to 7), and severe (numeric rating scale greater than 7) pain. On the right, the cryoneurolysis percentages do not total 100% due to a rounding error. P values are derived from a mixed model for repeated measures with covariates for the baseline value (where collected for that outcome), time, and the group-by-time interaction. PNB, peripheral nerve block.
Similarly, opioid consumption was lower for the cryoneurolysis group at each time point for days 1 to 7 (fig. 6). During the entire study period, cryoneurolysis lowered cumulative oxycodone use by 89%, with the treated group using 5 mg [0, 13 mg] compared with 45 mg [43, 135 mg] in controls (t test P = 0.013). There was no opioid used by any patient beyond 6 weeks.
Fig. 6.
Effects of percutaneous cryoneurolysis on opioid consumption after traumatic rib fracture. Oxycodone is a synthetic opioid and presented in milligrams. During this period, cryoneurolysis lowered cumulative opioid use by 89%, with the treated group using 5 mg [0, 13 mg] of oxycodone compared with 45 mg [43, 135 mg] in controls (P = 0.013). Regarding the risk of requiring any opioids at each time point, post hoc analysis results are presented as the percentage of each treatment group consuming any opioids at each time point. Data expressed as median (dark horizontal bars) with 25th to 75th percentiles (box), 10th to 90th percentiles (whiskers), mean (diamonds), and outliers below the 10th or above the 90th percentiles (circles). P values are derived from a mixed model for repeated measures with covariates for the baseline value (where collected for that outcome), time, and the group-by-time interaction.
Pain’s interference in physical and emotional functioning as measured with the Brief Pain Inventory (Interference subscale) was lower in the cryoneurolysis group to a statistically significant degree exclusively at 2 months (fig. 4). Cumulative awakenings due to pain for the entire treatment period were 1 [0, 4] for cryoneurolysis versus 13 [10, 17] for peripheral nerve block (t test P = 0.034). Finally, participants who received cryoneurolysis were discharged in a mean ± SD of 0.7 ± 1.0 days versus 1.4 ± 1.2 days for peripheral nerve block (t test P = 0.170). Post hoc analysis found that 55% of cryoneurolysis participants were discharged the same day as treatment, versus 22% who had received peripheral nerve block (Fisher exact test P < 0.001).
Protocol Deviations and Adverse Events
The only protocol deviations were one participant from each treatment group who had a clavicular fracture in addition to the rib fractures. The only adverse event was one participant who had received cryoneurolysis and was subsequently diagnosed with pneumonia on posttreatment day 2. The participant was successfully treated with oral antibiotics.
Discussion
This randomized, controlled, patient- and observer-masked study provides evidence that a single application of ultrasound-guided percutaneous intercostal nerve cryoneurolysis improves maximum inspired volume after traumatic rib fracture. This benefit was presumably due to improved analgesia afforded participants allocated to cryoneurolysis. The improvement in analgesia continued through the first month, resulting in a concurrent 89% decrease in cumulative oxycodone consumption (median 5 mg vs. 45 mg) and awakenings due to pain (median 1 vs. 13). No cryoneurolysis-related systemic side effects or complications were identified. While this study was not powered to detect differences in pulmonary complications due to pain-induced hypoventilation, cryoneurolysis could theoretically reduce the incidence of pneumonia and other morbidities. Should this ultimately prove accurate, it may reduce or even preclude the need for observation in the intensive care unit currently the recommendation for patients older than 65 yr with three or more fractures.8 In contrast, caution is warranted when treating bilateral rib fractures as delayed, bilateral, cryo-induced pneumothoraces could be life-threatening.
Cryoneurolysis decreased pain’s interference with physical and emotional functioning to a statistically significant degree only at month 2 (fig. 4). However, this study was not powered to detect differences in this secondary outcome. If the observed difference between the two groups at 14 and 30 days is found in a subsequent adequately powered trial, the improvement (23 to 30 points) would be far greater than the 7 points considered clinically relevant within the IMMPACT consensus statement.37
An important—and somewhat unexpected—finding was that no participant who had received cryoneurolysis experienced pain after 1 posttreatment month. In contrast, 50% of participants within the control group who responded at the 6-month timepoint (three of six) experienced pain. Due to an inability to contact three of the nine participants in the peripheral nerve block group along with the generally small sample size of the current study, this is most likely an artificially high percentage. However, assuming the three participants whom we were unable to contact were pain-free, the 33% incidence of persistent pain at 6 months would be approximately the same as reported in other investigations (28%).9 This may be evidence that a longer, more complete neural blockade treating acute pain reduces the risk of persistent chronic pain.9 At the 12-month time point, only one participant (of five contacted) who received peripheral nerve block was still experiencing pain, versus none (of eight contacted) from the cryoneurolysis group.
The current study results support growing evidence that for specific indications, percutaneous cryoneurolysis may be preferable over opioid- and local anesthetic–based analgesics. First, and perhaps most notably, cryoneurolysis offers a long-lasting effect that can persist for months after a single treatment.31 Cryoanalgesia also avoids the systemic side effects of opioids without any potential for misuse, dependence, overdose, or diversion. In comparison to continuous peripheral nerve blocks, cryoneurolysis eliminates the potential for local anesthetic–induced cardiac or neurologic toxicity, myotoxicity, catheter dislodgement, local anesthetic leakage, and infusion pump malfunction.42 Furthermore, cryoneurolysis significantly reduces the burden on both patients and healthcare providers, as it does not require the use of a portable infusion pump, local anesthetic reservoir, or perineural catheter to be carried, managed, or eventually removed. Notably, during the course of more than 50 yr, there has been only a single reported (suspected) instance of a cryoneurolysis-related infection,43 as opposed to a perineural catheter infection rate reported as high as 3%.42,44
Conversely, various attributes greatly limit the application of cryoneurolysis to acute pain, including its prolonged time requirement for administration; inconstant sensory, motor, and proprioceptive nerve block; inability to titrate effects; and a protracted—and variable—duration of action.31 Nonetheless, it appears to be a unique alternative to treat pain after traumatic rib fracture or other indications involving the thorax,36 with a duration of action at least similar to the duration of pain from rib fracture(s).
Two randomized controlled trials suggest an additional possible limitation: cryoneurolysis may be associated with a higher frequency of transient neuropathic pain between 3 and 6 months after open thoracotomy.45,46 However, in these studies, the surgeon manipulated the intercostal nerves before application of cryoneurolysis, as opposed to the current study, which utilized ultrasound-guided percutaneous cryoneurolysis, which does not require physical manipulation. In laboratory animals, physical nerve manipulation was required to induce chronic pain, suggesting an important difference in risk between direct surgical and percutaneous cryoneurolysis administration.47 As of the date of this writing, no chronic neuropathic pain has been associated with percutaneous administration of cryoneurolysis,48 and there is limited evidence that it might even decrease the risk of persistent chronic pain.36 Nevertheless, this issue deserves further investigation.49
Limitations of this study include a limited sample size of 20 participants all from a single enrolling center. Results from regression models with such small sample sizes should be viewed as exploratory. Furthermore, the optimal—or even minimal—freeze and defrost durations as well as the number of freeze–defrost cycles have yet to be determined.31 Decreasing these values would reduce the time limitation of cryoneurolysis, and future related research might increase the applicability of this modality.50 Therefore, the results of the current study may not be generalizable to other cryoneurolysis equipment and/or treatment protocols. Last, the control group received a relatively small volume (3 ml) of 0.5% ropivacaine applied to each intercostal nerve, which is the standard dose at our institution and similar to doses reported in the literature.51 This is based on the small size of the intercostal nerve and concern regarding local anesthetic toxicity due to the multiple nerves frequently targeted as well as the high rate of local anesthetic absorption in the intercostal region. The immediate increase in the maximum inspiratory volume and decrease in pain during spirometry (fig. 3) for the control group suggest that the block was effective. Due to the rapid uptake of local anesthetic in the intercostal regional—and resulting documented duration of intercostal nerve blocks lasting less than 24 h51—it is unsurprising that the maximum inspiratory volume decreased and pain during spirometry increased the day after treatment. Whether a larger dose of ropivacaine would improve analgesic potency or duration remains undetermined.
In conclusion, this study suggests that ultrasound-guided percutaneous cryoneurolysis improves maximum inspired lung volume while concurrently decreasing pain and opioid consumption after traumatic rib fracture. However, due to the limited number of participants of the current study, these results should be considered preliminary requiring confirmation in a trial with a larger sample size.
Acknowledgments
The authors appreciate the invaluable assistance of Baharin Abdullah, M.D. (Department of Anesthesiology, University of California-San Diego, La Jolla, California), and Grace Tong-Ventura, Pharm.D. (Investigational Drug Service, University of California-San Diego, San Diego, California).
Research Support
Epimed International (Dallas, Texas) provided unrestricted research funding and the cryoneurolysis devices used in this study. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by this company. Of note, this was an investigator-initiated project, and the senior author retained complete control of the study protocol; data collection, analysis, and interpretation; and the resulting manuscript. Epimed International was provided the initial protocol on which to comment, although no revisions were suggested. No information was subsequently provided to this company other than enrollment progress.
Competing Interests
Drs. Finneran and Ilfeld’s institution has received funding and/or product from Epimed International (Dallas, Texas) for other research studies, SPR Therapeutics (Cleveland, Ohio), Infutronix (Natick, Massachusetts), Masimo (Irvine, California), Varian Medical Systems (Palo Alto, California), and Avanos Medical (Irvine, California). Dr. Trescot. has served on an advisory board for Atricure, (Mason, Ohio) and is the chief medical officer for Stimwave Technologies (Pompano, Florida). Dr. Donohue has served on scientific advisory boards for Biogen (Cambridge, Massachusetts), Eli Lilly (Indianapolis, Indiana), and Neurotrack Technologies (Redwood City, California) and has consulted for Roche. His spouse is a full-time employee of Janssen (Beerse, Belgium). Dr. Schaar’s institution has received funding and/or product from Epimed International and Infutronix for other research studies. The other authors declare no competing interests.
Reproducible Science
Full protocol available at: bilfeld@health.ucsd.edu. Raw data available at: bilfeld@health.ucsd.edu.
Footnotes
This article is featured in “This Month in Anesthesiology,” page A1.
An abstract reporting on the initial eight participants was presented at the American Society of Regional Anesthesia Annual Spring Meeting in San Diego, California, March 22, 2024.
The article processing charge was funded by the authors.
Published online first on December 19, 2024.
Contributor Information
John J. Finneran, IV, Email: Jfinneran@health.ucsd.edu.
Leslie Kobayashi, Email: lmkobayashi@health.ucsd.edu.
Todd W. Costantini, Email: cost0086@umn.edu.
Jessica L. Weaver, Email: jlweaver@ucsd.edu.
Allison E. Berndtson, Email: aberndtson@health.ucsd.edu.
Laura Haines, Email: ladams@health.ucsd.edu.
Jay J. Doucet, Email: jdoucet@health.ucsd.edu.
Laura Adams, Email: ladams@health.ucsd.edu.
Jarrett E. Santorelli, Email: jsantorelli@health.ucsd.edu.
Jeanne Lee, Email: jgl003@ucsd.edu.
Andrea M. Trescot, Email: DrTrescot@gmail.com.
Michael C. Donohue, Email: mcdonohue@gmail.com.
Adam Schaar, Email: anschaar@health.ucsd.edu.
Brian M. Ilfeld, Email: bilfeld@health.ucsd.edu.
References
- 1.Bowman JA, Nuno M, Jurkovich GJ, Utter GH: Association of hospital-level intensive care unit use and outcomes in older patients with isolated rib fractures. JAMA Netw Open 2020; 3:e2026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler DW, Agarwal NN: The morbidity and mortality of rib fractures. J Trauma 1994; 37:975–9 [DOI] [PubMed] [Google Scholar]
- 3.Martin TJ, Eltorai AS, Dunn R, et al. : Clinical management of rib fractures and methods for prevention of pulmonary complications: A review. Injury 2019; 50:1159–65 [DOI] [PubMed] [Google Scholar]
- 4.Kerr-Valentic MA, Arthur M, Mullins RJ, Pearson TE, Mayberry JC: Rib fracture pain and disability: Can we do better? J Trauma 2003; 54:1058–63; discussion 1063 [DOI] [PubMed] [Google Scholar]
- 5.He Z, Zhang D, Xiao H, et al. : The ideal methods for the management of rib fractures. J Thorac Dis 2019; 11:S1078–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flagel BT, Luchette FA, Reed RL, et al. : Half-a-dozen ribs: The breakpoint for mortality. Surgery 2005; 138:717–23; discussion 723 [DOI] [PubMed] [Google Scholar]
- 7.Battle CE, Hutchings H, Evans PA: Risk factors that predict mortality in patients with blunt chest wall trauma: A systematic review and meta-analysis. Injury 2012; 43:8–17 [DOI] [PubMed] [Google Scholar]
- 8.Brasel KJ, Moore EE, Albrecht RA, et al. : Western Trauma Association critical decisions in trauma: Management of rib fractures. J Trauma Acute Care Surg 2017; 82:200–3 [DOI] [PubMed] [Google Scholar]
- 9.Gordy S, Fabricant L, Ham B, Mullins R, Mayberry J: The contribution of rib fractures to chronic pain and disability. Am J Surg 2014; 207:659–62; discussion 662 [DOI] [PubMed] [Google Scholar]
- 10.Simon B, Ebert J, Bokhari F, et al. ; Eastern Association for the Surgery of Trauma: Management of pulmonary contusion and flail chest: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012; 73:S351–61 [DOI] [PubMed] [Google Scholar]
- 11.Thiruvenkatarajan V, Cruz Eng H, Adhikary SD: An update on regional analgesia for rib fractures. Curr Opin Anaesthesiol 2018; 31:601–7 [DOI] [PubMed] [Google Scholar]
- 12.Bulger EM, Edwards T, Klotz P, Jurkovich GJ: Epidural analgesia improves outcome after multiple rib fractures. Surgery 2004; 136:426–30 [DOI] [PubMed] [Google Scholar]
- 13.Malekpour M, Hashmi A, Dove J, Torres D, Wild J: Analgesic choice in management of rib fractures: Paravertebral block or epidural analgesia? Anesth Analg 2017; 124:1906–11 [DOI] [PubMed] [Google Scholar]
- 14.Osinowo OA, Zahrani M, Softah A: Effect of intercostal nerve block with 0.5% bupivacaine on peak expiratory flow rate and arterial oxygen saturation in rib fractures. J Trauma 2004; 56:345–7 [DOI] [PubMed] [Google Scholar]
- 15.Truitt MS, Murry J, Amos J, et al. : Continuous intercostal nerve blockade for rib fractures: Ready for primetime? J Trauma 2011; 71:1548–52; discussion 1552 [DOI] [PubMed] [Google Scholar]
- 16.Yildiz M, Kozanhan B, Iyisoy MS, Canitez A, Aksoy N, Eryigit A: The effect of erector spinae plane block on postoperative analgesia and respiratory function in patients undergoing laparoscopic cholecystectomy: A double-blind randomized controlled trial. J Clin Anesth 2021; 74:110403. [DOI] [PubMed] [Google Scholar]
- 17.Lee CY, Robinson DA, Johnson CA, Jr., et al. : A randomized controlled trial of liposomal bupivacaine parasternal intercostal block for sternotomy. Ann Thorac Surg 2019; 107:128–34 [DOI] [PubMed] [Google Scholar]
- 18.Pedoto A, Noel J, Park BJ, Amar D: Liposomal bupivacaine versus bupivacaine hydrochloride for intercostal nerve blockade in minimally invasive thoracic surgery. J Cardiothorac Vasc Anesth 2021; 35:1393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao W, Zhou W, Chen X, Zhu J, Xue Q, Shi J: Analgesic effect of intercostal nerve block given preventively or at the end of operation in video-assisted thoracic surgery: A randomized clinical trial. Braz J Anesthesiol 2022; 72:574–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen VM, Schulze S, Hoier-Madsen K, Halkier E: Air-flow meter assessment of the effect of intercostal nerve blockade on respiratory function in rib fractures. Acta Chir Scand 1983; 149:119–20 [PubMed] [Google Scholar]
- 21.Qiu Y, Wu J, Huang Q, et al. : Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: A noninferiority randomised trial. Eur J Anaesthesiol 2021; 38:S97–S105 [DOI] [PubMed] [Google Scholar]
- 22.O’Connell KM, Patel KV, Powelson E, et al. : Use of regional analgesia and risk of delirium in older adults with multiple rib fractures: An Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2021; 91:265–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peek J, Smeeing DPJ, Hietbrink F, Houwert RM, Marsman M, de Jong MB: Comparison of analgesic interventions for traumatic rib fractures: A systematic review and meta-analysis. Eur J Trauma Emerg Surg 2019; 45:597–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashemzadeh S, Hashemzadeh K, Hosseinzadeh H, Aligholipour Maleki R, Golzari SE: Comparison thoracic epidural and intercostal block to improve ventilation parameters and reduce pain in patients with multiple rib fractures. J Cardiovasc Thorac Res 2011; 3:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gage A, Rivara F, Wang J, Jurkovich GJ, Arbabi S: The effect of epidural placement in patients after blunt thoracic trauma. J Trauma Acute Care Surg 2014; 76:39–45; discussion 45 [DOI] [PubMed] [Google Scholar]
- 26.Mohta M, Verma P, Saxena AK, Sethi AK, Tyagi A, Girotra G: Prospective, randomized comparison of continuous thoracic epidural and thoracic paravertebral infusion in patients with unilateral multiple fractured ribs–A pilot study. J Trauma 2009; 66:1096–101 [DOI] [PubMed] [Google Scholar]
- 27.Karmakar MK, Critchley LA, Ho AM, Gin T, Lee TW, Yim AP: Continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with multiple fractured ribs. Chest 2003; 123:424–31 [DOI] [PubMed] [Google Scholar]
- 28.Yeying G, Liyong Y, Yuebo C, et al. : Thoracic paravertebral block versus intravenous patient-controlled analgesia for pain treatment in patients with multiple rib fractures. J Int Med Res 2017; 45:2085–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truitt MS, Mooty RC, Amos J, Lorenzo M, Mangram A, Dunn E: Out with the old, in with the new: A novel approach to treating pain associated with rib fractures. World J Surg 2010; 34:2359–62 [DOI] [PubMed] [Google Scholar]
- 30.Horlocker TT, Wedel DJ, Rowlingson JC, et al. : Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010; 35:64–101 [DOI] [PubMed] [Google Scholar]
- 31.Ilfeld BM, Finneran JJ: Cryoneurolysis and percutaneous peripheral nerve stimulation to treat acute pain. Anesthesiology 2020; 133:1127–49 [DOI] [PubMed] [Google Scholar]
- 32.Hashemi M, Mahmood SMJ, Fernandez J, Oswald J: Cryoneurolysis of intercostal nerve for rib trauma and intercostal neuralgia in the emergency department: A multidisciplinary approach. J Emerg Med 2022; 63:376–81 [DOI] [PubMed] [Google Scholar]
- 33.Finneran JJ, Gabriel RA, Swisher MW, et al. : Ultrasound-guided percutaneous intercostal nerve cryoneurolysis for analgesia following traumatic rib fracture: A case series. Korean J Anesthesiol 2019; 73:455–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Restrepo RD, Wettstein R, Wittnebel L, Tracy M: Incentive spirometry: 2011. Respir Care 2011; 56:1600–4 [DOI] [PubMed] [Google Scholar]
- 35.Kastler A, Gruber H, Gizewski E, Loizides A: Ultrasound assessment of ice-ball formation by cryoneurolysis device in an ex vivo model. Reg Anesth Pain Med 2018; 43:631–3 [DOI] [PubMed] [Google Scholar]
- 36.Ilfeld BM, Finneran JJ, Swisher MW, et al. : Preoperative ultrasound-guided percutaneous cryoneurolysis for the treatment of pain after mastectomy: A randomized, participant- and observer-masked, sham-controlled study. Anesthesiology 2022; 137:529–42 [DOI] [PubMed] [Google Scholar]
- 37.Dworkin RH, Turk DC, Peirce-Sandner S, et al. : Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010; 149:177–93 [DOI] [PubMed] [Google Scholar]
- 38.Cleeland CS, Ryan KM: Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994; 23:129–38 [PubMed] [Google Scholar]
- 39.Butts CA, Brady JJ, Wilhelm S, et al. : Do simple beside lung function tests predict morbidity after rib fractures? Am J Surg 2017; 213:473–7 [DOI] [PubMed] [Google Scholar]
- 40.Hernandez N, de Haan J, Clendeninn D, et al. : Impact of serratus plane block on pain scores and incentive spirometry volumes after chest trauma. Local Reg Anesth 2019; 12:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch N, Salottolo K, Foster K, et al. : Comparative effectiveness analysis of two regional analgesia techniques for the pain management of isolated multiple rib fractures. J Pain Res 2019; 12:1701–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilfeld BM: Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg 2017; 124:308–35 [DOI] [PubMed] [Google Scholar]
- 43.Cahani D, Chacko J, Hahn B: Myonecrosis: A rare complication of cryoneurolysis. J Emerg Med 2019; 57:e73–6 [DOI] [PubMed] [Google Scholar]
- 44.Capdevila X, Bringuier S, Borgeat A: Infectious risk of continuous peripheral nerve blocks. Anesthesiology 2009; 110:182–8 [DOI] [PubMed] [Google Scholar]
- 45.Ju H, Feng Y, Yang BX, Wang J: Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. Eur J Pain 2008; 12:378–84 [DOI] [PubMed] [Google Scholar]
- 46.Mustola ST, Lempinen J, Saimanen E, Vilkko P: Efficacy of thoracic epidural analgesia with or without intercostal nerve cryoanalgesia for postthoracotomy pain. Ann Thorac Surg 2011; 91:869–73 [DOI] [PubMed] [Google Scholar]
- 47.Ilfeld BM, Wagner R: Cryoneurolysis: Interest and caution: Comment. Anesthesiology 2023; 139:112–3 [DOI] [PubMed] [Google Scholar]
- 48.Bittman RW, Peters GL, Newsome JM, et al. : Percutaneous image-guided cryoneurolysis. AJR Am J Roentgenol 2018; 210:454–65 [DOI] [PubMed] [Google Scholar]
- 49.Rathmell JP, Forrester JD, Schreiber K: Cryoneurolysis: Interest and caution. Anesthesiology 2022; 137:521–3 [DOI] [PubMed] [Google Scholar]
- 50.Garibyan L, Moradi Tuchayi S, Wang Y, et al. : Neural selective cryoneurolysis with ice slurry injection in a rat model. Anesthesiology 2020; 133:185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmoudi K, Rashidi M, Soltani F, Savaie M, Hedayati E, Rashidi P: Comparison of intercostal nerve block with ropivacaine and ropivacaine-dexmedetomidine for postoperative pain control in patients undergoing thoracotomy: A randomized clinical trial. Anesth Pain Med 2021; 11:e118667. [DOI] [PMC free article] [PubMed] [Google Scholar]