Abstract
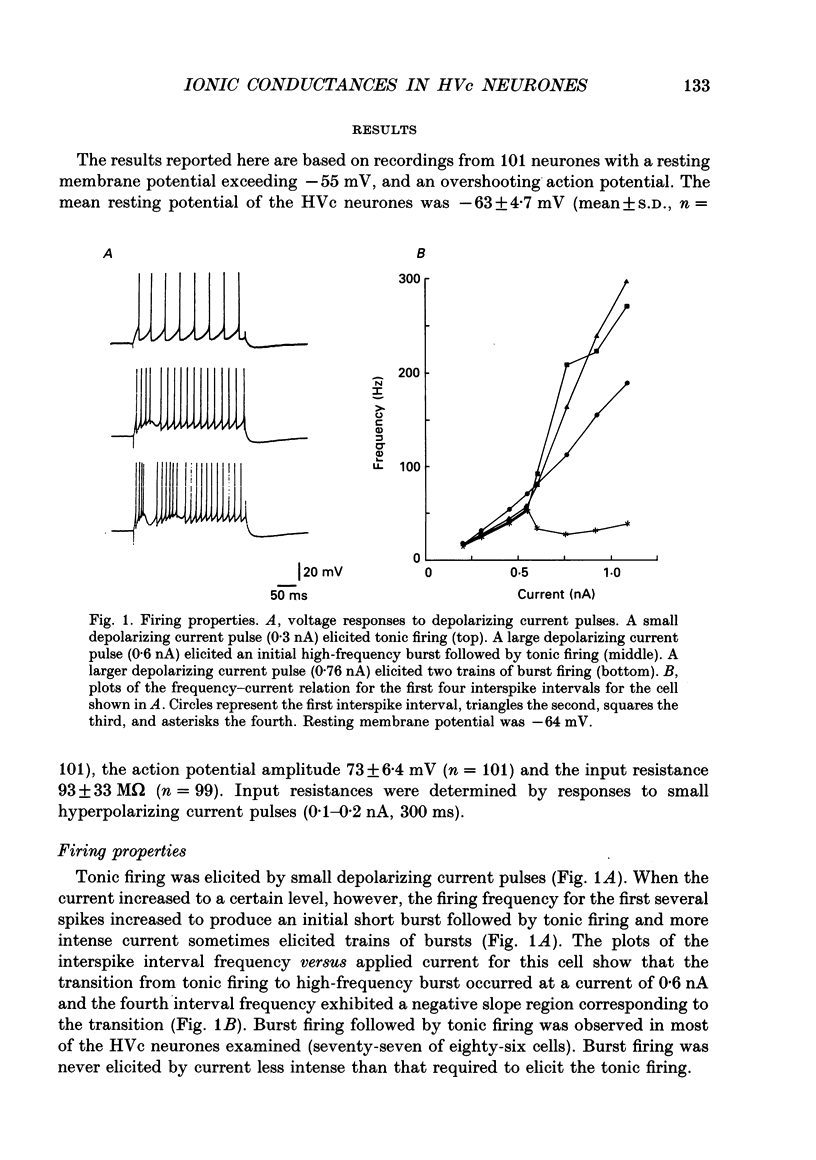
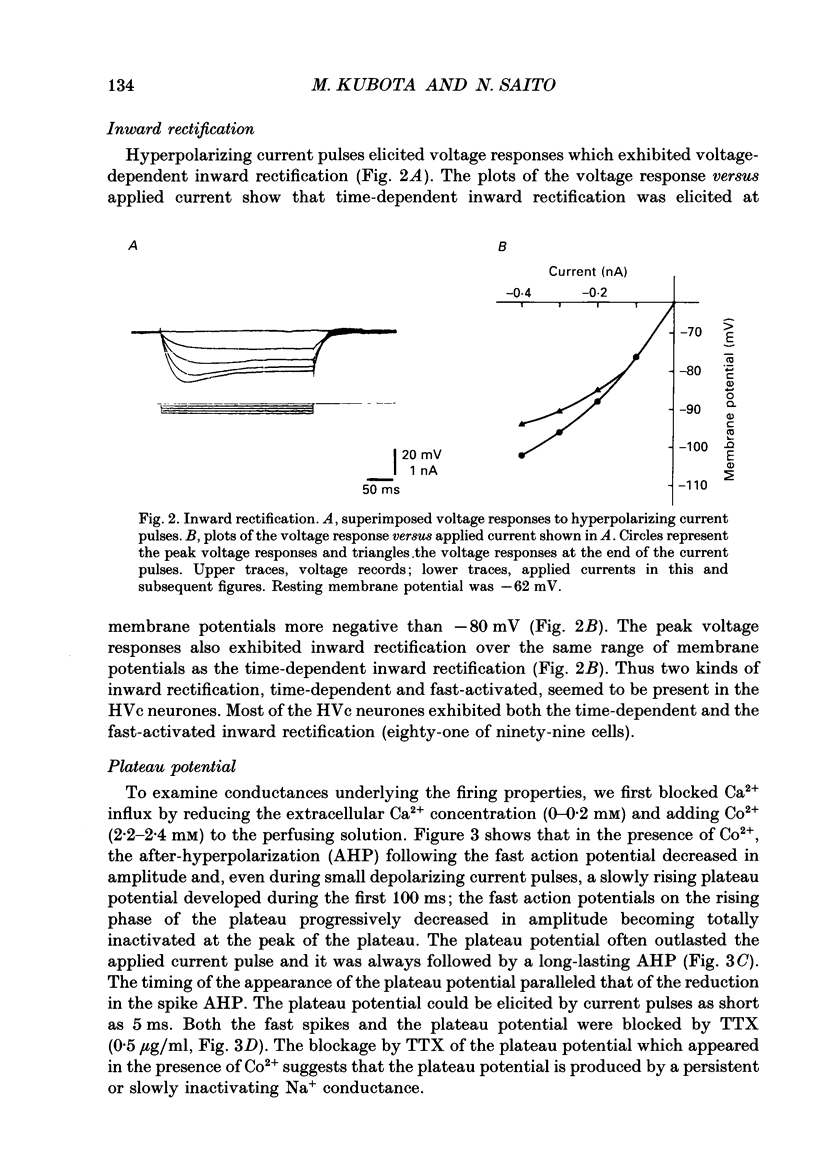
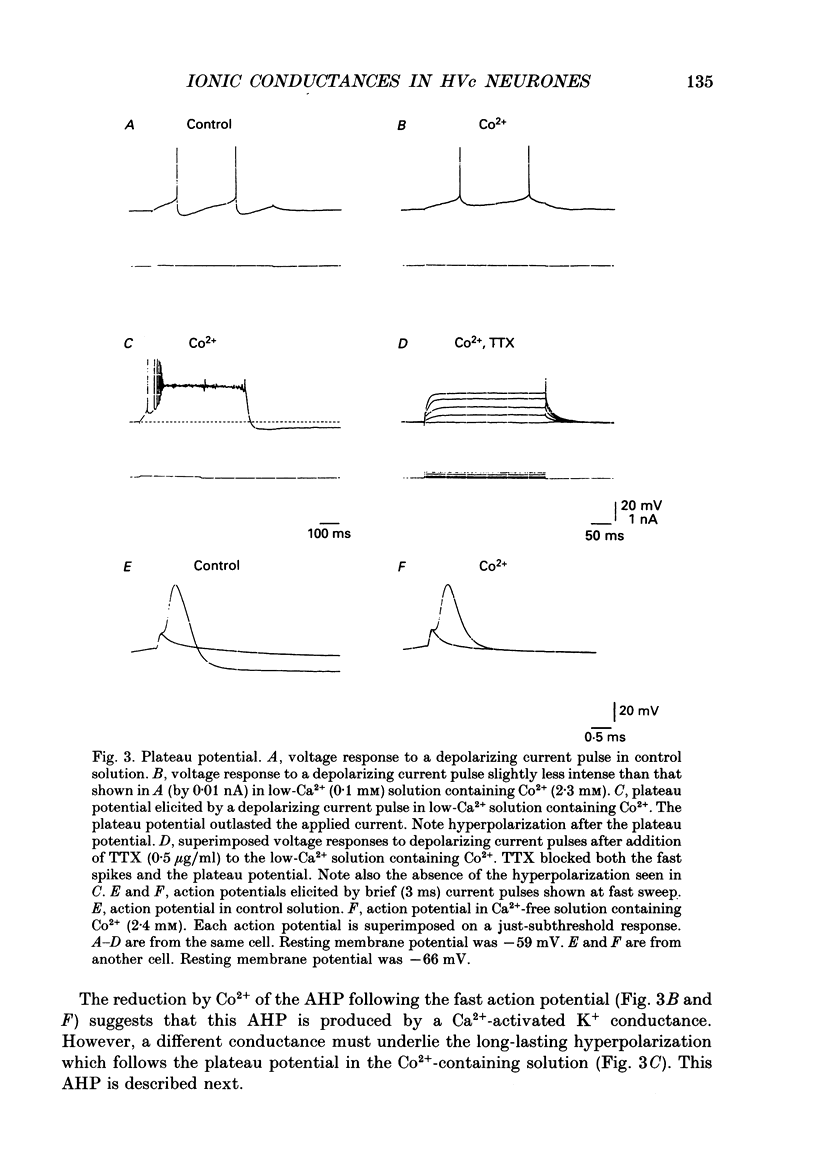
1. Intracellular recordings were made from zebra finch hyperstriatum ventrale pars caudale (HVc) neurones in in vitro slice preparations. 2. Small depolarizing current pulses elicited tonic firing while relatively large current pulses elicited an initial high-frequency burst followed by tonic firing. Time-dependent and fast-activated inward rectification were observed when a hyperpolarizing current pulse was applied. 3. Fast action potentials were abolished by tetrodotoxin (TTX, 0.5 micrograms/ml). When Co2+ (2.2-2.4 mM) was added to a low-Ca2+ (0-0.2 mM) solution, a plateau potential was elicited by a small depolarizing current pulse. These plateau potentials outlasted the applied current pulse and were abolished by TTX (0.5 micrograms/ml). 4. A long-lasting after-hyperpolarization (AHP), with a duration of tens of seconds, followed long trains of repetitive firing in control solution. Even in a low-Ca2+ (0-0.2 mM) solution containing Co2+ (2.2-2.4 mM) the long-lasting AHPs followed plateau potentials and were associated with an increase in input conductance. The long-lasting AHP, as well as the plateau potential, was blocked by TTX (0.5 micrograms/ml). 5. While TTX abolished fast action potentials, two types of active responses with different thresholds were elicited by depolarizing current in the presence of TTX (0.5 micrograms/ml), particularly when tetraethylammonium (5 mM) was also added to the solution. Both the low-threshold spike (LTS) and the high-threshold spike (HTS) were abolished by Co2+ (2.2-2.3 mM). 6. When Ba2+ (0.5-1 mM) was added to a solution containing TTX (0.5 micrograms/ml), an LTS elicited by a depolarizing current pulse became larger in amplitude. At membrane potentials more positive than -55 mV, an HTS, but not the LTS, was elicited. 7. These results suggest that HVc neurones have three Na(+)-dependent conductances: a fast Na+ conductance responsible for the fast spike, a persistent Na+ conductance responsible for the plateau potential, and a Na(+)-activated K+ conductance responsible for the long-lasting AHP; and that they have two Ca(2+)-dependent conductances: a low-threshold Ca2+ conductance responsible for the LTS, and a high-threshold Ca2+ conductance responsible for the HTS.
Full text
PDF

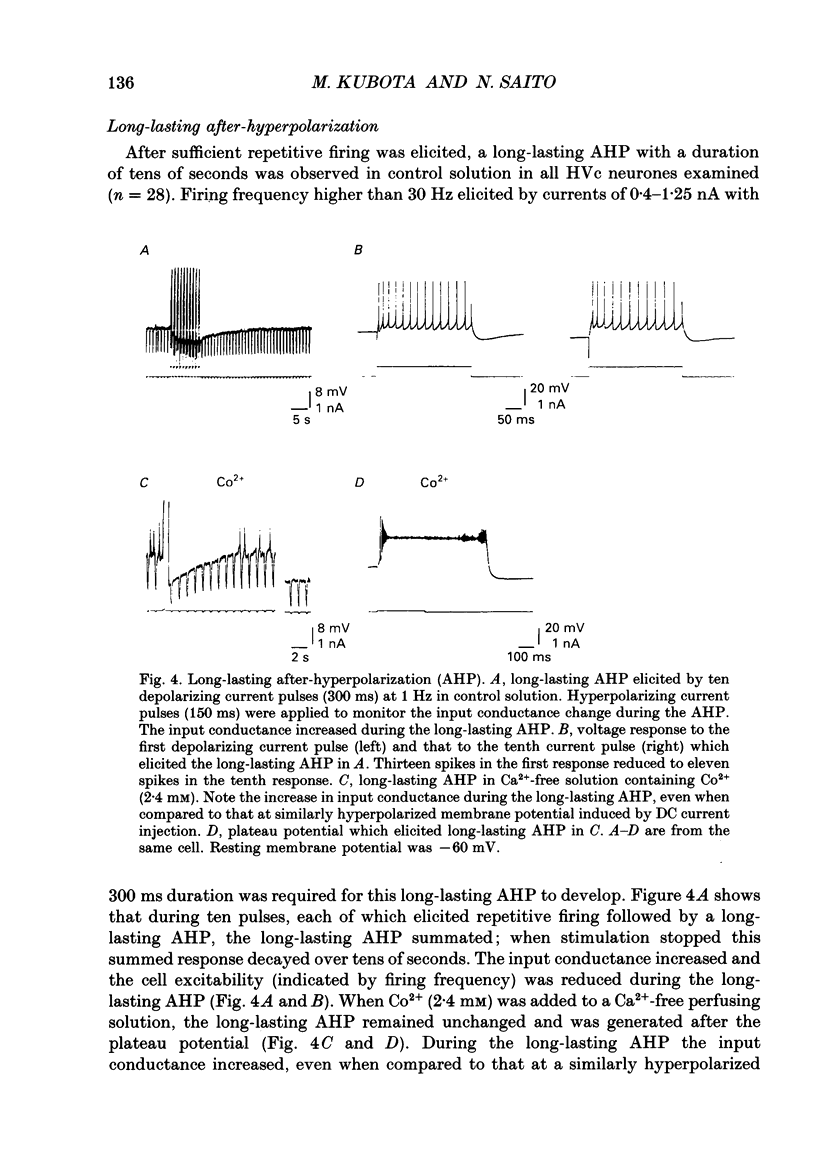
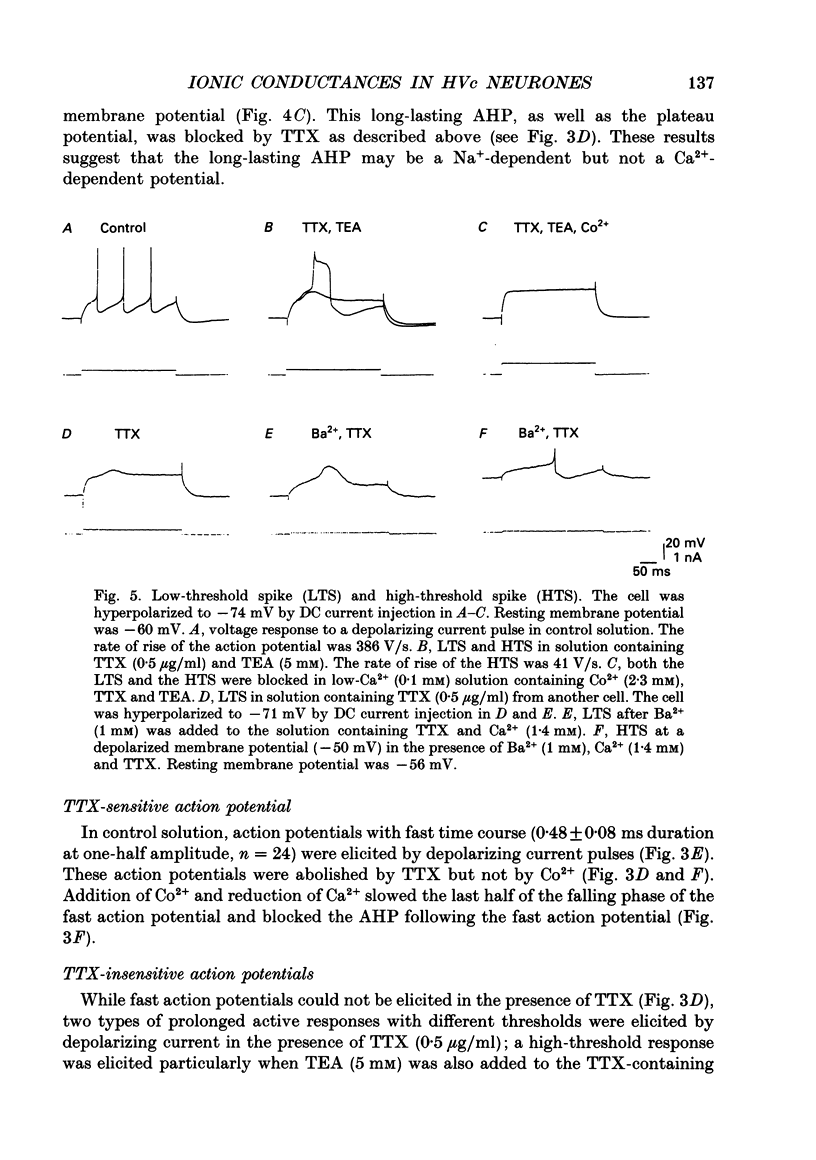




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Bader C. R., Bernheim L., Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985 Oct 10;317(6037):540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- Bottjer S. W., Halsema K. A., Brown S. A., Miesner E. A. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989 Jan 8;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Dryer S. E., Fujii J. T., Martin A. R. A Na+-activated K+ current in cultured brain stem neurones from chicks. J Physiol. 1989 Mar;410:283–296. doi: 10.1113/jphysiol.1989.sp017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber U., Greene R. W., McCarley R. W. Repetitive firing properties of medial pontine reticular formation neurones of the rat recorded in vitro. J Physiol. 1989 Mar;410:533–560. doi: 10.1113/jphysiol.1989.sp017548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hartung K. Potentiation of a transient outward current by Na+ influx in crayfish neurones. Pflugers Arch. 1985 May;404(1):41–44. doi: 10.1007/BF00581488. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Oertel D. Intrinsic properties of neurones in the dorsal cochlear nucleus of mice, in vitro. J Physiol. 1988 Feb;396:535–548. doi: 10.1113/jphysiol.1988.sp016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H. Extracellular activation and membrane conductances of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986 Mar;372:149–168. doi: 10.1113/jphysiol.1986.sp016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C., Gurney M. E. Auditory responses in the zebra finch's motor system for song. Brain Res. 1981 Sep 21;221(1):192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- Konishi M. Birdsong: from behavior to neuron. Annu Rev Neurosci. 1985;8:125–170. doi: 10.1146/annurev.ne.08.030185.001013. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland J. S. Neuronal control of bird song production. J Neurosci. 1987 Jan;7(1):23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf B. E., Davis S. S., DeVoogd T. J. Morphology of Golgi-impregnated neurons in hyperstriatum ventralis, pars caudalis in adult male and female canaries. J Comp Neurol. 1989 Jun 15;284(3):337–349. doi: 10.1002/cne.902840302. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Kelley D. B., Paton J. A. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982 Jun 1;207(4):344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980 Oct;44(4):827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Crill W. E. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol. 1989 Feb;61(2):233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Foehring R. C., Chubb M. C., Crill W. E. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol. 1988 Feb;59(2):450–467. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]