Abstract
1. Effects of adenosine 5'-triphosphate (ATP) on ionic currents of dispersed smooth muscle cells of the rabbit portal vein were investigated using the voltage-clamp procedure. 2. ATP (greater than or equal to 300 microM) produced transient and maintained inward currents. The former was inactivated within a few seconds, but the latter lasted more than several minutes. The transient but not the maintained current was blocked by pre-treatment with alpha,beta-methylene adenosine 5'-triphosphate (AMP-CPP). The amplitude of the latter was increased by ATP in a concentration-dependent manner. The following investigations were made on the ionic mechanism of the ATP-induced maintained inward current. 3. In 2.5 mM-Ca(2+)-containing tetraethylammonium chloride (TEA-Cl) solution (2.5 mM-Ca(2+)-TEA+ solution), the reversal potential for the ATP-induced inward current was close to the Cl- equilibrium potential, and in 140 mM-Na+ (nominally Ca(2+)-free or 0.3 mM-EGTA-containing) solution, the reversal potential was coincident with the Na+ equilibrium potential. 4. In 2.5 mM-Ca(2+)-TEA+ solution but not in 140 mM-Na+ solution and in physiological salt solution (PSS), niflumic acid (10 microM), a Cl- channel blocker, and Cl(-)-deficient perfusate in the pipette markedly inhibited the ATP-induced inward current. These results imply that in 2.5 mM-Ca(2+)-TEA+ solution the ATP-activated ion channel may admit Ca2+ which then accelerates the Ca(2+)-dependent Cl- current, but in 140 mM-Na+ solution and in PSS this channel may admit only Na+. 5. Intracellular perfusion of guanosine 5'-O-(3-thio triphosphate (GTP gamma S) did not provoke the current, but significantly increased the amplitude of the ATP-induced inward current in 2.5 mM-Ca(2+)-TEA+, 140 mM-Na+ and 2.5 mM-Ba(2+)-containing TEA+ (2.5 mM-Ba(2+)-TEA+) solutions. On the other hand, intracellular perfusion of guanosine 5'-O-(2-thiodiphosphate) (GDP beta S) reduced the amplitude of the ATP-induced inward current in the above solutions. 6. A low concentration of ATP (30 microM) transiently augmented the amplitude of the voltage-dependent Ca2+ current recorded in both 2.5 mM-Ca(2+)-TEA+ solution and PSS, but a high concentration of ATP (3 mM) consistently inhibited the voltage-dependent Ca2+ current in both solutions (4 mM-EGTA in the pipette). Such inhibition was partly prevented by application of 20 mM-EGTA in the pipette.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



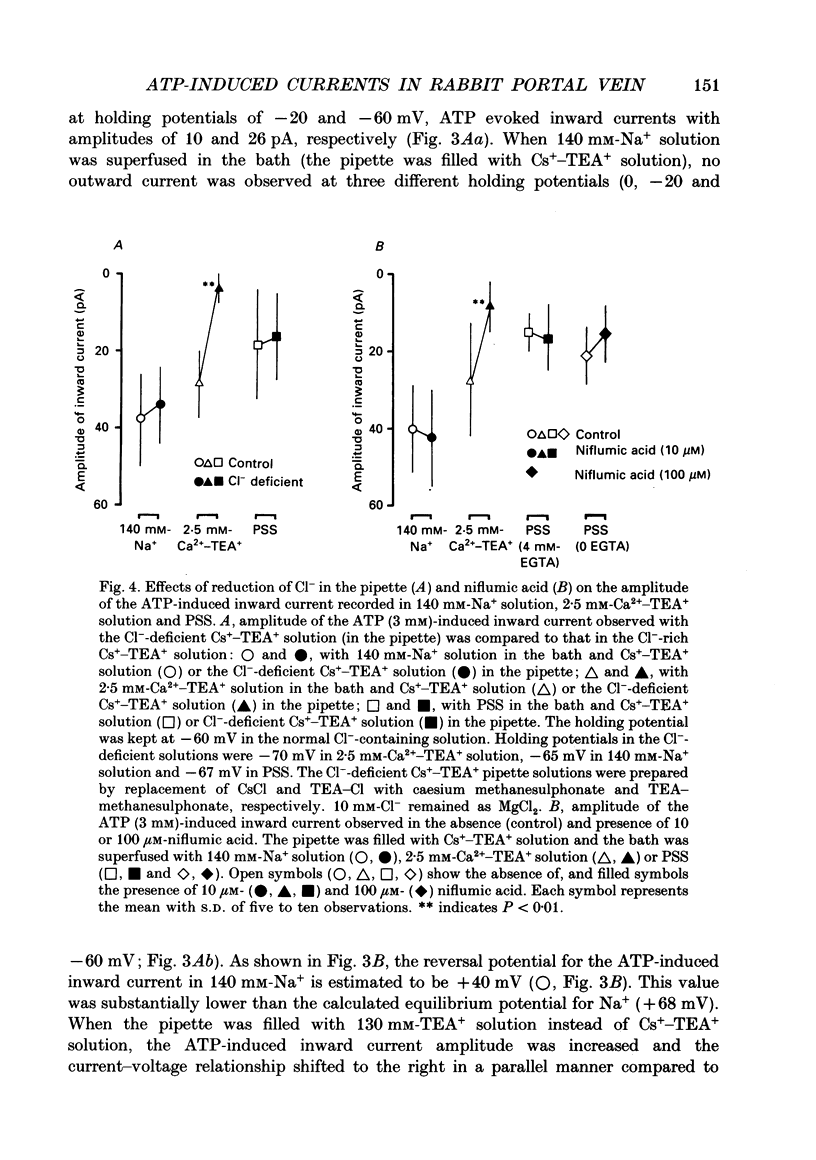

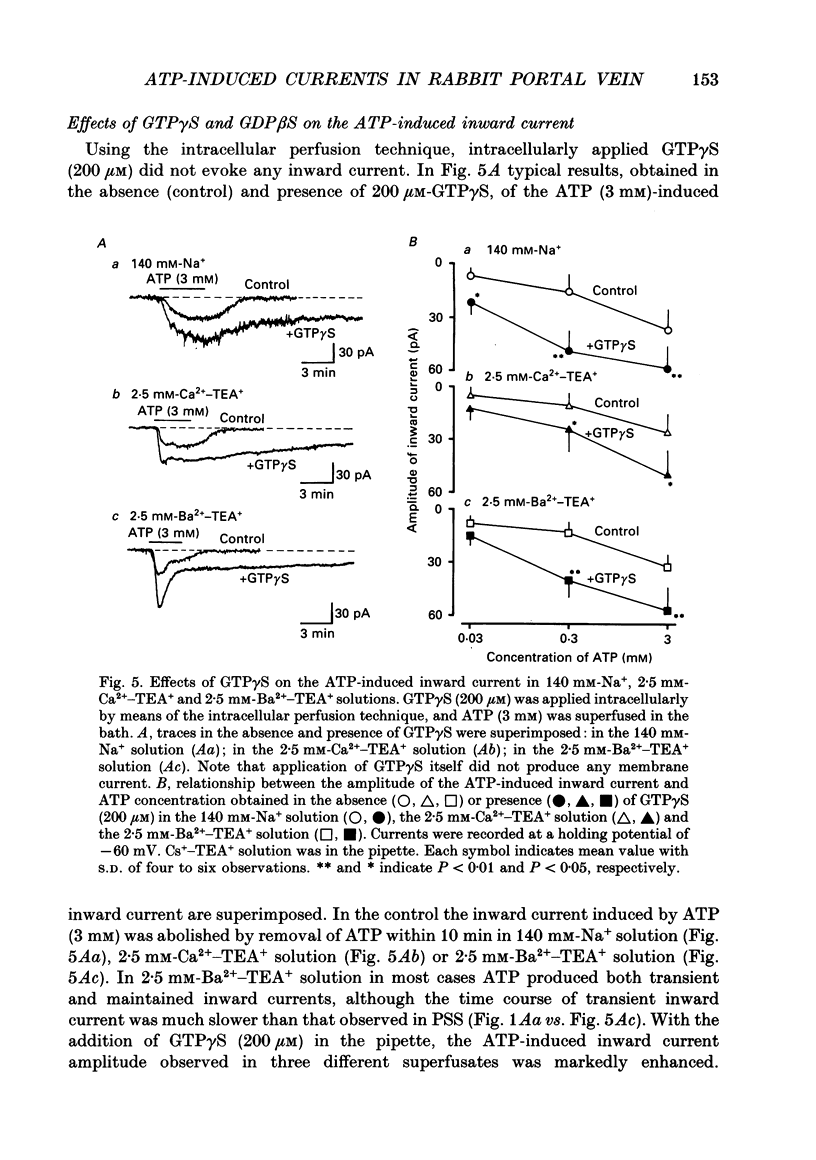

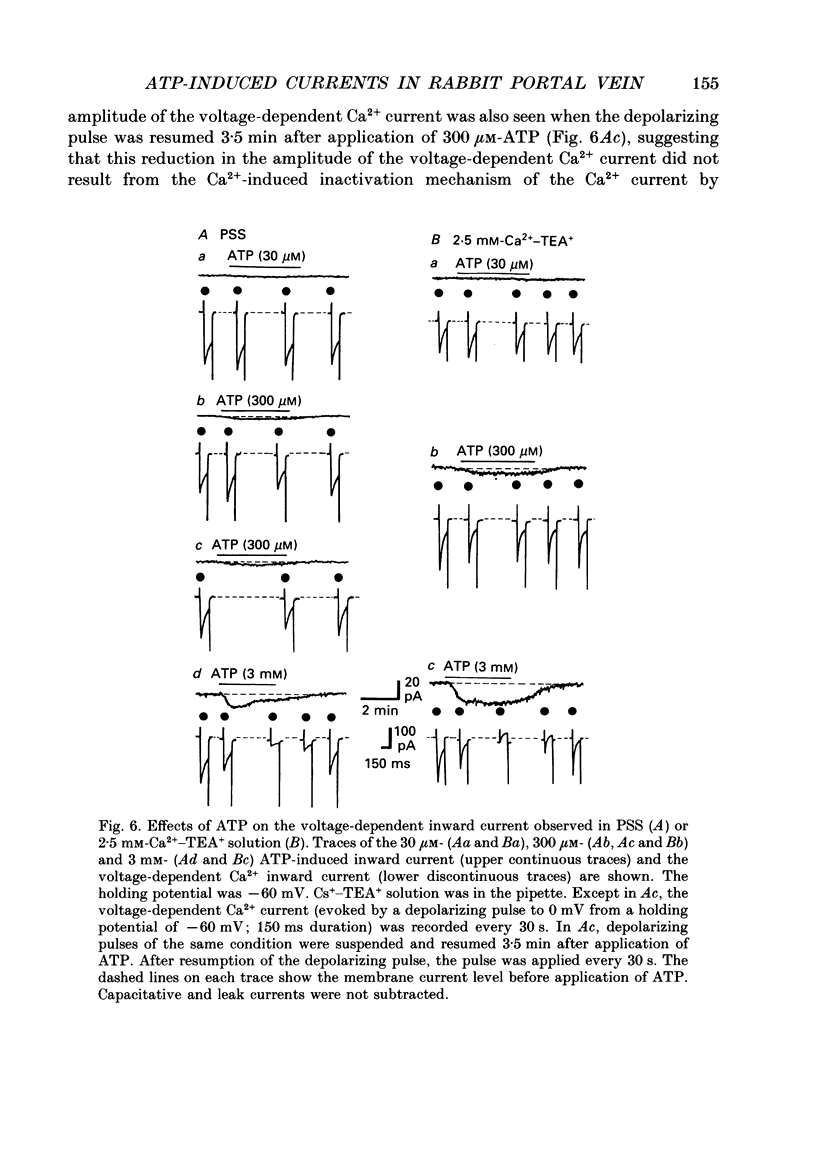
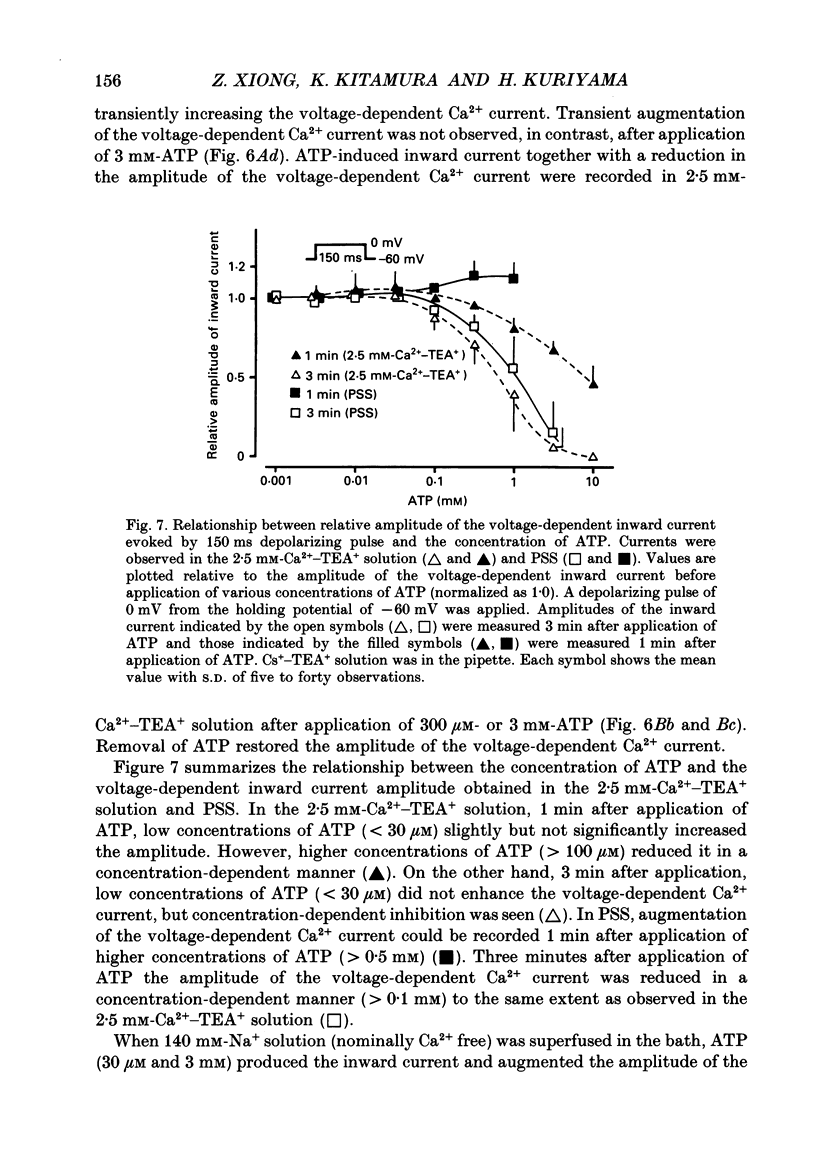






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989 Dec;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988 Oct;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Vivaudou M. B., Walsh J. V., Jr, Singer J. J. Acetylcholine increases voltage-activated Ca2+ current in freshly dissociated smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2092–2096. doi: 10.1073/pnas.84.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Martin C., Mironneau C., Mironneau J. An ATP-sensitive conductance in cultured smooth muscle cells from pregnant rat myometrium. Am J Physiol. 1989 Aug;257(2 Pt 1):C297–C305. doi: 10.1152/ajpcell.1989.257.2.C297. [DOI] [PubMed] [Google Scholar]
- Iino M., Kobayashi T., Endo M. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea-pig. Biochem Biophys Res Commun. 1988 Apr 15;152(1):417–422. doi: 10.1016/s0006-291x(88)80730-7. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N. Effects of divalent cations on acetylcholine-evoked membrane potential in the ionophore A23187 treated mouse pancreas. Pflugers Arch. 1984 Dec;402(4):465–472. doi: 10.1007/BF00583949. [DOI] [PubMed] [Google Scholar]
- Karashima T., Takata Y. The effects of ATP related compounds on the electrical activity of the rat portal vein. Gen Pharmacol. 1979;10(6):477–487. doi: 10.1016/0306-3623(79)90013-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Somlyo A. V., Somlyo A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988 Jun 16;153(2):625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. GTP-binding proteins mediate noradrenaline effects on calcium and chloride currents in rat portal vein myocytes. J Physiol. 1990 Sep;428:517–529. doi: 10.1113/jphysiol.1990.sp018225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1987 Aug;409(6):644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Modulation of ionic currents in smooth muscle balls of the rabbit intestine by intracellularly perfused ATP and cyclic AMP. Pflugers Arch. 1987 May;408(5):465–473. doi: 10.1007/BF00585070. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Noradrenaline activates a calcium-activated chloride conductance and increases the voltage-dependent calcium current in cultured single cells of rat portal vein. Br J Pharmacol. 1989 May;97(1):139–146. doi: 10.1111/j.1476-5381.1989.tb11934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sakai T., Terada K., Kitamura K., Kuriyama H. Ryanodine inhibits the Ca-dependent K current after depletion of Ca stored in smooth muscle cells of the rabbit ileal longitudinal muscle. Br J Pharmacol. 1988 Dec;95(4):1089–1100. doi: 10.1111/j.1476-5381.1988.tb11743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Vivaudou M. B., Clapp L. H., Walsh J. V., Jr, Singer J. J. Regulation of one type of Ca2+ current in smooth muscle cells by diacylglycerol and acetylcholine. FASEB J. 1988 Jun;2(9):2497–2504. doi: 10.1096/fasebj.2.9.2453389. [DOI] [PubMed] [Google Scholar]


