Abstract
The ascomycete Penicillium marneffei is an opportunistic human pathogen exhibiting a temperature-dependent dimorphic switch. At 25°C, P. marneffei grows as filamentous multinucleate hyphae and undergoes asexual development, producing uninucleate spores. At 37°C, it forms uninucleate yeast cells which divide by fission. We have cloned a gene encoding a Gα subunit of a heterotrimeric G protein from P. marneffei named gasA with high similarity to fadA in Aspergillus nidulans. Through the characterization of a ΔgasA strain and mutants carrying a dominant activating or a dominant interfering gasA allele, we show that GasA is a key regulator of asexual development but seems to play no role in the regulation of growth. A dominant activating gasA mutant whose mutation results in a G42-to-R change (gasAG42R) does not express brlA, the conidiation-specific regulatory gene, and is locked in vegetative growth, while a dominant interfering gasAG203R mutant shows inappropriate brlA expression and conidiation. Interestingly, the gasA mutants have no apparent defect in dimorphic switching or yeast-like growth at 37°C. Growth tests on dibutyryl cyclic AMP (dbcAMP) and theophylline suggest that a cAMP-protein kinase A cascade may be involved in the GasA signaling pathway.
Environmental signals leading to a variety of developmental processes and physiological responses are sensed and transduced to the cell interior through complex interactions at the cell membrane. Heterotrimeric guanine nucleotide-binding proteins (G proteins) act as signal transducers that couple cell surface receptors to cytoplasmic effector proteins in eukaryotes. Upon binding of the agonist with the corresponding serpentine transmembrane receptor, the Gα subunit becomes activated, exchanging GDP for GTP and dissociating from the βγ complex. Gα and Gβγ subunits can then interact with appropriate targets such as phosphodiesterases, protein kinases, adenylyl cyclases, phospholipases, and ion channels to trigger downstream signaling pathways (20, 27, 37, 44).
In fungi, G proteins affect a number of developmental and morphogenic processes and play essential roles during pathogenic and sexual development programs (4). The best-characterized fungal Gα proteins are Saccharomyces cerevisiae Gpa1 and Gpa2. Gpa1 is linked to cell-type-specific pheromone receptors encoded by the STE2 and STE3 genes and plays a negative role in the mitogen-activated protein kinase pheromone signaling pathway that leads to mating (32). Gpa2 is required for the induction of pseudohyphal growth in diploid yeast cells under nitrogen starvation and signals through a cyclic AMP (cAMP)-dependent protein kinase A (PKA) pathway (30, 35). Interestingly, Gpa2 is coupled to a G-protein-coupled receptor, Gpr1, which appears to be a glucose sensor (28, 33, 36). Gα subunits from other fungi with high homology to Gpa2 have also been implicated in morphogenesis through cAMP-PKA signaling. The corn smut fungus Ustilago maydis displays a dimorphic switch between a haploid yeast-like form and a pathogenic filamentous dikaryon. Haploid strains with the Gα subunit-encoding gpa3 gene deleted show a filamentous phenotype and are sterile; these phenotypes can be rescued by exogenous cAMP (19, 29, 38).
The gna1 and gna2 genes of Neurospora crassa were the first filamentous-fungal Gα-encoding genes identified. Based on sequence comparison and the ability to serve as a substrate for pertussis toxin, GNA1 belongs to the mammalian Gαi superfamily, whose members regulate various effectors, such as adenylyl cyclase, ion channels, and cyclic-GMP phosphodiesterase (27, 37, 45). GNA1 signaling mediates several cellular processes, including apical growth and the development of aerial hyphae. In addition, Δgna1 strains have lower intracellular cAMP levels than the wild type (24, 46). Analysis of other fungal Gαi proteins has further demonstrated the importance of this group of Gα proteins in development and morphogenesis. Disruption and site-directed mutagenesis of magB, a gene encoding a Gαi subunit in the rice blast fungus Magnaporthe grisea, affect vegetative growth, conidiation, and appressorium formation. Furthermore, the addition of cAMP induces appressorium formation in magB loss-of-function mutants (15, 34). The CPG1 protein of the chestnut blight fungus Cryphonectria parasitica shows high homology to the N. crassa GNA1 protein. Disruption of cpg1 greatly reduces virulence, growth, and conidiation and leads to elevated cAMP levels, suggesting that CPG1 functions as a negative regulator of adenylyl cyclase (10, 16).
In Aspergillus nidulans, a Gαi protein and a regulator of G-protein signaling domain protein, encoded by fadA and flbA, respectively, regulate the decision between growth and asexual development as well as the production of secondary metabolites such as the polyketide sterigmatocystin (21, 48). Asexual development involves the elaboration of a conidiophore through the differentiation of a number of specialized cell types (7, 11). BrlA and AbaA comprise the key transcriptional regulators controlling this process, and the induction of BrlA is sufficient to drive rudimentary conidiation (1, 2). AflR, on the other hand, has been shown to be a sterigmatocystin biosynthesis pathway-specific transcription factor (47). Both brlA and aflR require FlbA and FadA for their normal expression. The signal transduction pathway connecting FadA with its downstream effectors BrlA and AflR has recently been shown to be partially mediated by PkaA, the catalytic subunit of a PKA (43).
The ascomycete Penicillium marneffei is an opportunistic human pathogen endemic in Asia that exhibits a temperature-dependent dimorphic switch (12, 17, 23). At 25°C, multinucleate, septate hyphae are produced by apical growth and lateral branching. Exposure of hyphae to an air interface induces asexual development at this temperature to produce asexual spores on conidiophores similar to those of A. nidulans. It has been shown that both a brlA and an abaA homologue are required for asexual development in P. marneffei (A. R. Borneman, M. J. Hynes, and A. Andrianopoulos, unpublished data, and reference 5). At 37°C, P. marneffei hyphae undergo a process known as arthroconidiation, in which septation and nuclear division become coupled. The hyphae lay down double septa, which consequently allow cell separation to produce single uninucleate yeast cells, which divide by fission (8). These two developmental programs in P. marneffei have been shown to share elements controlling morphogenesis. At 25°C, deletion of the abaA gene leads to defects in conidial development and a failure to produce conidia. At 37°C, deletion of the abaA gene results in uncoupling of the nuclear and cell division cycles such that the yeast cells contain more than one nucleus and have aberrant morphology (5). Therefore, AbaA plays a role in both of these distinct developmental programs, and this suggests that upstream regulators of the conidiation pathway may also be important regulators of dimorphic switching.
This work describes the cloning of the P. marneffei gasA gene encoding a Gα subunit that is highly similar to A. nidulans FadA. We have investigated the role of GasA in the hyphal growth, asexual development, and yeast-like growth of P. marneffei. Through the characterization of a ΔgasA strain and strains carrying a dominant activating or a dominant interfering mutation, we show that GasA plays a key role in the regulation of asexual development. In contrast, gasA mutants have no apparent defect in dimorphic switching or yeast-like growth. We have also investigated possible signal transduction pathways connecting GasA and its downstream effectors BrlA and AbaA and show that in P. marneffei, a cAMP-PKA pathway may be involved in the GasA signaling pathway.
MATERIALS AND METHODS
Fungal strains, media, and growth conditions.
The P. marneffei wild-type strain FRR2161 was obtained from J. Pitt (CSIRO Food Industries, Sydney, Australia). Transformation of the SPM4 strain, which is unable to grow on nitrate as a sole nitrogen source and is a uracil auxotroph, was performed by the previously described protoplast method (6). The gasAG42R (resulting in a G42-to-R change) and gasAG203R strains (TS-24-5-4 and TS28-4-8, respectively) were generated by transformation of strain SPM4 with pSZ5030 and pSZ5089, respectively, and selection for pyrG+; TS-24-5-4 contains three copies of pSZ5030 and TS28-4-8 contains five copies of pSZ5089. In the same way, pSZ5029, which contains the gasA wild-type allele, was used to create gasA+ strains containing from one to six copies of pSZ5029. The ΔgasA strain (TS27-3-29) was generated by transformation of strain SPM4 with 500 ng of a gel-purified XhoI/SphI fragment from pSZ5031 and selection for pyrG+.
P. marneffei strains were grown on Aspergillus nitrogen-free medium (ANM) supplemented with 10 mM gamma-aminobutyric acid (GABA) at 25°C and on S. cerevisiae synthetic-dextrose (SD) medium at 37°C (3, 13). These media were supplemented with 0.1 mM dibutyryl cAMP (dbcAMP; Sigma) and 10 mM theophylline, as required. Where appropriate, the strains were tested on media containing 10 mM uracil to confirm that the observed phenotypes were unrelated to poor expression of the pyrG selectable marker. For microscopic analysis, P. marneffei strains were grown at 25°C for 3 days on SD medium containing 0.1% glucose to induce conidiation and at 37°C for 4 days on brain heart infusion (BHI) broth (Oxoid) to favor the formation of single yeast cells.
Due to the lack of conidiation in the gasAG42R strain, yeast cells grown for 4 days on SD plates at 37°C were used as an inoculum for all strains. Vegetative hyphal samples were grown in SD medium with shaking for 2 or 3 days at 25°C as stated for each individual experiment. Asexually developing cultures were prepared by growing the 2-day vegetative cultures on ANM-GABA plates containing 0.1% glucose for an additional 4 days to allow conidiation. Yeast cultures were grown in BHI liquid medium for 6 days as previously described (6).
Molecular techniques.
Plasmid DNA was isolated by using a high-purity plasmid kit (Roche Diagnostics). Genomic DNA from P. marneffei was isolated as previously described (6). RNA was prepared by using a FastRNA kit (Bio 101, Inc.) as previously described (6). Southern and Northern blotting were performed with Amersham Hybond N+ membranes according to the manufacturer's instructions. Filters were hybridized with [α-32P]dATP-labeled probes by standard methods (40). Northern blots were probed with a histone H3 gene probe as a loading control (14).
Degenerate PCR was performed on genomic DNA of strain FRR2161 with the sense primer GAGES (5′CGGGATCCGGNGCNGGNGARWSNGGNAA3′) and the antisense primer GαPCR3 (5′GAGGTCACGTTCTCGAAGCARTRDAWCCA3′), which correspond to highly conserved domains in G-protein α subunits (27). PCR conditions consisted of an initial denaturation step of 94°C for 2 min, after which Taq DNA polymerase was added and DNA was amplified for 35 cycles in a Mastercycler gradient thermocycler (Eppendorf). The cycling parameters were as follows: 30 s at 94°C, 30 s at 45°C, and 1 min at 72°C, with a final extension of 10 min at 72°C. The products were cloned into pGEMTeasy (Promega). A 600-bp insert with high homology to A. nidulans fadA was used to probe a 5- to 6-kb BglII/SalI size-selected FRR2161 genomic library in pBluescript II SK(+) (Stratagene). One positive clone (pSZ4809) was chosen for subcloning and sequencing. Sequencing was performed by the Australian Genome Research Facility and analyzed with Sequencher 3.1.1 (Genes Codes Corporation). Database searches and sequence comparisons were performed via the Australian National Genomic Information Service. The phylogenetic tree was generated by using the Dayhoff PAM matrix and the neighbor-joining method in the Phylogeny Inference package (version 3.57c; University of Washington, Seattle [http://evolution.genetics.washington.edu/phylip.html]). U. maydis Gpa4 was used as an outgroup (4).
A GeneEditor in vitro site-directed mutagenesis kit (Promega) was used to create the dominant activating gasAG42R mutation. Mutagenesis was performed on an XhoI/SacII pBluescript II SK(+) subclone (pSZ4904) with the mutagenic primer gasA.dom (5′CTCTAGGTGCTCGCGAATCTGGAAA3′; base mismatches are underlined) to generate pSZ4967. This product was sequenced and used to recreate a full-length gasA clone in pALX223 which contained the pyrG gene for direct selection in the P. marneffei strain SPM4 (A. Andrianopoulos, unpublished data), generating pSZ5030. The dominant interfering gasAG203R mutation was generated by inverse PCR on an AgeI subclone (pSZ4921) with the mutagenic primers gasA.domneg.up (5′CAACGATCCGAACGTAAGAAGTGG3′) and gasA.domneg.lo (5′TCGACCAACGTCAAACATGCGGTAG3′) to yield pSZ5088. This fragment was sequenced and used to recreate a full-length gasA clone in pALX223, generating pSZ5089. Additionally, the full-length gasA wild-type allele was cloned into pALX223, generating pSZ5029. The dominant activating fadAG42R and dominant interfering fadAG203R alleles, kindly provided by T. H. Adams (48), were subcloned into pALX223 to generate pSZ5027 and pSZ5028, respectively. To disrupt gasA, a 2.2-kb EcoRV/SacII fragment containing the pyrG cassette of pAB4626 (Borneman et al., unpublished) was cloned between the internal SacII site and the HindIII end-filled site of pSZ4809, generating plasmid pSZ5031.
Microscopy.
P. marneffei strains were grown on slides covered with thin layers of solid medium resting in liquid medium (5). The slides were mounted with the addition of 500 μg of 4′,6′-diamidino-2-phenylindole (DAPI)/μl and viewed by using either differential interference contrast or epifluorescence optics on a Reichart Jung Polyvar II microscope. Images were captured digitally with a SPOT charge-coupled-device camera (Diagnostic Instruments) and processed with Adobe Photoshop software.
Nucleotide sequence accession number.
The GenBank accession number of the P. marneffei gasA gene is AF448796.
RESULTS
P. marneffei gasA and A. nidulans fadA genes are highly conserved.
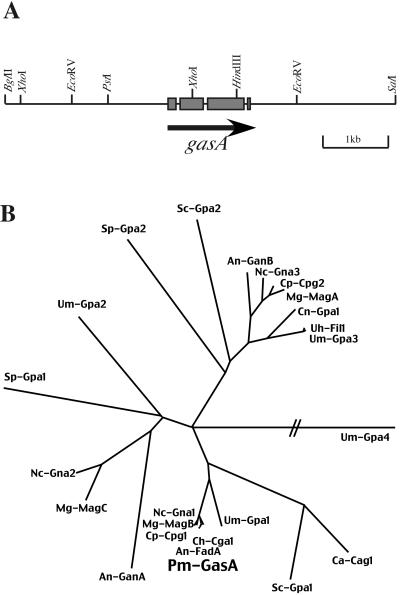
To isolate genes encoding Gα subunits from P. marneffei, we used a degenerate-primer PCR-based approach. Sequence analysis of one class of PCR products showed significant homology to fadA of A. nidulans. Southern blot analysis with the amplification product as a probe indicated a gene present as a single copy in P. marneffei (data not shown). This gene was designated gasA (Gα subunit), and the amplification product was used to screen a partial genomic DNA library. A 5.5-kb BglII/SalI fragment was isolated, and a 3.5-kb EcoRV subclone was sequenced (Fig. 1A). The gene structure was predicted by comparison with the genomic sequence of the A. nidulans fadA gene, and the gene contained three introns which had conserved positions in the two species. The gasA gene is therefore predicted to encode a 353-amino-acid (aa) protein with 96% identity with the Gα subunit FadA of A. nidulans and 93% identity with Cochliobolus heterostrophus CGA1 and C. parasitica CPG1. Like FadA, CGA1, and CPG1, GasA shows the characteristic Gα subdomains P (aa 27 to 50), G′ (aa 200 to 217), and G (aa 261 to 273) as well as the presence of both a potential myristoylation site (MGXXXS) at the N terminus and the consensus site CXXX for ADP ribosylation by pertussis toxin (27, 44). These features have been taken as evidence that members of this group are homologous to the mammalian Gαi subunits and show that gasA encodes an α subunit of a heterotrimeric G protein. Phylogenetic analysis of GasA and other fungal Gα proteins revealed three major subgroups within the family of Gα proteins, with GasA in the same group as FadA (Fig. 1B).
FIG. 1.
The gasA gene of P. marneffei encodes a Gα subunit of a heterotrimeric G protein. (A) Gene structure and partial restriction map of the gasA gene corresponding to pSZ4809. The region predicted to encode the GasA protein is indicated by gray boxes representing the exons, interrupted by three introns. The solid black arrow shows the direction of transcription. (B) Relationship of fungal Gα subunits. The tree is based on amino acid sequence alignments and was constructed as described in Materials and Methods. Abbreviations of species and GenBank gene accession numbers are as follows: An-FadA, A. nidulans FadA (Q00743); An-GanA, A. nidulans GanA (AF142058); An-GanB, A. nidulans GanB (AF198116); Ca-Cag1, Candida albicans Cag1 (M88113); Ch-Cga1, Cochliobolus heterostrophus CGA1 (AF070446); Cn-Gpa1, Cryptococcus neoformans Gpa1 (AAD46575); Cp-Cpg1, C. parasitica CPG1 (L32176); Cp-Cpg2, C. parasitica CPG2 (L32177); Mg-MagA, M. grisea MagA (AF011340); Mg-MagB, M. grisea MagB (AF011341); Mg-MagC, M. grisea MagC (AF011342); Nc-Gna1, N. crassa GNA1 (L11453); Nc-Gna2, N. crassa GNA2 (L114532); Nc-Gna3, N. crassa GNA3 (AF281862); Pm-GasA, P. marneffei GasA (AF448796); Sc-Gpa1, S. cerevisiae Gpa1 (AAA34650); Sc-Gpa2, S. cerevisiae Gpa2 (AAA34651); Sp-Gpa1, Schizosaccharomyces pombe Gpa1 (M64268); Sp-Gpa2, S. pombe Gpa2 (D13366); Uh-Fil1, Ustilago hordei Fil1 (U76672); Um-Gpa1, U. maydis Gpa1 (U85775); Um-Gpa2, U. maydis Gpa2 (U857756); Um-Gpa3, U. maydis Gpa3 (U85777); Um-Gpa4, U. maydis Gpa4 (U85778n). Hatched lines indicate nonproportional length.
gasA is expressed differentially during different growth stages.
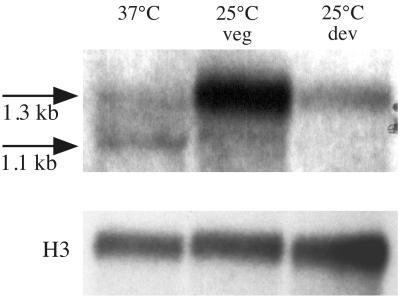
RNA from the wild-type strain was isolated from vegetative hyphal cells grown in liquid medium for 2 days at 25°C, from asexually developing (conidiating) cultures at 25°C, and from yeast cells at 37°C (see Materials and Methods). Northern blot analysis showed a single highly expressed gasA transcript of 1.3 kb during vegetative growth which was strongly downregulated during asexual development. In the yeast phase, two transcripts, of 1.1 and 1.3 kb, were detected (Fig. 2).
FIG. 2.
Northern blot analysis of gasA expression. Total RNA from the P. marneffei wild-type strain (FRR2161) was isolated from yeast cultures (37°C), vegetative mycelia grown in liquid at 25°C for 2 days (25°C veg), and asexually developing cultures (25°C dev), as indicated in Material and Methods. RNA from each of the three growth stages (37°C, 25°C veg, and 25°C dev) was hybridized with probes specific for either gasA or histone H3 (H3), which was used as a loading control. Arrows indicate the two gasA transcripts of 1.1 and 1.3 kb.
GasA-mediated signaling negatively affects asexual development.
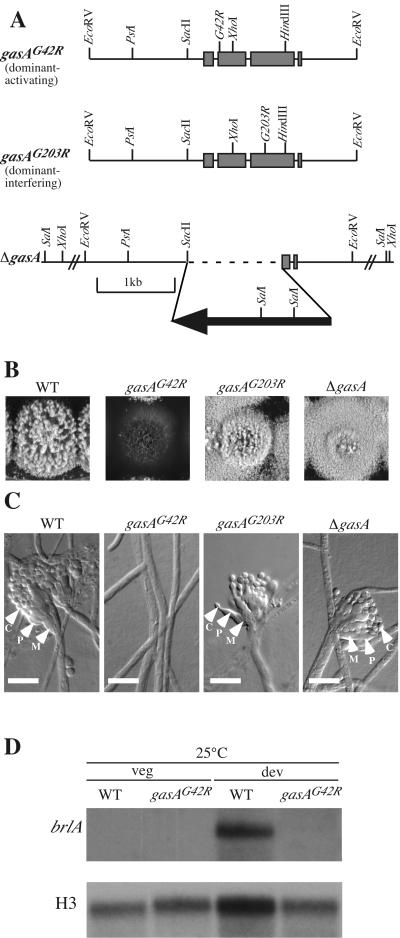
To examine the function of GasA, a number of mutant gasA alleles were generated. A dominant activated (gasAG42R) mutant allele was produced by site-directed mutagenesis to convert glycine 42 to arginine, as this particular glycine is required for GTPase activity and is therefore predicted to result in a GTP-bound Gα constitutively activating signaling (27, 31). The plasmid containing gasAG42R (pSZ5030) was transformed into SPM4, and transformants were isolated by direct selection for pyrG+ (Fig. 3A). The presence and the copy number of the plasmid were determined by Southern blot analysis of genomic DNA (data not shown). Four representative transformants of gasAG42R, ranging in copy number from 1 to 20, were analyzed further. Dominant activated gasAG42R transformants displayed thick aerial hyphae and failed to differentiate conidiophore structures and therefore completely lacked conidia (Fig. 3B and C). No effect was observed in transformants containing multiple copies of the gasA wild-type allele (pSZ5029). This suggests that GasA-mediated signaling negatively affects asexual development. Additionally, the transformants showed an increased production of a red pigment which is characteristic of the vegetative filamentous form of P. marneffei (42; data not shown). We also transformed SPM4 with the plasmid containing the A. nidulans fadAG42R allele (pSZ5027), isolating transformants by direct selection for pyrG+. These transformants displayed the same phenotype as that of transformants carrying the gasAG42R allele, suggesting functional interchangeability between GasA and FadA.
FIG. 3.
Analysis of the gasAG42R, gasAG203R, and ΔgasA strains at 25°C. (A) Partial restriction map of the dominant activated gasAG42R (pSZ5030) and dominant interfering gasAG203R (pSZ5089) alleles used to generate the respective eponymous strains. The gasA locus in the ΔgasA strain is shown after insertion of the gasA deletion construct (pSZ5031). Hatched lines indicate nonproportional length. (B) Colonial morphologies of the P. marneffei wild-type (FRR2161), dominant activated gasAG42R, dominant interfering gasAG203R, and ΔgasA strains. The strains were grown on ANM-GABA for 14 days at 25°C. All strains conidiated normally except for the gasAG42R mutant, which is aconidial and exhibited flat colony morphology with thickaerial hyphae. (C) Microscopic examination of the P. marneffei wild-type (FRR2161), dominant activated gasAG42R, dominant interfering gasAG203R, and ΔgasA strains. The strains were grown on SD containing 0.1% glucose for 3 days at 25°C. All strains exhibited wild-type conidiophores except for the gasAG42R mutant, which showed no signs of asexual development. Scale bars, 20 μm. Arrowheads highlight the conidiophore structures (M, metulae; P, phialides; C, conidia). (D) Northern blot analysis of the P. marneffei wild-type strain (FRR2161) and the dominant activating gasAG42R mutant. Total RNA was isolated from vegetative mycelia grown in liquid at 25°C for 2 days (veg) and from asexually developing cultures (dev). Northern blots were probed with either brlA or histone H3 (H3). WT, wild type.
The brlA gene of P. marneffei is necessary for asexual development, as is brlA of A. nidulans (Borneman et al., unpublished, and reference 1). We examined the expression of brlA in the dominant activated gasAG42R strain, which fails to conidiate. A brlA transcript was not detected in RNA from vegetative hyphal cells of either the wild type or the gasAG42R mutant grown at 25°C (Fig. 3D). In developmental cultures, the brlA transcript was strongly expressed in the wild type but not in the gasAG42R transformant (Fig. 3D).
A dominant interfering gasAG203R mutation results in inappropriate asexual development.
To further examine the role of GasA in the regulation of conidiation, a dominant interfering (gasAG203R) mutant allele was generated. For this purpose, we converted glycine 203 to arginine by site-directed mutagenesis, which is predicted to prevent the conformational switch that accompanies GTP binding and is necessary for Gβγ release, thereby maintaining the association of the Gαβγ complex and leading to a dominant inactivation of signaling (27, 31). The plasmid containing gasAG203R (pSZ5089) was transformed into SPM4, and transformants were isolated by direct selection for pyrG+ (Fig. 3A). The presence and the copy number of the plasmid were determined by Southern blot analysis of genomic DNA (data not shown). Four representative gasAG203R transformants, ranging in copy number from 3 to 10, were analyzed further. In addition, a construct in which part of the gasA coding sequence was replaced with the A. nidulans pyrG gene was used to transform SPM4 and inactivate the gasA gene by homologous gene replacement (Fig. 3A). In 5 transformants out of 65 screened by Southern blotting, the endogenous copy was deleted (data not shown).
At 25°C, the dominant interfering gasAG203R transformants and the ΔgasA strain showed a colonial phenotype similar to that of the wild type. The radial growth rate was the same as that of the wild type; however, the gasAG203R and ΔgasA strains were slower in producing aerial hyphae, leading to a slight delay in conidiation (Fig. 3B and C). Additionally, both mutants showed reductions in the production of the red pigment (42; data not shown).
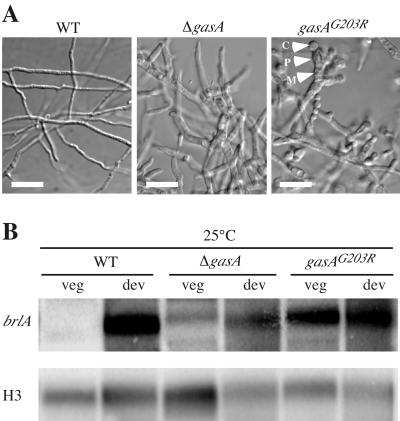
If GasA negatively regulates asexual development, a gasAG203R mutant would be expected to enter asexual development under conditions where the wild type does not. To test this hypothesis, we examined growth in liquid culture, where the wild type exhibits no conidiation. Unlike the wild type, the dominant interfering gasAG203R mutant produced a large number of relatively normal conidiophore structures after 3 days at 25°C (Fig. 4A). The ΔgasA strain failed to produce conidiophores; however, the hyphal cells were larger in diameter than those of the wild type, resembling those observed in the dominant interfering gasAG203R mutant (Fig. 4A). We also transformed SPM4 with the plasmid containing the A. nidulans fadAG203R allele (pSZ5028), isolating transformants by direct selection for pyrG+. In liquid culture, these transformants displayed the same phenotype as that of the transformants carrying the gasAG203R allele, providing further evidence for the functional interchangeability of GasA and FadA (data not shown). To support the microscopic observations, RNAs from the wild-type, gasAG203R, and ΔgasA strains were isolated from vegetative hyphal cells grown in liquid culture for 3 days at 25°C. No brlA mRNA was detected in the wild type. As expected, a brlA transcript was readily detectable in the gasAG203R mutant. In the ΔgasA strain, a brlA transcript was detected but was clearly less abundant than in the gasAG203R mutant (Fig. 4B).
FIG. 4.
Analysis of the gasAG203R and ΔgasA strains in liquid cultures at 25°C. (A) Microscopic examination of the P. marneffei wild-type (FRR2161), dominant interfering gasAG203R, and ΔgasA strains. The strains were grown in liquid at 25°C for 3 days. Scale bars, 20 μm. Arrowheads highlight the conidiophore structures (M, metulae; P, phialides; C, conidia). (B) Northern blot analysis of the P. marneffei wild-type (FRR2161), dominant interfering gasAG203R, and ΔgasA strains. Total RNA was isolated from vegetative mycelia grown in liquid at 25°C for 3 days (veg) and from asexually developing cultures (dev). Northern blots were probed with either brlA or histone H3 (H3). WT, wild type.
GasA is not required for yeast-like growth at 37°C.
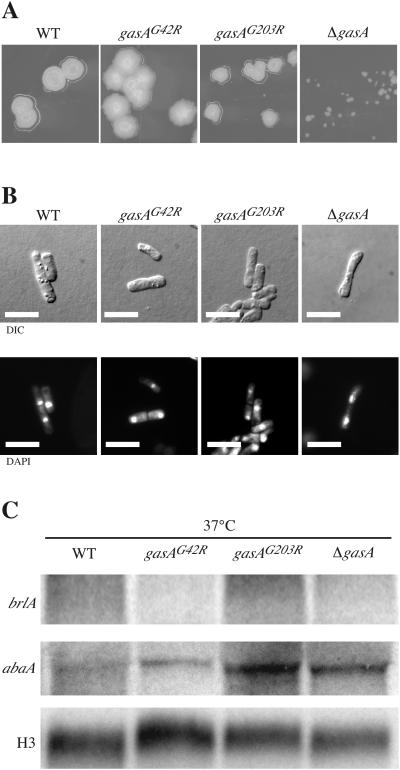
In order to investigate if GasA plays a role in hyphal-yeast morphogenetic transition as well as the maintenance of yeast-like growth at 37°C, we first compared the colony morphology of the wild type with those of the dominant activated gasAG42R, dominant interfering gasAG203R, and ΔgasA strains. All of the strains formed normal yeast-like colonies; however, the ΔgasA strain produced colonies which were smaller in diameter. Upon examination of four additional ΔgasA strains, it was determined that the pyrG selectable marker which replaced the gasA coding region in these deletion strains was being poorly expressed, resulting in reduced growth. Therefore, it can be concluded that gasA does not appear to play a direct role in yeast-like growth.
In the wild type at 37°C, hyphal compartments became uninucleate, as nuclear and cell division became coupled and the cells of these filaments separated to form arthroconidial yeast cells that propagated by fission. This developmental pathway was not affected in any of the gasA mutants, which were capable of switching to yeast-like growth and producing morphologically wild-type yeast cells (Fig. 5B).
FIG. 5.
Analysis of the gasAG42R, gasAG203R, and ΔgasA strains at 37°C. (A) Colonial morphologies of the P. marneffei wild-type (FRR2161), dominant activated gasAG42R, dominant interfering gasAG203R, and ΔgasA strains. The strains were grown on SD medium at 37°C for 4 days. All strains exhibited normal yeast-like colonies except for the ΔgasA strain, which showed smaller colonies. This phenotype was found not to result from the inactivation of gasA (see the text). (B) Microscopic examination of the P. marneffei wild-type (FRR2161), dominant activated gasAG42R, dominant interfering gasAG203R, and ΔgasA strains. The strains were grown on BHI broth at 37°C for 4 days. All strains exhibited wild-type yeast cells. Scale bars, 20 μm. Differential interference contrast (DIC) and DAPI-stained epifluorescence of nuclei (DAPI) are shown. (C) Northern blot analysis of the P. marneffei wild-type (FRR2161), dominant activating gasAG42R, dominant interfering gasAG203R, and ΔgasA strains. Total RNA was isolated from yeast cultures grown in liquid at 37°C for 6 days. Northern blots were probed with either brlA, abaA, or histone H3 (H3). WT, wild type.
Expression of brlA and abaA in the gasA mutants at 37°C.
P. marneffei does not express brlA during yeast-like growth at 37°C (Borneman et al., unpublished). The abaA gene, however, is expressed at 37°C, and deletion of abaA results in the misregulation of arthroconidiation such that the transitional hyphal cells and the yeast cells contain more than one nucleus and have aberrant morphology (5). As described above, the dominant interfering gasAG203R mutant shows inappropriate brlA expression at 25°C in liquid culture. It was therefore of interest to determine whether any of the gasA mutants, especially the gasAG203R mutant, showed altered brlA and abaA expression at 37°C. As for the wild type, the brlA transcript was not detected in any of the gasA mutants. The abaA transcript was detected in all the strains (Fig. 5C).
GasA in P. marneffei may signal through a cAMP-PKA cascade.
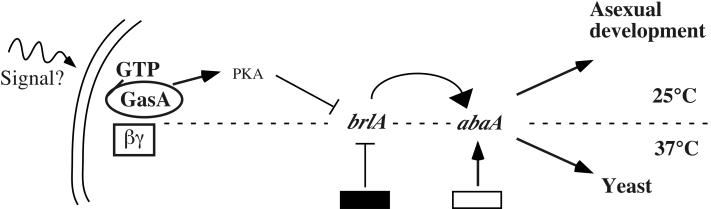
We investigated the signal transduction pathways connecting GasA and its downstream effectors BrlA and AbaA. To test if a cAMP-PKA cascade mediates GasA signaling in P. marneffei, we assessed the phenotypes of the different mutants in the presence of 0.1 mM dbcAMP and 10 mM theophylline, a phosphodiesterase inhibitor. Growth on dbcAMP and theophylline inhibited conidiation and led to a fluffy phenotype after 10 days at 25°C for the wild-type, dominant interfering gasAG203R, and ΔgasA strains. The dominant activated gasAG42R mutant did not show any additional phenotype in the presence of these compounds (data not shown). These observations suggest that GasA signals through a cAMP-PKA cascade to inhibit asexual development (Fig. 6).
FIG. 6.
Proposed model for signal transduction pathways regulating development and growth in P. marneffei. GasA signaling at 25°C involves a cAMP-PKA pathway in the regulation of asexual development and the expression of the core regulatory factors BrlA and AbaA. GasA has no apparent role during yeast cell morphogenesis at 37°C. Therefore, we hypothesize the presence of a brlA repressor (black box) as well as an abaA activator (white box) which are independent of GasA signaling at this temperature.
DISCUSSION
The P. marneffei gasA gene encodes a Gα subunit of a heterotrimeric G protein. GasA has the highest level of amino acid identity with the Gα subunits FadA of A. nidulans, CGA1 of Cochliobolus heterostrophus, CPG1 of C. parasitica, MagB of M. grisea, and GNA1 of N. crassa. All of these fungal Gα subunits are homologous to members of the mammalian Gαi family and have been shown to regulate a number of developmental and morphological pathways (22, 24, 27, 34, 48). We have shown in this study that a P. marneffei mutant carrying a dominant activating gasAG42R allele is locked in vegetative growth and cannot enter asexual development. The same phenotype has been described for an A. nidulans mutant carrying the corresponding fadAG42R allele (48). This suggests that GasA signaling in P. marneffei at 25°C antagonizes conidiophore development and that GasA is a key regulator of asexual development in P. marneffei, as FadA is in A. nidulans. This hypothesis is further supported by the observation that a P. marneffei mutant carrying a dominant interfering gasAG203R allele conidiates abundantly in liquid culture. Furthermore, P. marneffei mutants carrying a dominant activating fadAG42R or a dominant interfering fadAG203R mutation are phenotypically similar to mutants carrying the corresponding gasA mutations. This shows that the P. marneffei gasA gene encodes a G-protein α subunit which appears to be a functional homologue of the A. nidulans fadA gene. The facts that hyphal cells of the ΔgasA mutant show conidiophore-like characteristics but that this strain does not conidiate in liquid culture suggest that GasA acts together with a Gβγ subunit and that both the Gα subunit and the Gβγ subunit antagonize conidiophore development. It is only in a dominant interfering gasAG203R mutant, where signaling of both the Gα and the Gβγ subunit is impaired, that inappropriate conidiation is evident. This finding is consistent with the observation that in liquid cultures, brlA, which codes for one of the key transcriptional regulators controlling asexual development, is strongly expressed in the dominant interfering gasAG203R mutant, less abundantly expressed in the ΔgasA mutant, and not expressed in the wild type. Similar results have been reported for A. nidulans, in which a mutant carrying the dominant interfering fadAG203R allele conidiates in submerged culture while the ΔfadA strain does not (48). Moreover, deletion of sfaD, which encodes a Gβ subunit, also leads to hyperactive sporulation (39). Signaling by the Gβγ subunit has also been suggested to occur in M. grisea, where both a mutant carrying a dominant activating magBG42R allele and a magB loss-of-function mutant exhibit severe reductions in conidiation. In contrast, the magBG203R mutation has no significant effect on conidiation. Thus, it is proposed that Gβγ in M. grisea remains active in both null and constitutively active magBG42R mutants and may be responsible for negatively regulating development (15).
GasA signaling negatively regulates asexual development and must be turned off in order for P. marneffei to enter asexual development. Northern blot analysis showed that the level of gasA transcription was downregulated during asexual development compared to that observed during vegetative hyphal growth, and this appears to be part of the mechanism by which the level of GasA signaling is controlled. However, in A. nidulans, both the mRNA and the protein encoded by fadA can be detected at relatively constant levels throughout the A. nidulans life cycle (48). These findings suggest that a mechanism regulating the expression of gasA in P. marneffei does not operate for fadA in A. nidulans.
FadA signaling in A. nidulans not only antagonizes conidiophore development but also plays a key role in the regulation of hyphal proliferation. Activation of FadA is thought to be initiated by the binding of a growth factor ligand to a putative G-protein-coupled receptor (48). A dominant activating fadAG42R mutation stimulates growth and leads to colonies which eventually autolyze. The ΔfadA mutant produces smaller colonies than those of the wild type, and the dominant interfering fadAG203R mutant shows a more severe growth defect than that of the ΔfadA strain, suggesting that the Gα subunit FadA is a positive regulator of growth (48). In addition to FadA, hyphal proliferation is also partly regulated by the Gβγ subunit, because deletion of the sfaD gene encoding the Gβ subunit retards growth (39). In contrast, hyphal proliferation in P. marneffei at 25°C showed no significant differences between the different GasA mutants. Although the growth rate differences may be very subtle, a more likely explanation is that GasA functions predominantly in activating a signaling pathway that prevents asexual development and has only a minor or no role in the regulation of growth. A similar situation has been noted for M. grisea (15). These findings imply the presence of another signal transduction pathway with a more pronounced role in regulating growth. The presence of two different signal transduction pathways regulating mycelial proliferation and asexual development and involving the Gα subunits GNA1 and GNA3 has been reported for N. crassa. During vegetative growth, Δgna1 strains exhibit slower apical extension of hyphae and slower growth. Asexual development is not affected dramatically, but Δgna1 mutants form more conidia per aerial hypha than does the wild type (46), whereas Δgna3 mutants produce short aerial hyphae and conidiate prematurely, yielding a dense conidiation pattern, and, unlike the wild type, conidiate in submerged culture (26).
It has previously been shown that the conidiation and dimorphic switching pathways share transcriptional regulatory components (5, 18, 41). In P. marneffei, the abaA gene is required for both conidiation and correct yeast cell morphogenesis (5). It was therefore important to investigate the role of the signaling component GasA at 37°C. Our inability to distinguish the different gasA mutants from the wild type at the colonial and microscopic levels at 37°C suggests that GasA plays no role in dimorphic switching or yeast-like growth. The significance of the two gasA transcripts detected at 37°C was therefore not investigated further. At 37°C, a brlA transcript could not be detected in the dominant interfering gasAG203R mutant, which expresses brlA inappropriately at 25°C. This result suggests that an as yet unknown factor must specifically repress brlA transcription at 37°C. Alternatively, an unknown factor required for brlA expression may be present at 25°C but missing at 37°C. These suggestions lead to the hypothesis that at 37°C, abaA may be activated independently of GasA and BrlA. This is in contrast to the situation at 25°C, where abaA expression is mainly dependent on BrlA (Fig. 6).
A number of fungal G proteins closely related to GasA, such as N. crassa GNA1 and M. grisea MagB, are suggested to signal through a cAMP-PKA cascade, and FadA signaling in A. nidulans has recently been shown to be mediated through PkaA, the catalytic subunit of PKA (34, 43, 46). In P. marneffei, elevation of the intracellular cAMP level through the addition of dbcAMP and theophylline to the growth medium revealed an inhibition of conidiation in the wild type, the dominant interfering gasAG203R mutant, and the ΔgasA strain. This result suggests that GasA signals via the production of cAMP and the activation of PKA to inhibit the expression of brlA and, subsequently, asexual development (Fig. 6) and implies that GasA may function as a Gαs subunit that stimulates adenylyl cyclase despite its sequence similarity to mammalian Gαi subunits. Therefore, GasA is another example of the group of fungal Gα proteins with similarity to those of the Gαi class but which more likely function as activators of adenylyl cyclase, as do FadA of A. nidulans and GNA1 of N. crassa (25, 43), while CPG1 of C. parasitica seems to work as a bona fide Gαi protein (9). As the discrepancy between the sequences and functions of several fungal Gαi subunits becomes more apparent, the subdivision of the fungal Gαi class into two subclasses may be warranted.
We show in this study that the Gα subunit GasA of P. marneffei is a key regulator of asexual development. We propose that GasA signaling involves a cAMP-PKA pathway. Of particular significance is the fact that GasA does not play a role in dimorphic switching or yeast-like growth. We are currently characterizing two additional P. marneffei Gα subunits to give further insight into the complex regulation of mycelial proliferation and asexual development in P. marneffei at 25°C and to explain the possible role of these Gα subunits in dimorphic switching and yeast-like growth at 37°C.
Acknowledgments
We thank T. H. Adams for providing the A. nidulans fadAG42R and fadAG203R alleles.
This work was supported by a grant from the Australian Research Council. S.Z. was supported by an International Postgraduate Research Scholarship (IPRS) and a Melbourne Research Scholarship (MRS).
REFERENCES
- 1.Adams, T. H., M. T. Boylan, and W. E. Timberlake. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353-362. [DOI] [PubMed] [Google Scholar]
- 2.Andrianopoulos, A., and W. E. Timberlake. 1994. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol. Cell. Biol. 14:2503-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, et al. (ed.). 1994. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 4.Bolker, M. 1998. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25:143-156. [DOI] [PubMed] [Google Scholar]
- 5.Borneman, A. R., M. J. Hynes, and A. Andrianopoulos. 2000. The abaA homologue of Penicillium marneffei participates in two developmental programmes: conidiation and dimorphic growth. Mol. Microbiol. 38:1034-1047. [DOI] [PubMed] [Google Scholar]
- 6.Borneman, A. R., M. J. Hynes, and A. Andrianopoulos. 2001. An STE12 homolog from the asexual, dimorphic fungus Penicillium marneffei complements the defect in sexual development of an Aspergillus nidulans steA mutant. Genetics 157:1003-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylan, M. T., P. M. Mirabito, C. E. Willett, C. R. Zimmerman, and W. E. Timberlake. 1987. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Mol. Cell. Biol. 7:3113-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, Y. F., and T. C. Chow. 1990. Ultrastructural observations on Penicillium marneffei in natural human infection. Ultrastruct. Pathol. 14:439-452. [DOI] [PubMed] [Google Scholar]
- 9.Chen, B., S. Gao, G. H. Choi, and D. L. Nuss. 1996. Extensive alteration of fungal gene transcript accumulation and elevation of G-protein-regulated cAMP levels by a virulence-attenuating hypovirus. Proc. Natl. Acad. Sci. USA 93:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi, G. H., B. Chen, and D. L. Nuss. 1995. Virus-mediated or transgenic suppression of a G-protein α subunit and attenuation of fungal virulence. Proc. Natl. Acad. Sci. USA 92:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clutterbuck, A. J. 1969. A mutational analysis of conidial development in Aspergillus nidulans. Genetics 63:317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper, C. R., Jr., and M. R. McGinnis. 1997. Pathology of Penicillium marneffei. An emerging acquired immunodeficiency syndrome-related pathogen. Arch. Pathol. Lab. Med. 121:798-804. [PubMed] [Google Scholar]
- 13.Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51-56. [DOI] [PubMed] [Google Scholar]
- 14.Ehinger, A., S. H. Denison, and G. S. May. 1990. Sequence, organization and expression of the core histone genes of Aspergillus nidulans. Mol. Gen. Genet. 222:416-424. [DOI] [PubMed] [Google Scholar]
- 15.Fang, E. G., and R. A. Dean. 2000. Site-directed mutagenesis of the magB gene affects growth and development in Magnaporthe grisea. Mol. Plant-Microbe Interact. 13:1214-1227. [DOI] [PubMed] [Google Scholar]
- 16.Gao, S., and D. L. Nuss. 1996. Distinct roles for two G protein α subunits in fungal virulence, morphology, and reproduction revealed by targeted gene disruption. Proc. Natl. Acad. Sci. USA 93:14122-14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrison, R. G., and K. S. Boyd. 1973. Dimorphism of P. marneffei as observed by electron microscopy. Can. J. Microbiol. 19:1305-1309. [DOI] [PubMed] [Google Scholar]
- 18.Gavrias, V., A. Andrianopoulos, C. J. Gimeno, and W. E. Timberlake. 1996. Saccharomyces cerevisiae Tec1 is required for pseudohyphal growth. Mol. Microbiol. 19:1255-1263. [DOI] [PubMed] [Google Scholar]
- 19.Gold, S., G. Duncan, K. Barrett, and J. Kronstad. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8:2805-2816. [DOI] [PubMed] [Google Scholar]
- 20.Hamm, H. E., and A. Gilchrist. 1996. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 8:189-196. [DOI] [PubMed] [Google Scholar]
- 21.Hicks, J. K., J.-H. Yu, N. P. Keller, and T. H. Adams. 1997. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 16:4916-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz, B. A., A. Sharon, S. W. Lu, V. Ritter, T. M. Sandrock, O. C. Yoder, and B. G. Turgeon. 1999. A G protein α subunit from Cochliobolus heterostrophus involved in mating and appressorium formation. Fungal Genet. Biol. 26:19-32. [DOI] [PubMed] [Google Scholar]
- 23.Imwidthaya, P. 1994. Update of Penicillosis marneffei in Thailand. Mycopathologia 127:135-137. [DOI] [PubMed] [Google Scholar]
- 24.Ivey, F. D., P. N. Hodge, G. E. Turner, and K. A. Borkovich. 1996. The Gαi homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7:1283-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivey, F. D., Q. Yang, and K. A. Borkovich. 1999. Positive regulation of adenylyl cyclase activity by a Gαi homolog in Neurospora crassa. Fungal Genet. Biol. 26:48-61. [DOI] [PubMed] [Google Scholar]
- 26.Kays, A. M., P. S. Rowley, R. A. Baasiri, and K. A. Borkovich. 2000. Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20:7693-7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaziro, Y., H. Itoh, T. Kozasa, M. Nakafuku, and T. Satoh. 1991. Structure and function of signal-transducing GTP-binding proteins. Annu. Rev. Biochem. 60:349-400. [DOI] [PubMed] [Google Scholar]
- 28.Kraakman, L., K. Lemaire, P. Ma, A. W. Teunissen, M. C. Donaton, P. Van Dijck, J. Winderickx, J. H. de Winde, and J. M. Thevelein. 1999. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32:1002-1012. [DOI] [PubMed] [Google Scholar]
- 29.Kruger, J., G. Loubradou, E. Regenfelder, A. Hartmann, and R. Kahmann. 1998. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260:193-198. [DOI] [PubMed] [Google Scholar]
- 30.Kubler, E., H. U. Mosch, S. Rupp, and M. P. Lisanti. 1997. Gpa2p, a G-protein α-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272:20321-20323. [DOI] [PubMed] [Google Scholar]
- 31.Kurjan, J., J. P. Hirsch, and C. Dietzel. 1991. Mutations in the guanine nucleotide-binding domains of a yeast Gα protein confer a constitutive or uninducible state to the pheromone response pathway. Genes Dev. 5:475-483. [DOI] [PubMed] [Google Scholar]
- 32.Leberer, E., D. Y. Thomas, and M. Whiteway. 1997. Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev. 7:59-66. [DOI] [PubMed] [Google Scholar]
- 33.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, S. H., and R. A. Dean. 1997. G protein α-subunit genes control growth, development and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 10:1075-1086. [DOI] [PubMed] [Google Scholar]
- 35.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by Gpa2, a G-protein α homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz, M. C., X. Pan, T. Harashima, M. E. Cardenas, Y. Xue, J. P. Hirsch, and J. Heitman. 2000. The G protein-coupled receptor Gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154:609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neer, E. J. 1995. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80:249-257. [DOI] [PubMed] [Google Scholar]
- 38.Regenfelder, E., T. Spellig, A. Hartmann, S. Lauenstein, M. Bölker, and R. Kahmann. 1997. G-proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 16:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen, S., J. H. Yu, and T. H. Adams. 1999. The Aspergillus nidulans sfaD gene encodes a G protein β subunit that is required for normal growth and repression of sporulation. EMBO J. 18:5592-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Schweizer, A., S. Rupp, B. N. Taylor, M. Rollinghoff, and K. Schroppel. 2000. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol. Microbiol. 38:435-445. [DOI] [PubMed] [Google Scholar]
- 42.Segretain, G. 1959. Penicillium marneffei n. sp., agent d'une mycose du systeme reticulo-endothelial. Mycopathol. Mycol. Appl. 11:327-353. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon, M. I., M. P. Strathmann, and N. Gautam. 1991. Diversity of G proteins in signal transduction. Science 252:802-808. [DOI] [PubMed] [Google Scholar]
- 45.Turner, G. E., and K. A. Borkovich. 1993. Identification of a G protein α subunit from Neurospora crassa that is a member of the Gi family. J. Biol. Chem. 268:14805-14811. [PubMed] [Google Scholar]
- 46.Yang, Q., and K. A. Borkovich. 1999. Mutational activation of a Gαi causes uncontrolled proliferation of aerial hyphae and increased sensitivity to heat and oxidative stress in Neurospora crassa. Genetics 151:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, J. H., R. A. Butchko, M. Fernandes, N. P. Keller, T. J. Leonard, and T. H. Adams. 1996. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29:549-555. [DOI] [PubMed] [Google Scholar]
- 48.Yu, J. H., J. Wieser, and T. H. Adams. 1996. The Aspergillus Flba RGS domain protein antagonizes G-protein signalling to block proliferation and allow development. EMBO J. 15:5184-5190. [PMC free article] [PubMed] [Google Scholar]