Abstract
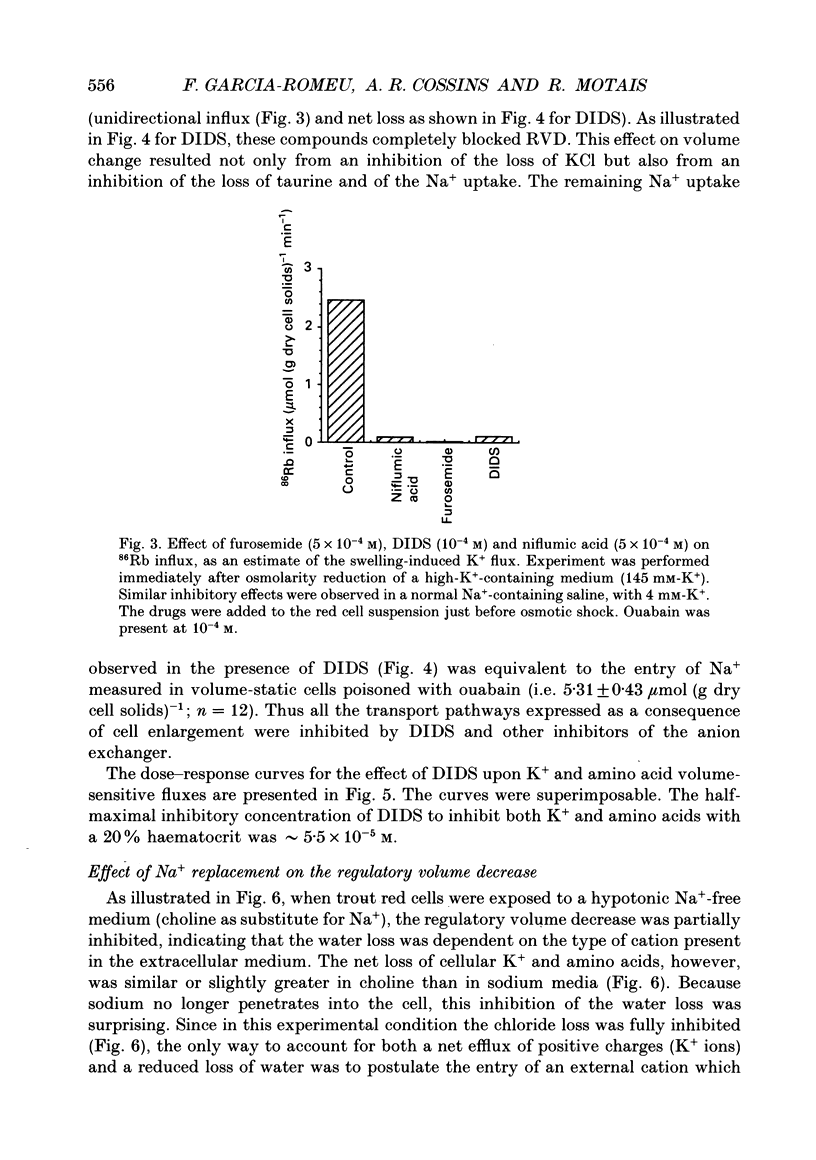
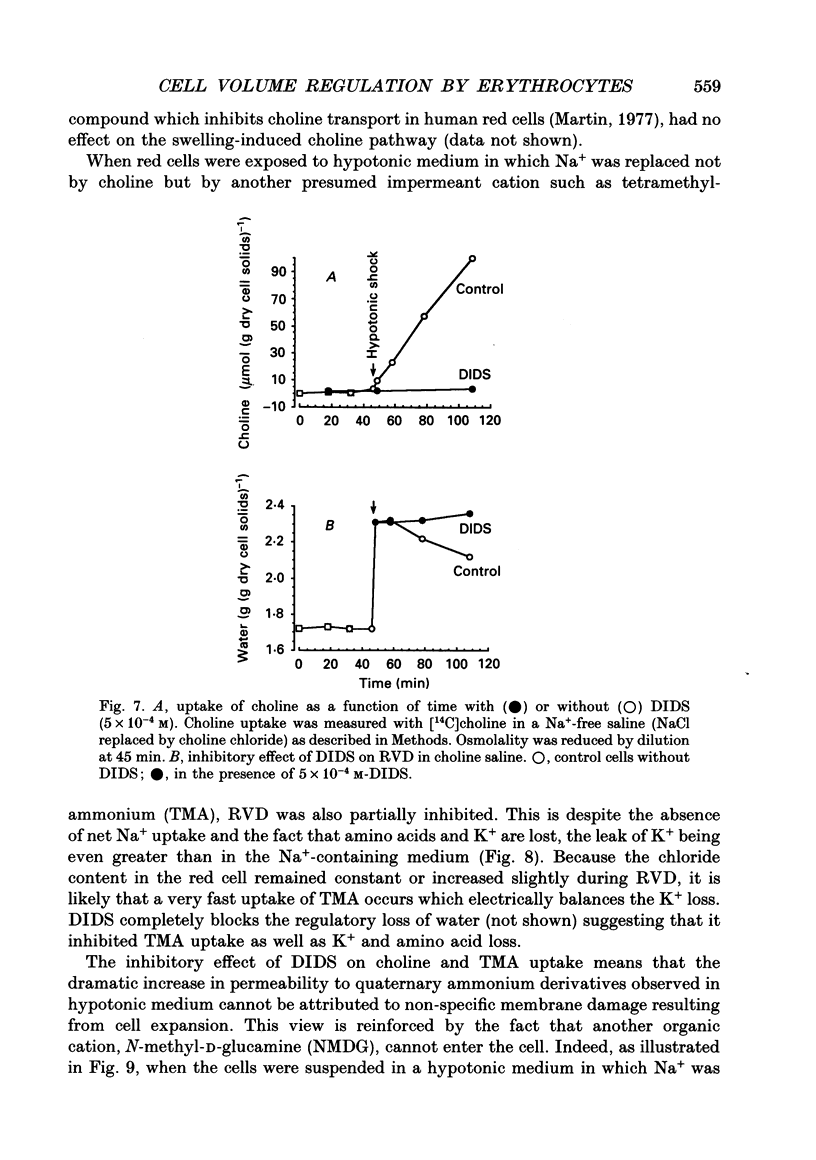
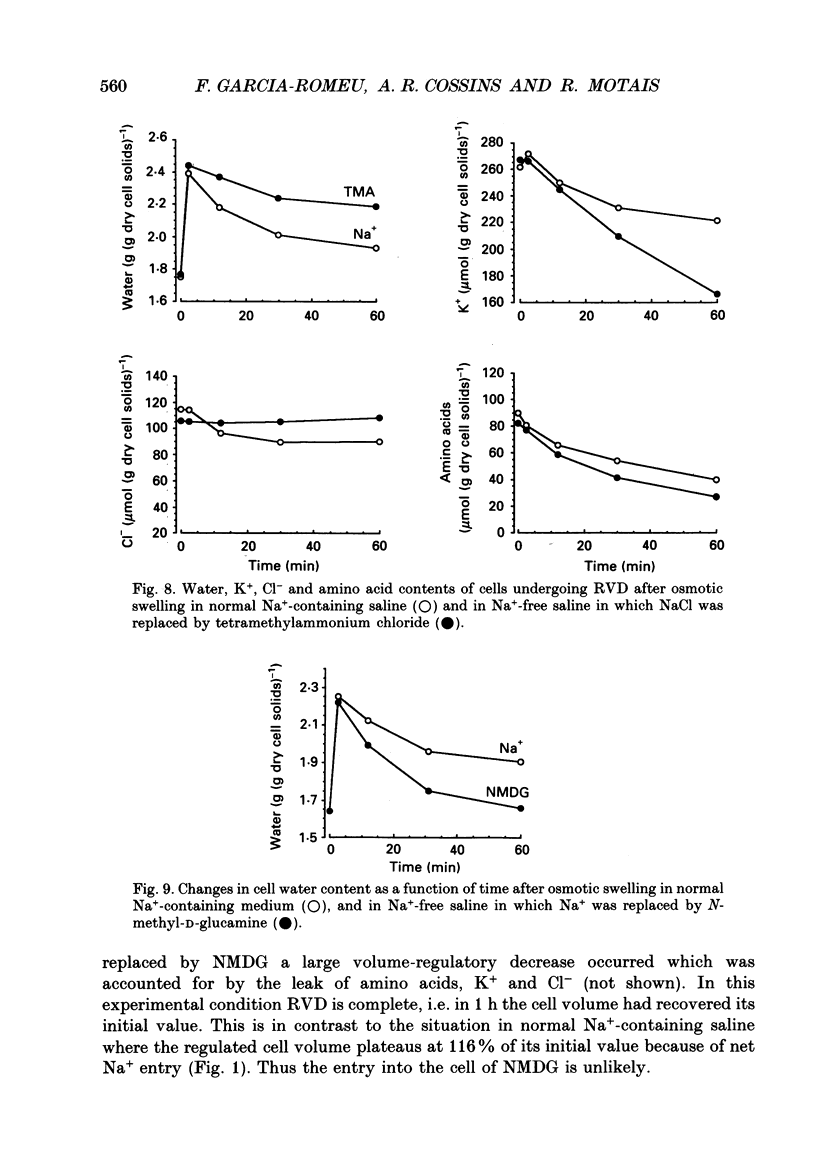
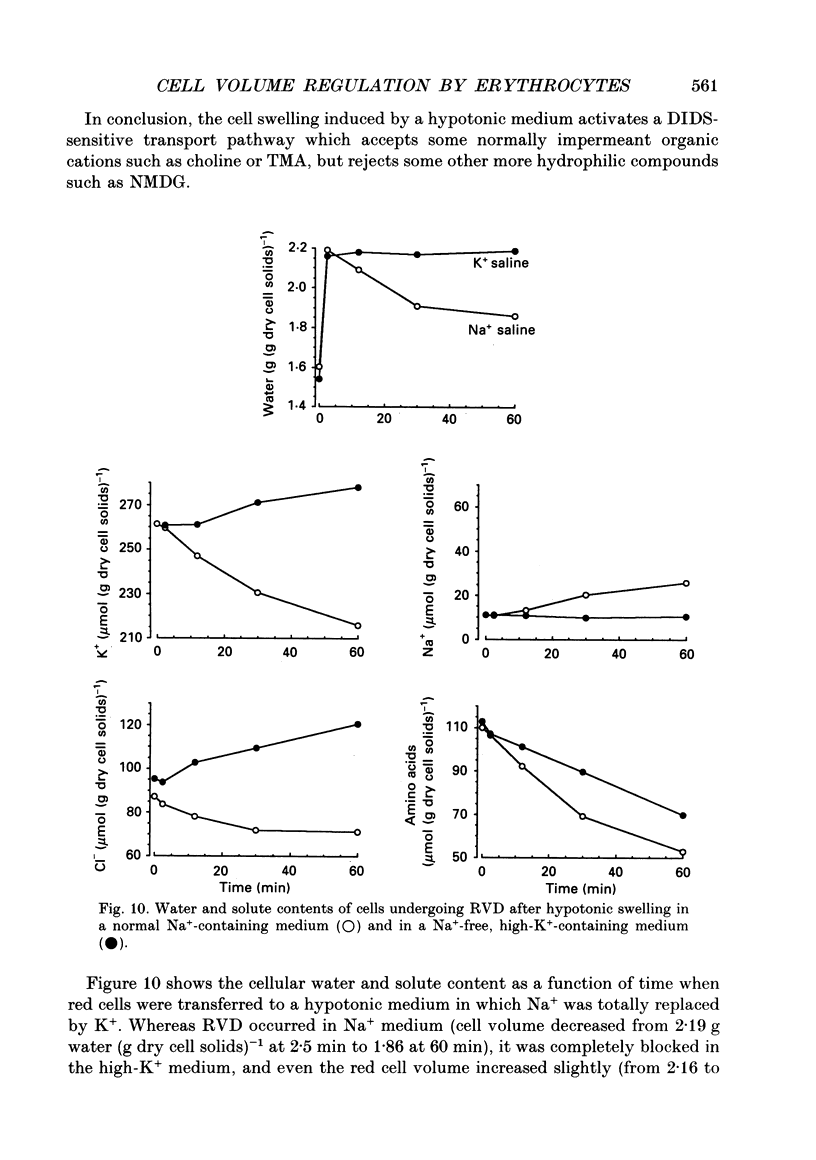
1. An osmolality reduction of the suspending medium leads to osmotic swelling of trout erythrocytes, which is followed by a volume readjustment towards the original level. The regulatory volume decrease (RVD) was not complete after 1 h. 2. During RVD the cells lost K+ and Cl- but gained Na+. This entry of Na+, which is about half the K+ loss, explains the incomplete volume recovery (it was complete when Na+ was replaced by impermeant N-methyl-D-glucamine). The cells also lose large quantities of taurine, which accounts for about 53% of the volume recovery. In addition RVD is accompanied by the activation of a pathway allowing some large organic cations which are normally impermeant, such as choline or tetramethyl-ammonium, to rapidly penetrate the cells. 3. The swelling-activated K+ loss is not significantly affected by replacement of Cl- by NO3-, indicating that K+ moves through a Cl(-)-independent K+ pathway. Furosemide, DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid) and niflumic acid inhibit the K+ loss. From experiments performed in high-K(+)-containing media, it appears that these compounds block the K+ flux, not by inhibiting Cl- movements but by interfering with the K+ pathway. 4. All the volume-activated pathways (K+, Na+, taurine, choline) are fully inhibited by furosemide and by inhibitors of the anion exchanger such as DIDS and niflumic acid. The concentration required for 50% inhibition (IC50) of both inorganic cations and taurine appears to be similar. It is proposed that DIDS interacts with a unique target which controls all the volume-sensitive transport systems.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorante J. S., Cala P. M. Activation of electroneutral K flux in Amphiuma red blood cells by N-ethylmaleimide. Distinction between K/H exchange and KCl cotransport. J Gen Physiol. 1987 Aug;90(2):209–227. doi: 10.1085/jgp.90.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. Hormone-induced co-transport with specific pharmacological properties in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 May;350:137–157. doi: 10.1113/jphysiol.1984.sp015193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Catecholamine-induced transport systems in trout erythrocyte. Na+/H+ countertransport or NaCl cotransport? J Gen Physiol. 1986 Apr;87(4):551–566. doi: 10.1085/jgp.87.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Control of cell volume and ion transport by beta-adrenergic catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1987 Jan;382:123–144. doi: 10.1113/jphysiol.1987.sp016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne P. K., Cossins A. R. Sodium and potassium transport in trout (Salmo gairdneri) erythrocytes. J Physiol. 1984 Feb;347:361–375. doi: 10.1113/jphysiol.1984.sp015070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd T. A., Cha C. J., Forster R. P., Goldstein L. Free amino acids in tissues of the skate Raja erinacea and the stingray Dasyatis sabina: effects of environmental dilution. J Exp Zool. 1977 Mar;199(3):435–442. doi: 10.1002/jez.1401990318. [DOI] [PubMed] [Google Scholar]
- Brazy P. C., Gunn R. B. Furosemide inhibition of chloride transport in human red blood cells. J Gen Physiol. 1976 Dec;68(6):583–599. doi: 10.1085/jgp.68.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnara C., Tosteson D. C. Cell volume, K transport, and cell density in human erythrocytes. Am J Physiol. 1987 Mar;252(3 Pt 1):C269–C276. doi: 10.1152/ajpcell.1987.252.3.C269. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by flounder red blood cells in anisotonic media. J Gen Physiol. 1977 May;69(5):537–552. doi: 10.1085/jgp.69.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa M., Fabry M. E., Blumenfeld N., Nagel R. L. Volume-stimulated, Cl(-)-dependent K+ efflux is highly expressed in young human red cells containing normal hemoglobin or HbS. J Membr Biol. 1987;97(2):97–105. doi: 10.1007/BF01869416. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Cervera M., Marco R. A convenient micromethod for the assay of primary amines and proteins with fluorescamine. A reexamination of the conditions of reaction. Anal Biochem. 1979 Nov 1;99(2):379–391. doi: 10.1016/s0003-2697(79)80022-6. [DOI] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. Inhibition of anion permeability by amphiphilic compounds in human red cell: evidence for an interaction of niflumic acid with the band 3 protein. J Membr Biol. 1979 Apr 20;46(2):125–153. doi: 10.1007/BF01961377. [DOI] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976 Mar;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman K. G., Goldstein L. Cell volume regulation by skate erythrocytes: role of potassium. Am J Physiol. 1990 May;258(5 Pt 2):R1217–R1223. doi: 10.1152/ajpregu.1990.258.5.R1217. [DOI] [PubMed] [Google Scholar]
- Fincham D. A., Wolowyk M. W., Young J. D. Volume-sensitive taurine transport in fish erythrocytes. J Membr Biol. 1987;96(1):45–56. doi: 10.1007/BF01869333. [DOI] [PubMed] [Google Scholar]
- Forster R. P., Goldstein L. Amino acids and cell regulation. Yale J Biol Med. 1979 Nov-Dec;52(6):497–515. [PMC free article] [PubMed] [Google Scholar]
- Fugelli K., Thoroed S. M. Taurine transport associated with cell volume regulation in flounder erythrocytes under anisosmotic conditions. J Physiol. 1986 May;374:245–261. doi: 10.1113/jphysiol.1986.sp016077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugelli K., Zachariassen K. E. The distribution of taurine, gamma-aminobutyric acid and inorganic ions between plasma and erythrocytes in flounder (Platichthys flesus) at different plasma osmolalities. Comp Biochem Physiol A Comp Physiol. 1976;55(2A):173–177. doi: 10.1016/0300-9629(76)90088-8. [DOI] [PubMed] [Google Scholar]
- Guizouarn H., Scheuring U., Borgese F., Motais R., Garcia-Romeu F. Effects of anions on the Na(+)-H+ exchange of trout red blood cells. J Physiol. 1990 Sep;428:79–94. doi: 10.1113/jphysiol.1990.sp018201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., McManus T. J. Effect of norepinephrine on swelling-induced potassium transport in duck red cells. Evidence against a volume-regulatory decrease under physiological conditions. J Gen Physiol. 1985 May;85(5):649–667. doi: 10.1085/jgp.85.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Evidence for the presence of volume-sensitive KCl transport in 'young' human red cells. Biochim Biophys Acta. 1986 Jun 26;858(2):317–320. doi: 10.1016/0005-2736(86)90338-x. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H. Amino acid transport and cell volume regulation in Ehrlich ascites tumour cells. J Physiol. 1983 May;338:613–625. doi: 10.1113/jphysiol.1983.sp014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Livne A., Hoffmann E. K. Cytoplasmic acidification and activation of Na+/H+ exchange during regulatory volume decrease in Ehrlich ascites tumor cells. J Membr Biol. 1990 Mar;114(2):153–157. doi: 10.1007/BF01869096. [DOI] [PubMed] [Google Scholar]
- Parker J. C. Volume-responsive sodium movements in dog red blood cells. Am J Physiol. 1983 May;244(5):C324–C330. doi: 10.1152/ajpcell.1983.244.5.C324. [DOI] [PubMed] [Google Scholar]
- Siebens A. W., Kregenow F. M. Volume-regulatory responses of Amphiuma red cells in anisotonic media. The effect of amiloride. J Gen Physiol. 1985 Oct;86(4):527–564. doi: 10.1085/jgp.86.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symposium on cell volume regulation. J Exp Zool. 1981 Mar;215(3):235–384. doi: 10.1002/jez.1402150302. [DOI] [PubMed] [Google Scholar]


