Abstract
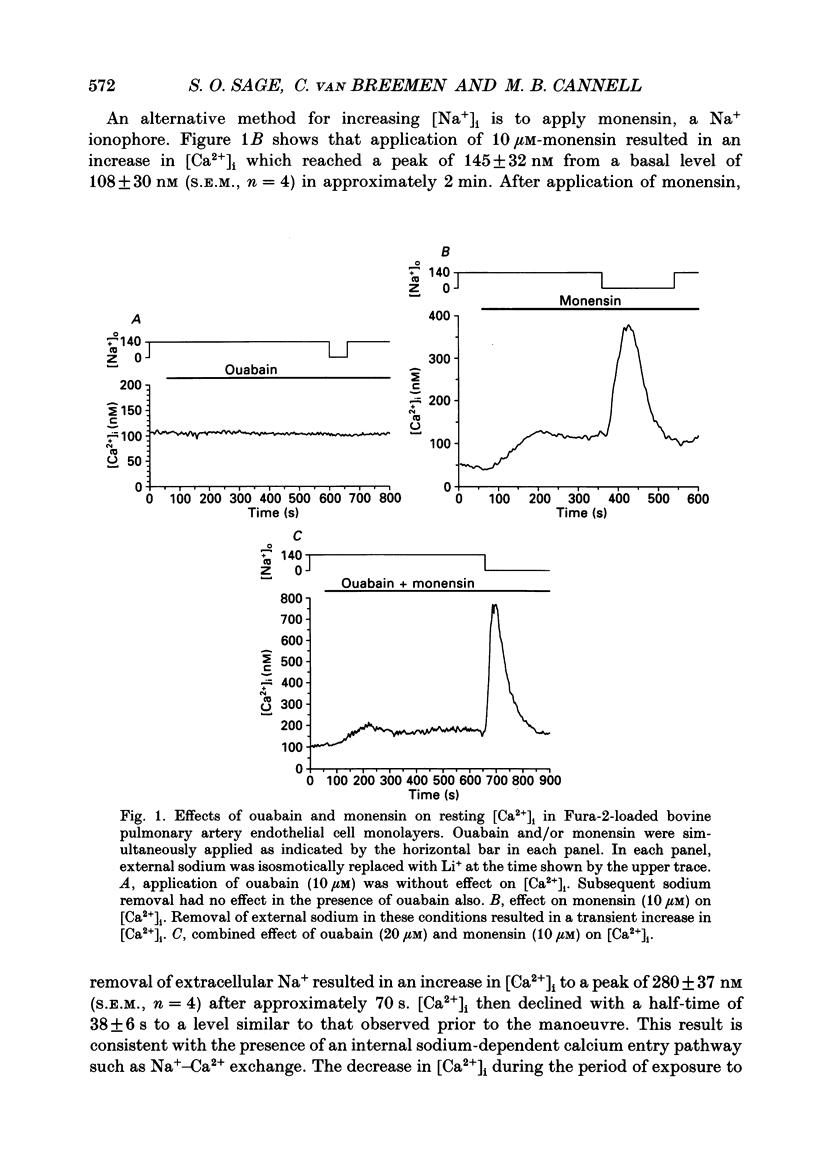
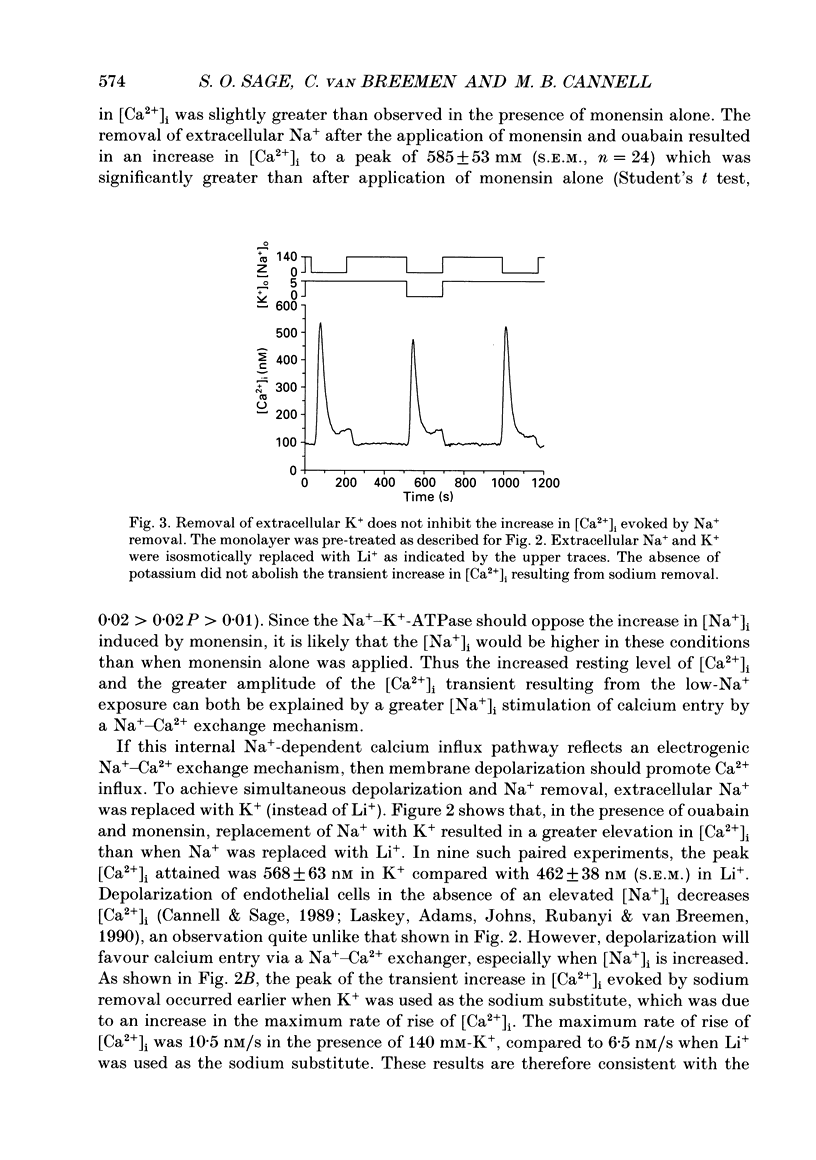
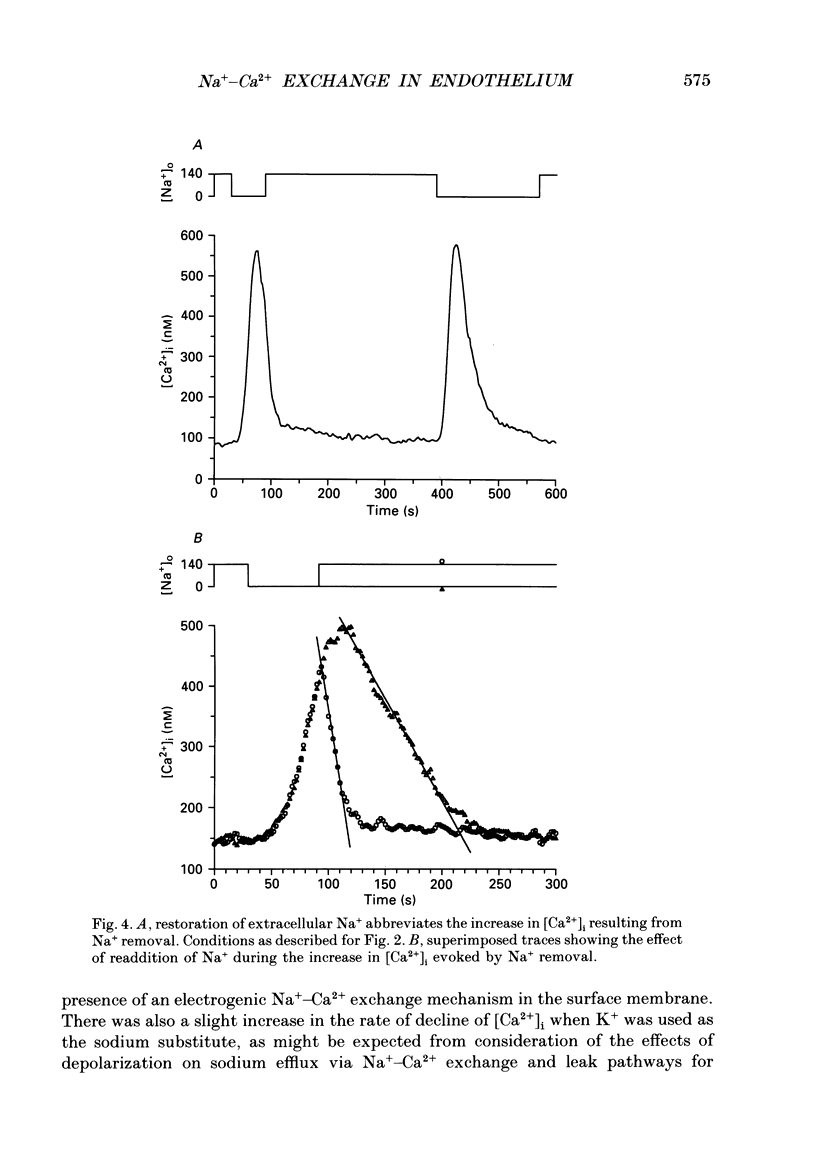
1. Intracellular free calcium ([Ca2+]i) was measured in cultured bovine pulmonary artery endothelial cell monolayers loaded with the fluorescent calcium indicator Fura-2. 2. Resting [Ca2+]i was 112 +/- 10 nM. Application of ouabain (20 microM) was without effect on [Ca2+]i for periods of up to 1 h. Monensin (10 microM) resting [Ca2+]i to 145 +/- 32 nM over approximately 2 min. In the presence of ouabain (20 microM), 10 microM-monensin increased [Ca2+]i to 146 +/- 15 nM. 3. Removal of extracellular sodium was without effect in resting cells or cells exposed to ouabain alone. However, in the presence of monensin, replacement of extracellular Na+ with Li+ resulted in a prompt increase in [Ca2+]i to a peak of 280 +/- 37 nM, which then returned towards resting levels. When Na+ was removed in the presence of both ouabain and monensin, [Ca2+]i reached a peak of 585 +/- 53 nM. 4. When extracellular Na+ was replaced with K+, to achieve simultaneous Na+ removal and depolarization, [Ca2+]i reached a peak of 568 +/- 63 nM, compared with a peak of 462 +/- 38 nM when Li+ was used as a Na+ substitute in paired experiments. The transient increase in [Ca2+]i evoked by sodium removal peaked earlier when K+ was used as the sodium substitute, showing that depolarization increased the rate of calcium influx into the cell when sodium was removed from the bathing medium. 5. Removal of extracellular K+ had no effect on the low-Na(+)-evoked increase in [Ca2+]i. 6. Returning extracellular Na+ during the increase in [Ca2+]i resulting from Na+ removal increased the rate of return of [Ca2+]i towards basal levels. In the absence of Na+, [Ca2+]i took 41 +/- 5 s to decline from 400 to 200 nM, and this was reduced to 26 +/- 6 s (n = 4, S.E.M.) when Na+ was returned to the bathing solution. 7. These results indicate endothelial cells possess a voltage-dependent Na(+) -Ca2+ exchange mechanism in the surface membrane. However, this mechanism does not appear to be of primary importance in the maintenance of resting [Ca2+]i since cells were able to restore a low [Ca2+]i in the absence of extracellular Na+. The evidence for the existence of a Na(+) -Ca2+ exchanger in the surface membrane of endothelial cells and the possibility that this mechanism may contribute to calcium entry and/or extrusion during agonist-evoked responses is discussed.
Full text
PDF


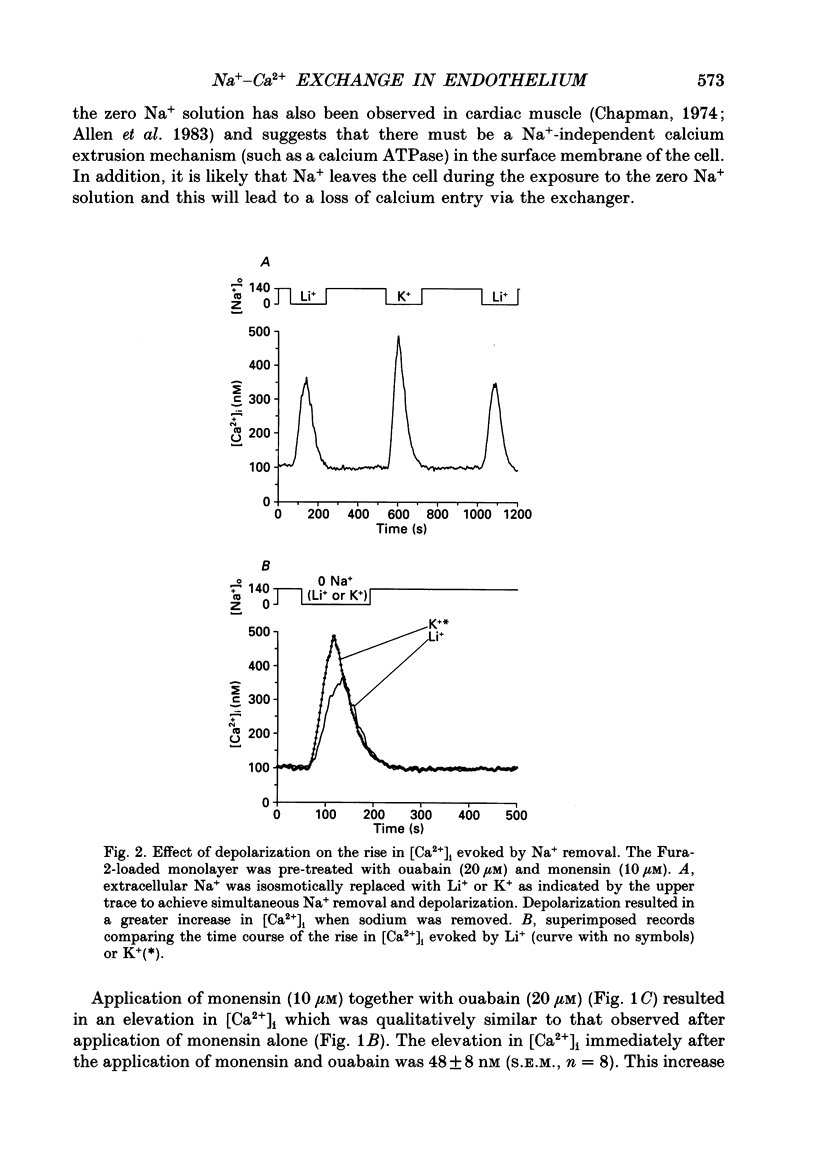





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Lab M. J., Orchard C. H. The effects of low sodium solutions on intracellular calcium concentration and tension in ferret ventricular muscle. J Physiol. 1983 Dec;345:391–407. doi: 10.1113/jphysiol.1983.sp014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The energetics and kinetics of sodium-calcium exchange in barnacle muscles, squid axons, and mammalian heart: the role of ATP. Soc Gen Physiol Ser. 1984;38:129–147. [PubMed] [Google Scholar]
- Brass L. F. Ca2+ homeostasis in unstimulated platelets. J Biol Chem. 1984 Oct 25;259(20):12563–12570. [PubMed] [Google Scholar]
- Bregestovski P., Bakhramov A., Danilov S., Moldobaeva A., Takeda K. Histamine-induced inward currents in cultured endothelial cells from human umbilical vein. Br J Pharmacol. 1988 Oct;95(2):429–436. doi: 10.1111/j.1476-5381.1988.tb11663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. A., Brugnara C., Canessa M., Gimbrone M. A., Jr Bradykinin and vasopressin stimulate Na+-K+-Cl- cotransport in cultured endothelial cells. Am J Physiol. 1986 Jun;250(6 Pt 1):C888–C895. doi: 10.1152/ajpcell.1986.250.6.C888. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Eisner D. A., Lederer W. J., Valdeolmillos M. Effects of membrane potential on intracellular calcium concentration in sheep Purkinje fibres in sodium-free solutions. J Physiol. 1986 Dec;381:193–203. doi: 10.1113/jphysiol.1986.sp016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. A study of the contractures induced in frog atrial trabeculae by a reduction of the bathing sodium concentration. J Physiol. 1974 Mar;237(2):295–313. doi: 10.1113/jphysiol.1974.sp010483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Takamatsu H., Toyoda T., Sawada S., Tsuji H., Ijichi H. Effect of inhibition of Na+-K+ ATPase on the prostacyclin generation of cultured human vascular endothelial cells. Life Sci. 1987 Jan 26;40(4):351–357. doi: 10.1016/0024-3205(87)90136-6. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E. Regulation of Na-K-Cl cotransport in endothelial cells by atrial natriuretic factor. Am J Physiol. 1989 Jul;257(1 Pt 1):C36–C44. doi: 10.1152/ajpcell.1989.257.1.C36. [DOI] [PubMed] [Google Scholar]
- Requena J., Mullins L. J., Whittembury J., Brinley F. J., Jr Dependence of ionized and total Ca in squid axons on Nao-free or high-Ko conditions. J Gen Physiol. 1986 Jan;87(1):143–159. doi: 10.1085/jgp.87.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S. Isolation and culture of pulmonary endothelial cells. Environ Health Perspect. 1984 Jun;56:103–114. doi: 10.1289/ehp.8456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. Effects of ionic substitution on [Ca2+]i rises evoked by thrombin and PAF in human platelets. Eur J Pharmacol. 1986 Aug 22;128(1-2):99–107. doi: 10.1016/0014-2999(86)90563-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer J., Blaustein M. P. Platelet free calcium concentrations measured with fura-2 are influenced by the transmembrane sodium gradient. Cell Calcium. 1989 Feb-Mar;10(2):101–113. doi: 10.1016/0143-4160(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Winquist R. J., Bunting P. B., Schofield T. L. Blockade of endothelium-dependent relaxation by the amiloride analog dichlorobenzamil: possible role of Na+/Ca++ exchange in the release of endothelium-derived relaxant factor. J Pharmacol Exp Ther. 1985 Dec;235(3):644–650. [PubMed] [Google Scholar]
- Yasui K., Kimura J. Is potassium co-transported by the cardiac Na-Ca exchange? Pflugers Arch. 1990 Jan;415(4):513–515. doi: 10.1007/BF00373636. [DOI] [PubMed] [Google Scholar]
- Zimlichman R., Goldstein D. S., Zimlichman S., Keister H. R. Effects of ouabain on cytosolic calcium in lymphocytes, platelets and adrenomedullary cells. J Hypertens. 1987 Oct;5(5):605–609. doi: 10.1097/00004872-198710000-00016. [DOI] [PubMed] [Google Scholar]


