Abstract
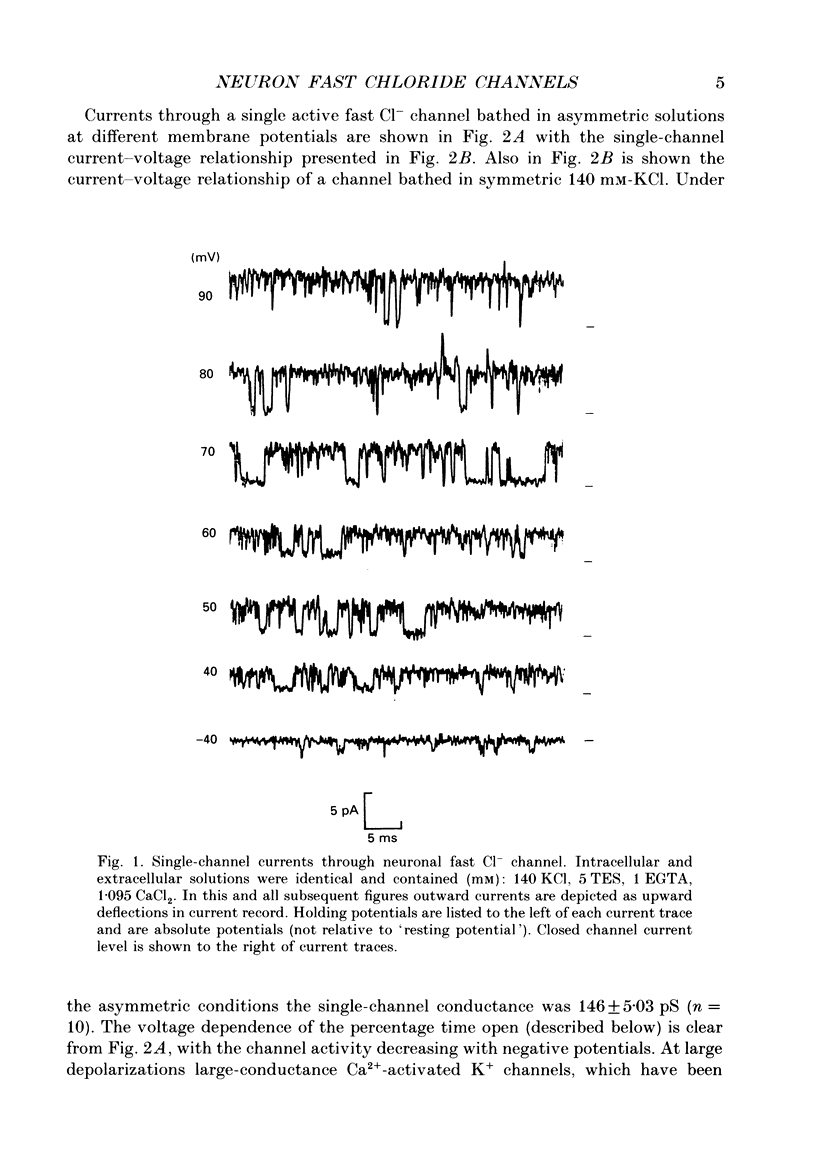
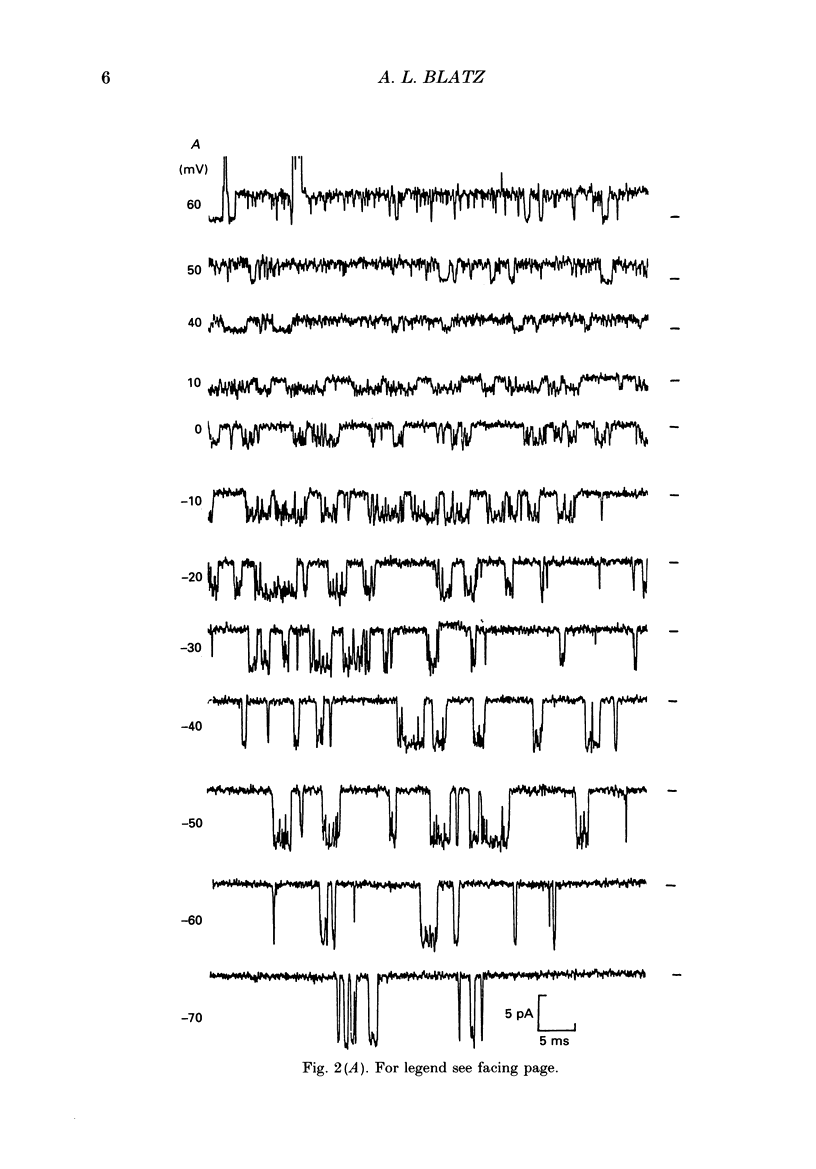
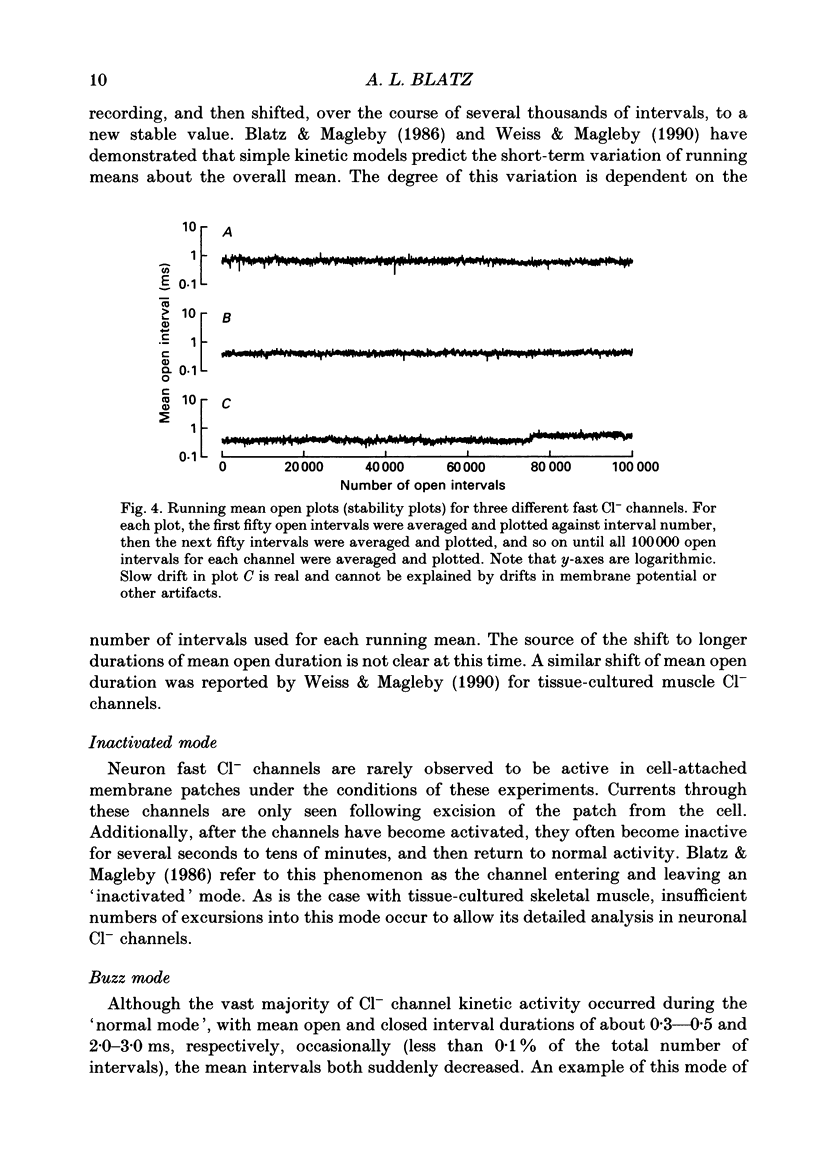
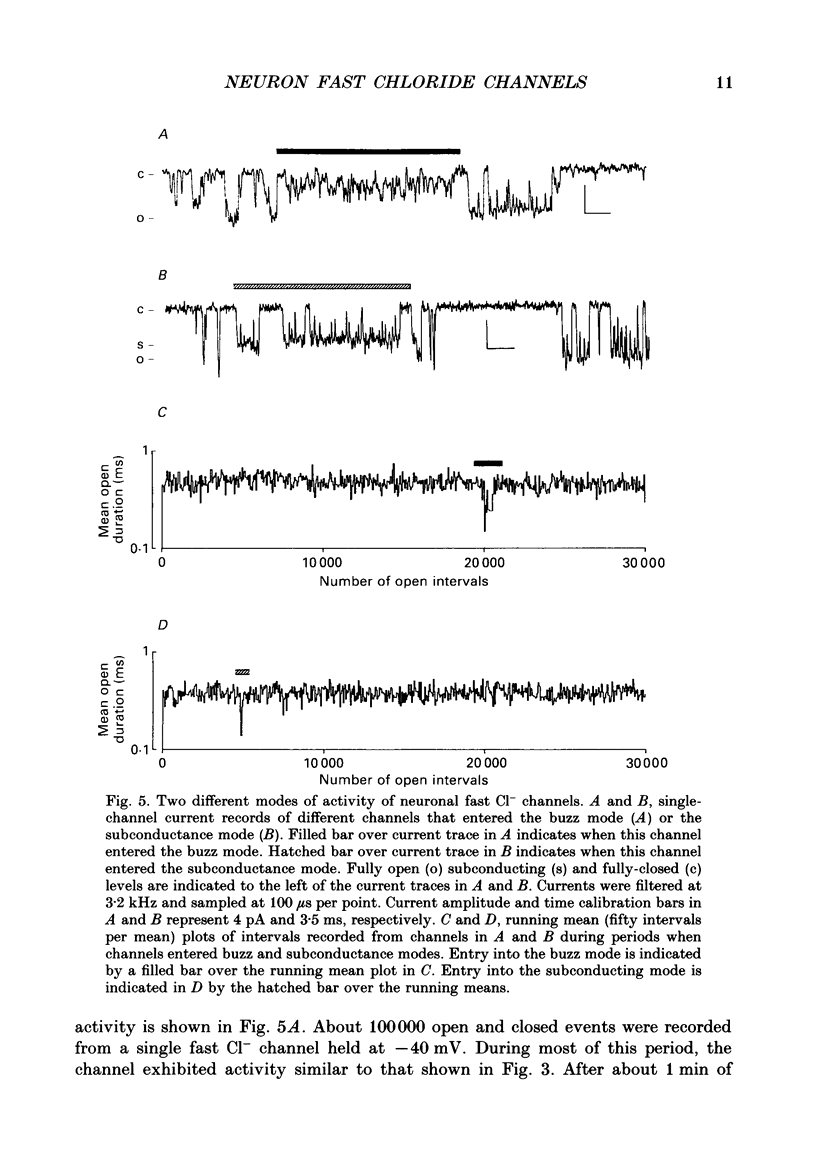
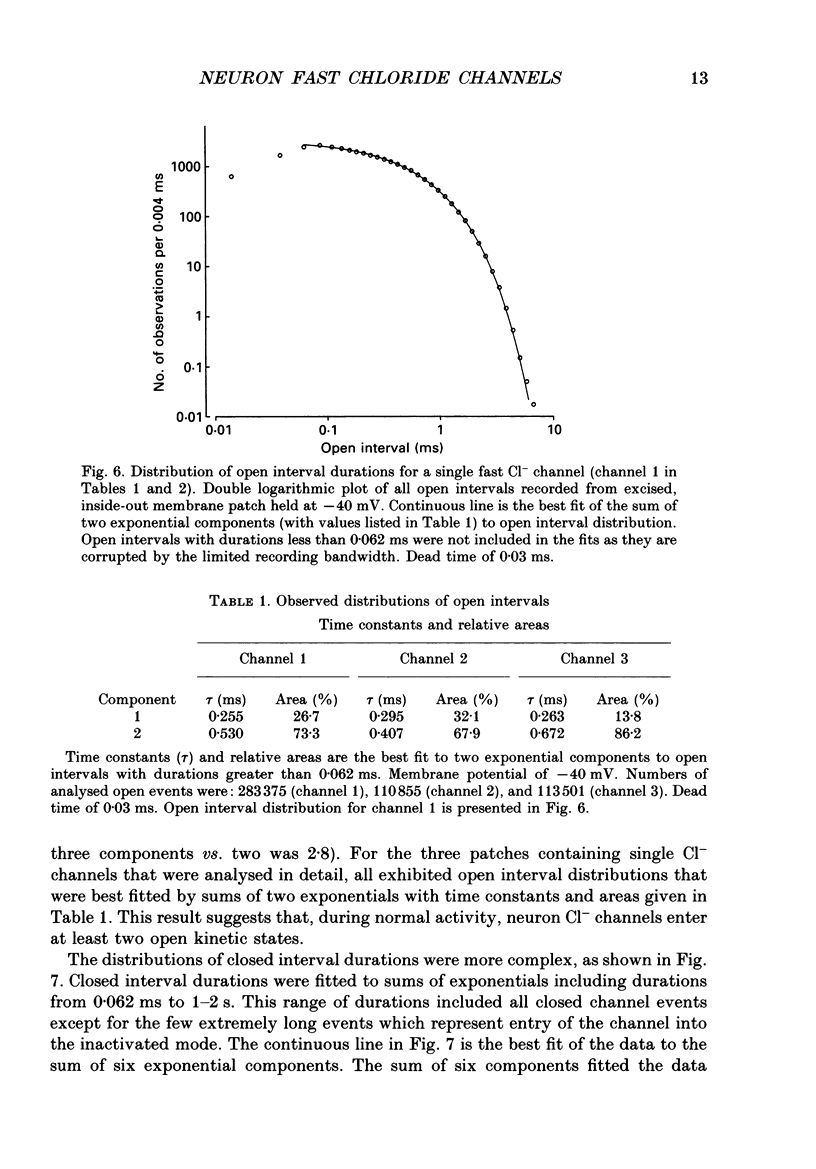
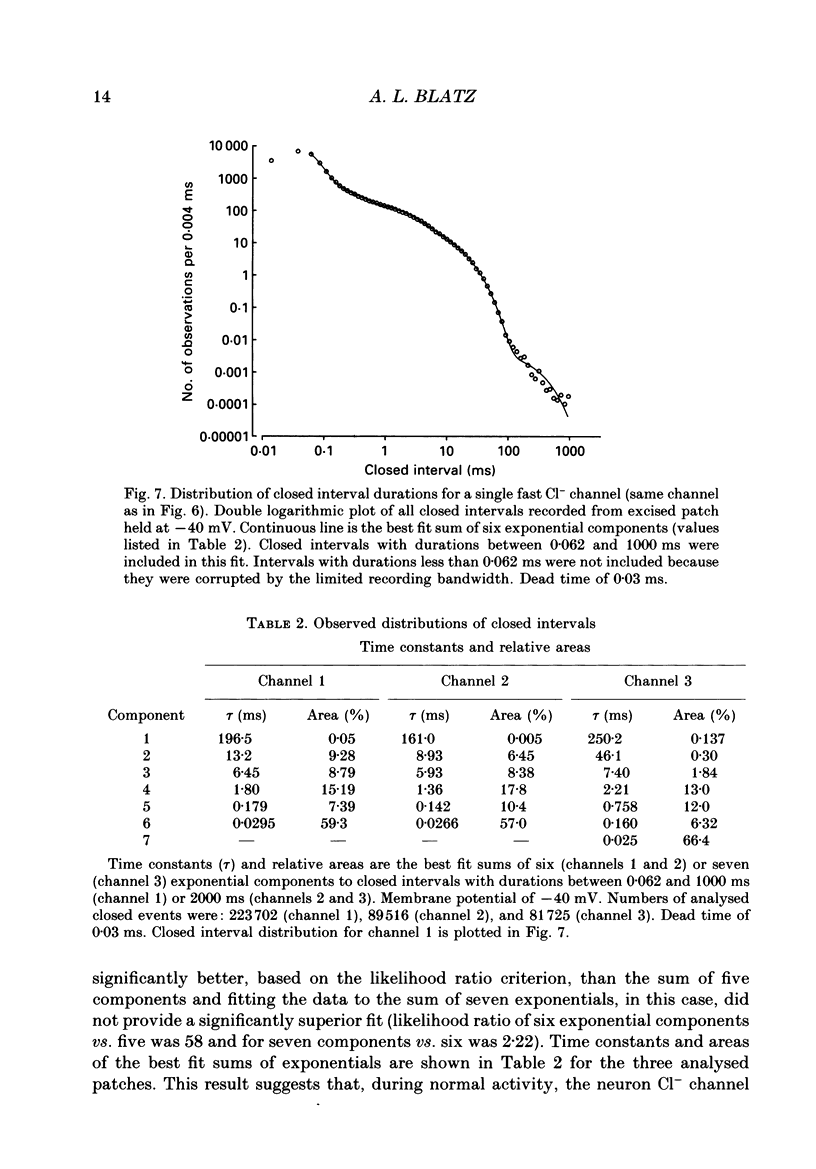
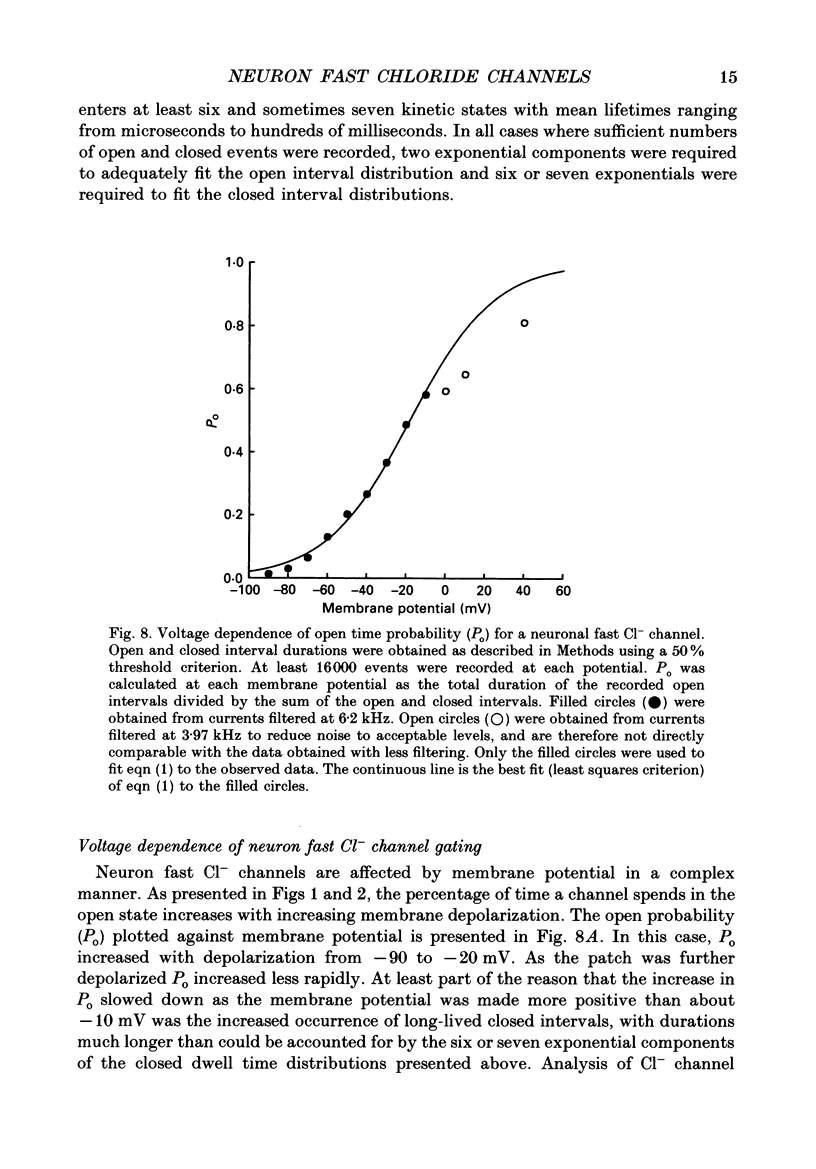
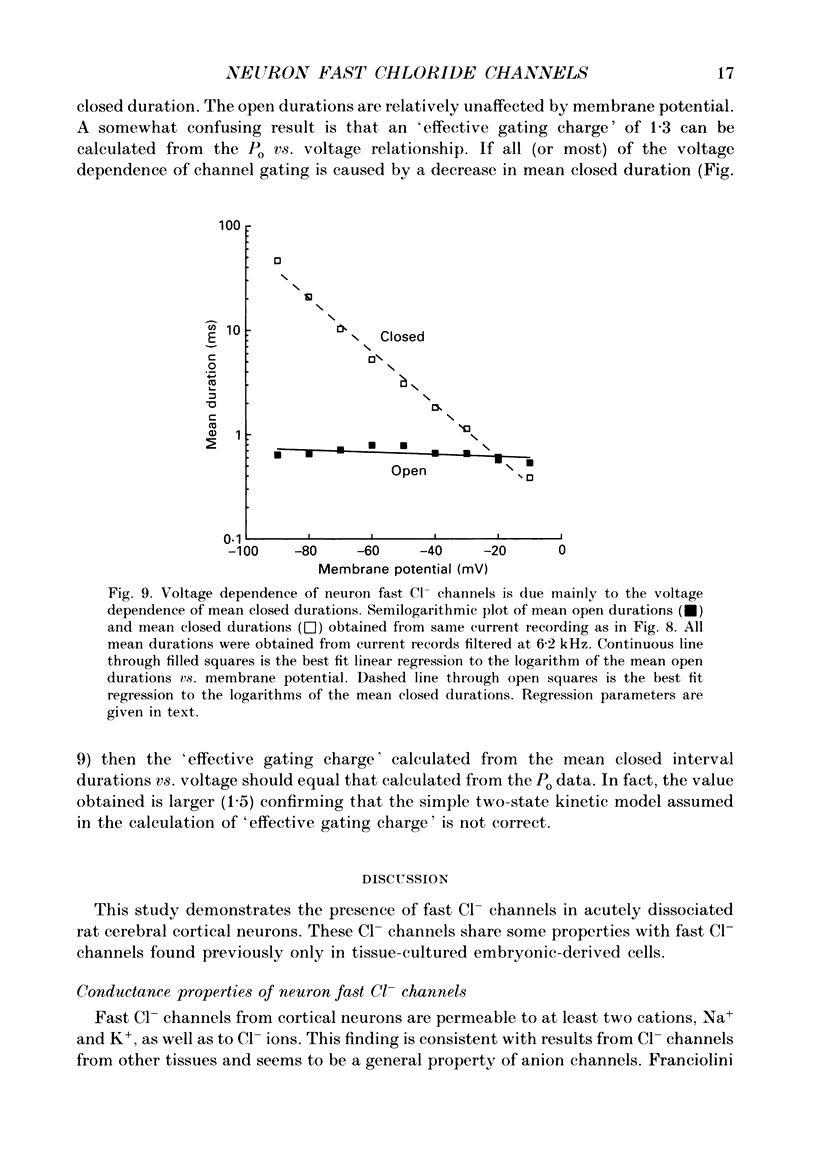
1. Properties of Cl- channels from surface membranes of acutely dissociated rat cerebral cortical neurons were studied with the patch clamp technique. These channels were present in the majority of excised inside-out membrane patches. 2. Cl- channels were rarely observed in cell-attached membrane patches, and usually several minutes elapsed following excision of the patch before Cl- channels became active. 3. Under asymmetric ionic conditions (1000 mM-KCli, 140 mM-KClo), neuronal Cl- channels are fairly selective for Cl- over K+ and Na+, with permeability ratios, determined by reversal potential shifts of 4.8 for both PCl/PK and PCl/PNa. 4. Neuronal Cl- channel kinetic activity remained stable over periods of time long enough to collect up to 500,000 open and closed intervals. Occasionally, the channels entered altered modes of activity. In the 'buzz mode' the open and closed interval durations became much shorter than normal for several hundreds of intervals. In the 'subconductance mode' the channel opened to a current level about two-thirds of the normal level. 5. Using the method of maximum likelihood, sums of exponentials were fitted to the distributions of open and closed interval durations. Open interval distributions required at least two exponential components with time constants of less than 1 ms. At least six or seven exponential components were required to fit the closed interval distributions with time constants ranging from 30 microseconds to several hundreds of milliseconds. This suggests that neuronal Cl- channels enter at least two open and six or seven closed kinetic states during normal activity. 6. Cl- channels often entered long-duration closed states of several minutes which could not be accounted for by the sums of exponentials fitted to the distribution of closed interval durations. 7. Neuronal Cl- channels exhibit a marked voltage dependence with the percentage of time the channels are open increasing with depolarization. Most of the observed voltage dependence can be accounted for by a decrease in the mean closed interval duration with depolarization. The mean open interval was relatively independent of voltage. 8. These results suggest a high degree of similarity in kinetic behaviour and conductance properties between the fast Cl- channels of tissue-cultured rat skeletal muscle and fast Cl- channels in acutely dissociated rat cerebral cortical neurons.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Adjacent interval analysis distinguishes among gating mechanisms for the fast chloride channel from rat skeletal muscle. J Physiol. 1989 Mar;410:561–585. doi: 10.1113/jphysiol.1989.sp017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Ion conductance and selectivity of single calcium-activated potassium channels in cultured rat muscle. J Gen Physiol. 1984 Jul;84(1):1–23. doi: 10.1085/jgp.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single chloride-selective channels active at resting membrane potentials in cultured rat skeletal muscle. Biophys J. 1985 Jan;47(1):119–123. doi: 10.1016/S0006-3495(85)83884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single voltage-dependent chloride-selective channels of large conductance in cultured rat muscle. Biophys J. 1983 Aug;43(2):237–241. doi: 10.1016/S0006-3495(83)84344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag A. H. Muscle chloride channels. Physiol Rev. 1987 Apr;67(2):618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Franciolini F., Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987 Oct;90(4):453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolini F., Petris A. Chloride channels of biological membranes. Biochim Biophys Acta. 1990 May 7;1031(2):247–259. doi: 10.1016/0304-4157(90)90009-2. [DOI] [PubMed] [Google Scholar]
- Franciolini F., Petris A. Single chloride channels in cultured rat neurones. Arch Biochem Biophys. 1988 Feb 15;261(1):97–102. doi: 10.1016/0003-9861(88)90108-7. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., McBurney R. N., Smith S. M., Zorec R. Caesium ions activate chloride channels in rat cultured spinal cord neurones. J Physiol. 1987 Nov;392:231–251. doi: 10.1113/jphysiol.1987.sp016778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Magleby K. L. Voltage dependence and stability of the gating kinetics of the fast chloride channel from rat skeletal muscle. J Physiol. 1990 Jul;426:145–176. doi: 10.1113/jphysiol.1990.sp018131. [DOI] [PMC free article] [PubMed] [Google Scholar]


