Abstract
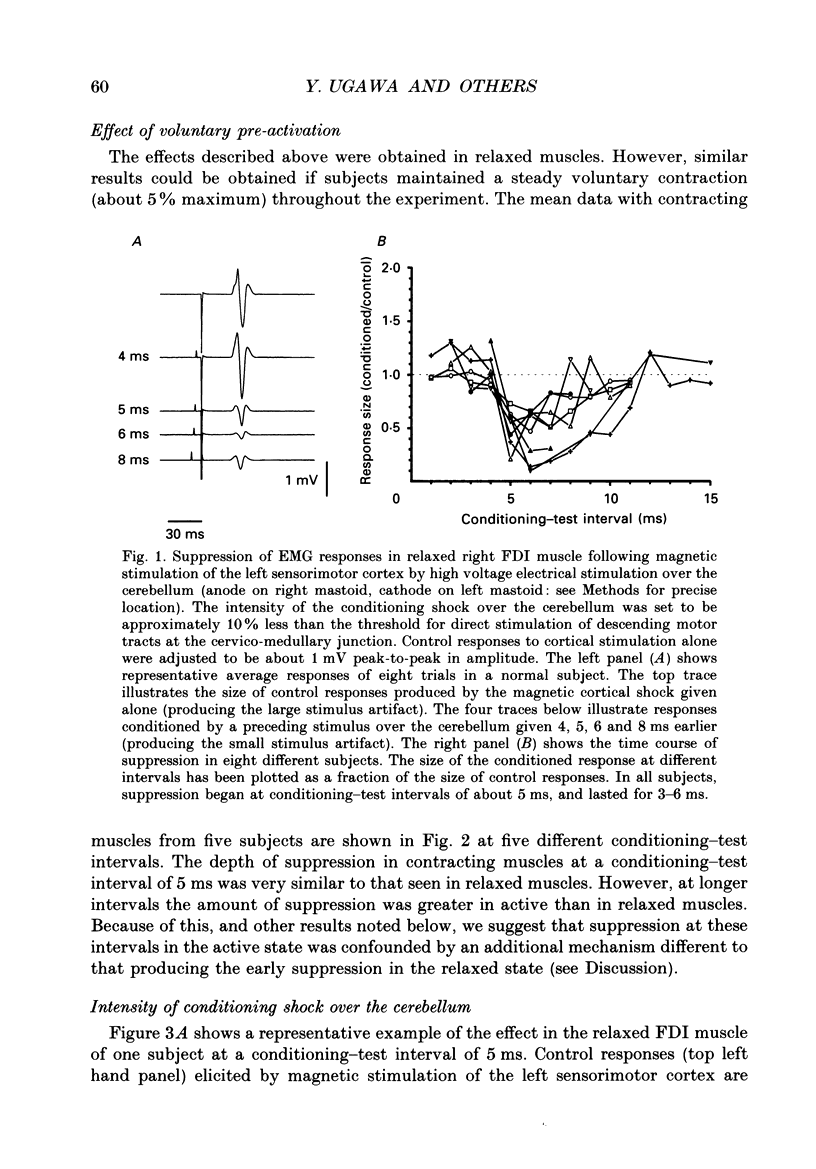
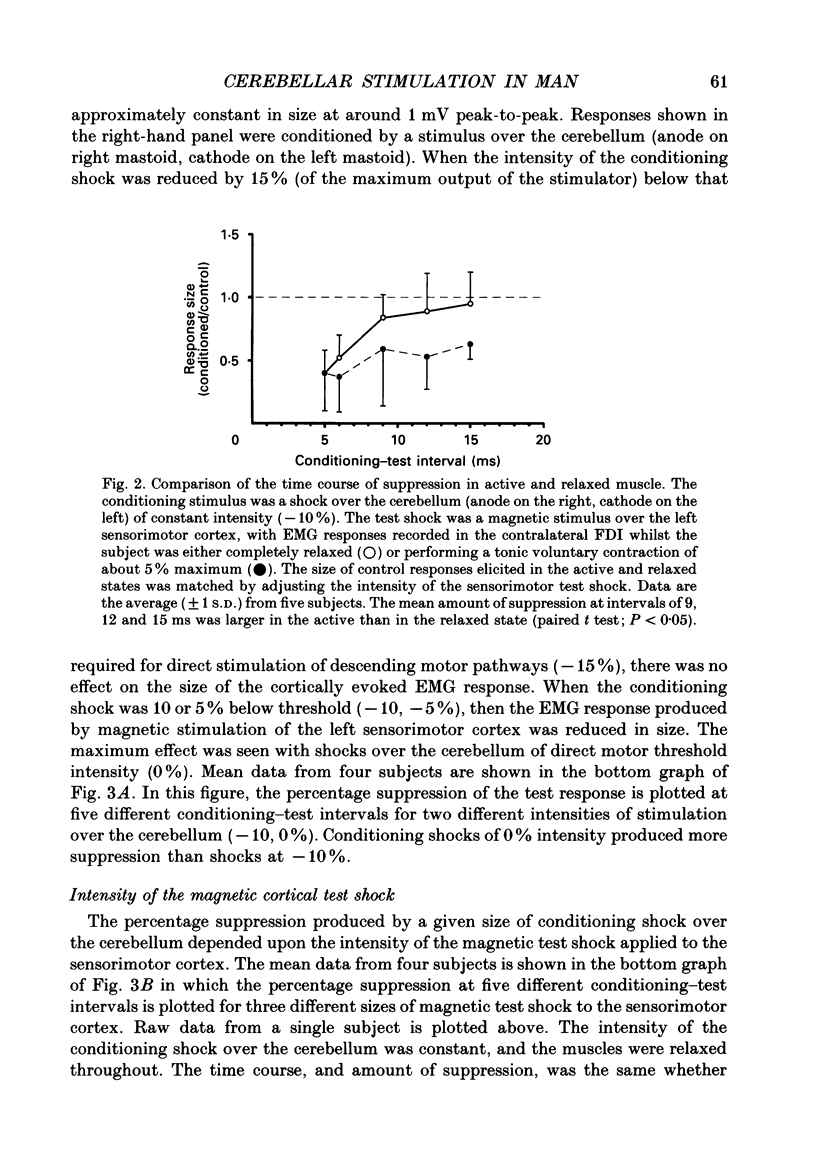
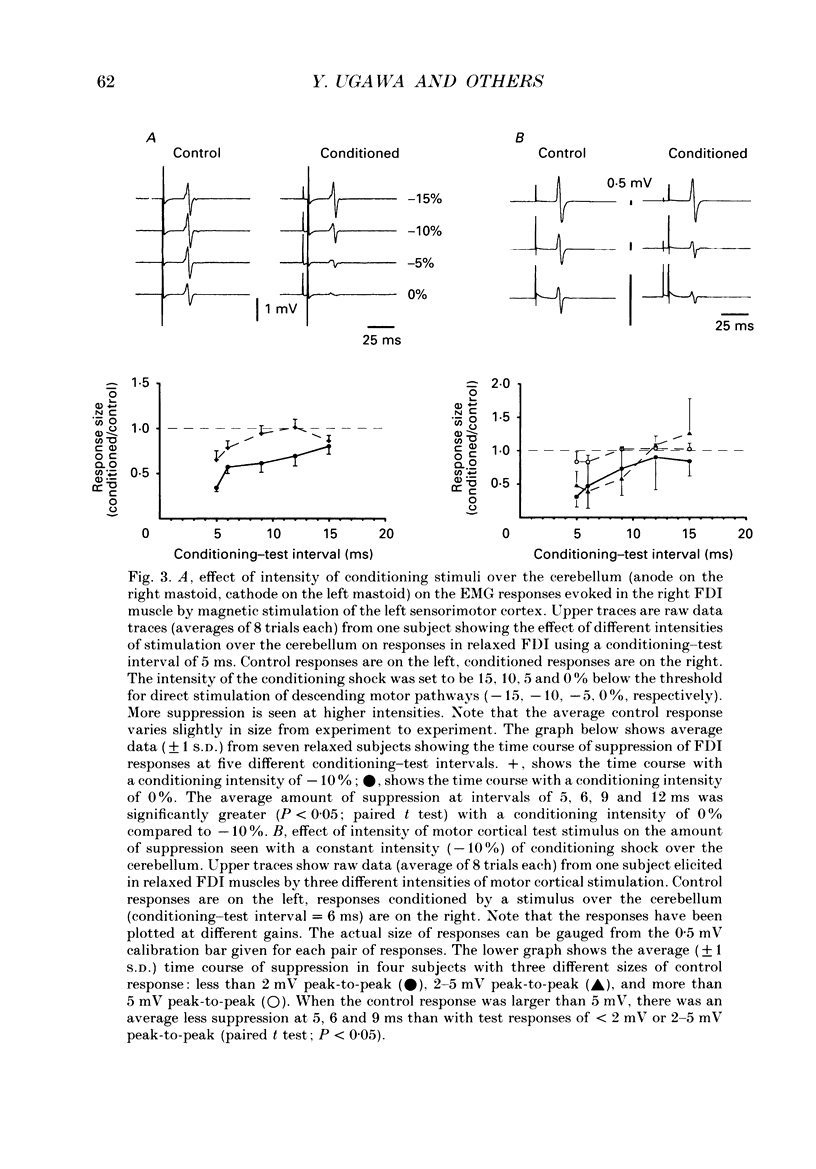
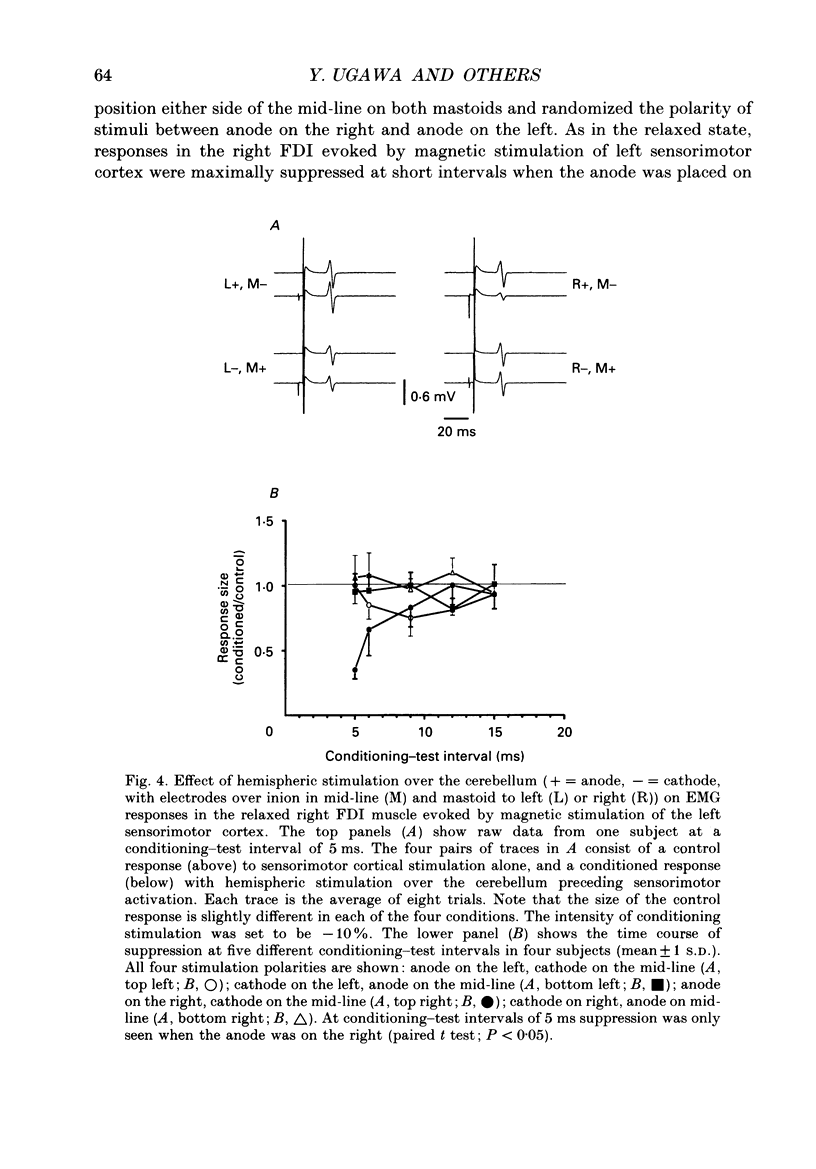
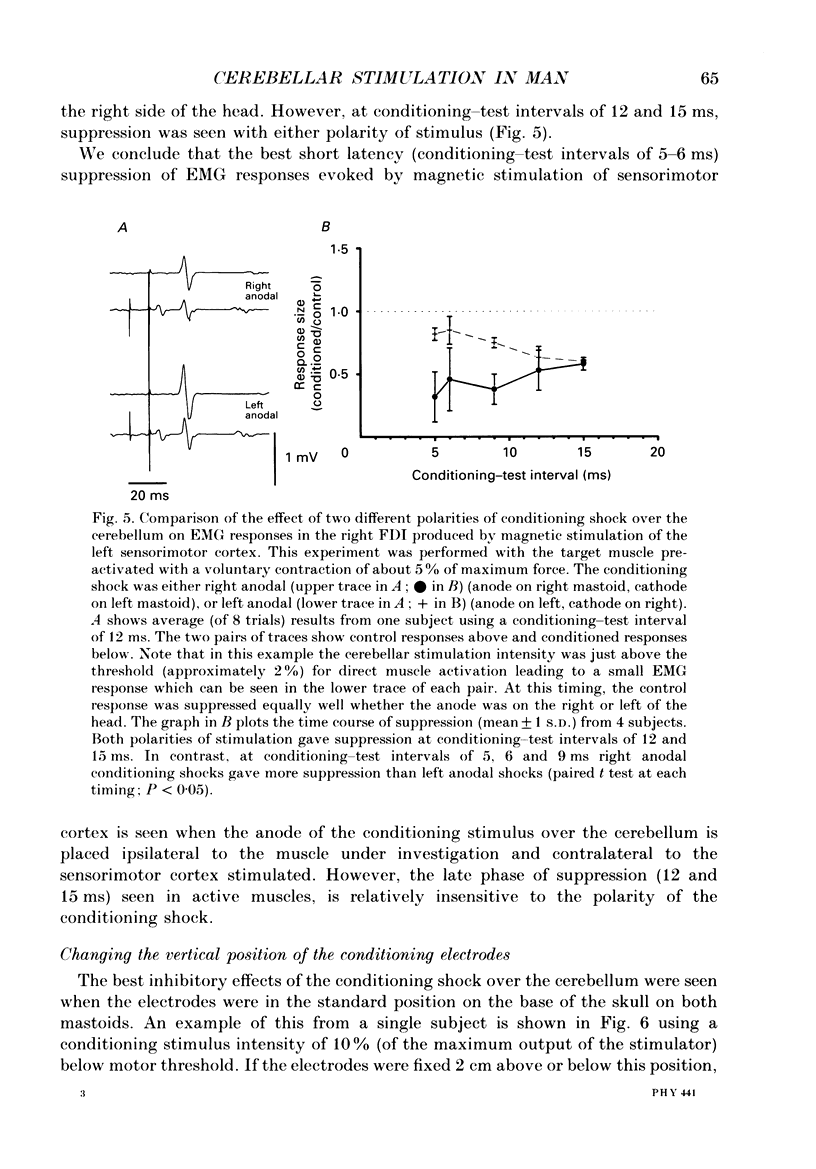
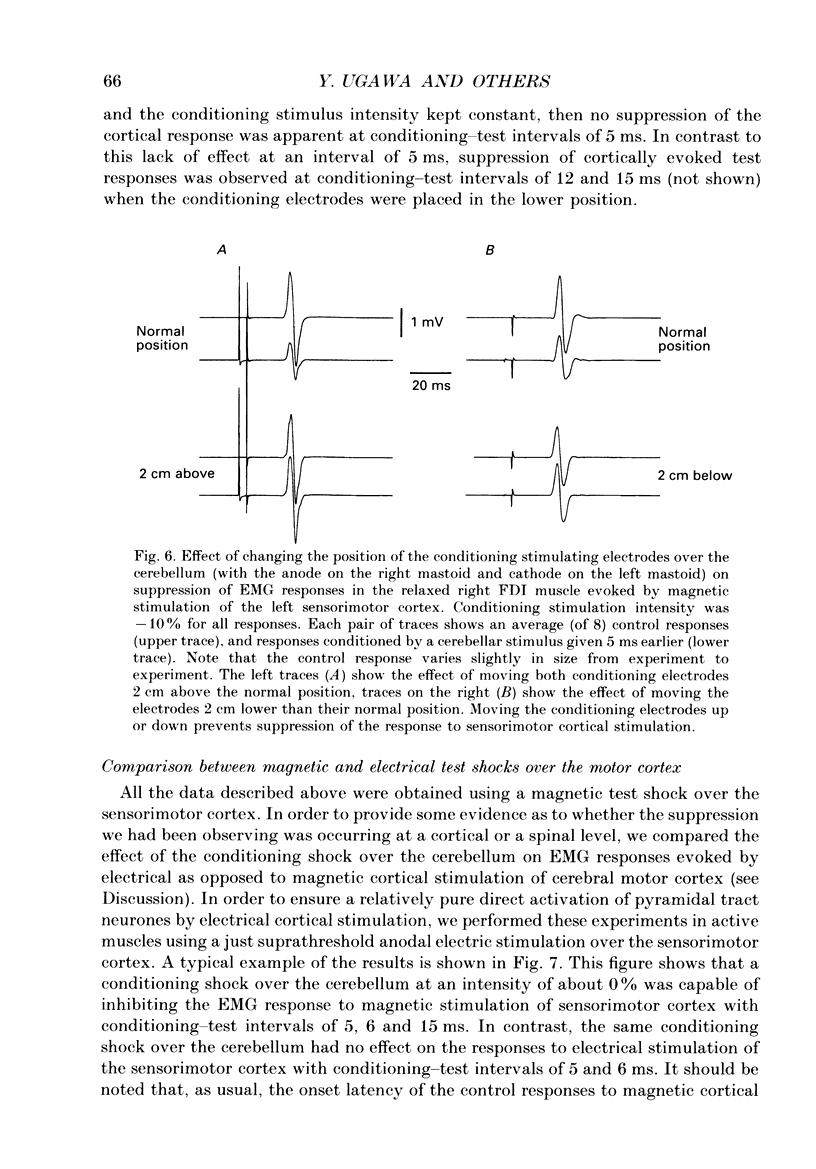
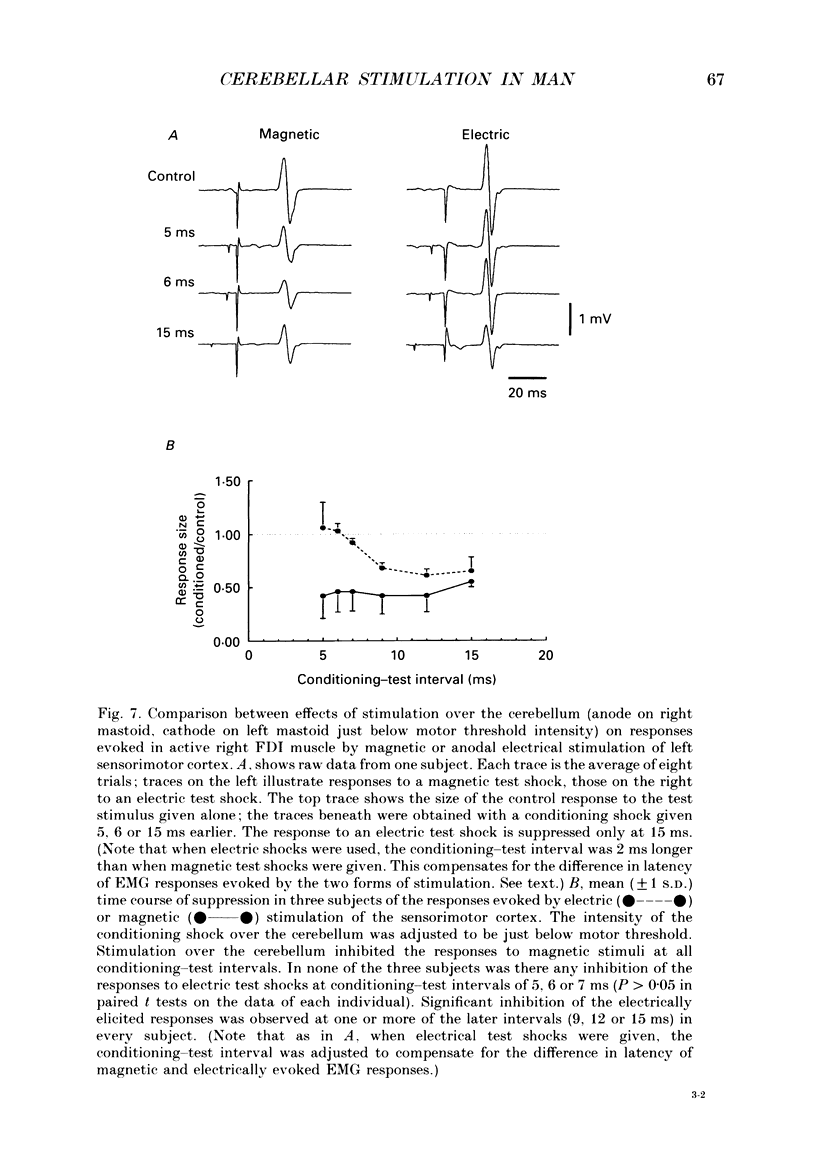
1. We have stimulated over the cerebellum of intact human subjects by applying single electrical stimuli through electrodes placed on the back of the head, approximately at the level of the inion. The intensity of stimulation used was below that required to produce direct EMG responses in pre-activated muscles of the hand. 2. In ten subjects the effect of the stimulus over the cerebellum was to reduce the size of the EMG response in first dorsal interosseous muscle evoked by a magnetic stimulus to the cerebral cortex. In all subjects the onset of the period of suppression occurred when the test magnetic cortical shock followed the conditioning cerebellar shock by 5 ms. The duration of the suppression lasted from 3 to 7 ms. 3. The amount of suppression was related to the intensity of stimulation over the cerebellum. At 15% below the threshold for direct motor activation there was no effect; increasing suppression was evident at 10, 5 and 0% below motor threshold. 4. With a conditioning-test interval of 5-6 ms the suppression was the same whether the target muscle was relaxed or active. With longer conditioning-test intervals (12 and 15 ms) the amount of suppression was greater in active than relaxed muscles. 5. The short-latency suppression was greatest when the stimulating anode was ipsilateral to the target muscle and contralateral to the stimulated sensorimotor cortex. The later period of suppression was insensitive to the polarity of stimulation. When the stimulating electrodes were moved 2 cm caudally or cranially the short latency suppression disappeared whereas the longer latency suppression was still observed with the electrodes in the lower position. 6. Different results were obtained when the test EMG response was produced by an electrical (rather than magnetic) stimulus over the sensorimotor cortex. The short latency effect was no longer visible whereas the longer latency effect was the same as when testing with a magnetic cortical stimulus. 7. We suggest that a single electrical stimulus across the base of the skull (particularly with the anode over one cerebellar hemisphere) produces a short latency (5-6 ms) disfacilitation of the contralateral motor cortex through activation of cerebellar structures. A later (12 and 15 ms), less specific suppression which is present when testing in active muscles is thought to be mediated by a different mechanism and probably produces its effect at the level of the spinal cord.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Cooper I. S., Amin I., Riklan M., Waltz J. M., Poon T. P. Chronic cerebellar stimulation in epilepsy. Clinical and anatomical studies. Arch Neurol. 1976 Aug;33(8):559–570. doi: 10.1001/archneur.1976.00500080037006. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Harrison L. M., Stephens J. A. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol. 1989 Nov;418:13–23. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. R., Corbishley C. M., Richardson P. S. The uptake of radiolabelled precursors of mucus glycoconjugates by secretory tissues in the feline trachea. J Physiol. 1990 Jan;420:19–30. doi: 10.1113/jphysiol.1990.sp017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Dressler D., Maertens de Noordhout A., Marsden C. D., Nakashima K., Rothwell J. C., Thompson P. D. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989 May;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Riescher H., Struppler A., Rothwell J. C., Marsden C. D. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. J Physiol. 1991 Feb;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Eyre J. A., Lemon R. N., Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990 Jun;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Effects on Purkinje cells of surface stimulation of the cerebellum. J Physiol. 1957 Jan 23;135(1):73–92. doi: 10.1113/jphysiol.1957.sp005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Levy W. J., Jr Clinical experience with motor and cerebellar evoked potential monitoring. Neurosurgery. 1987 Jan;20(1):169–182. [PubMed] [Google Scholar]
- Merton P. A., Morton H. B. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980 May 22;285(5762):227–227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Ugawa Y., Rothwell J. C., Day B. L., Thompson P. D., Marsden C. D. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991 Apr;29(4):418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Uno M., Yoshida M., Hirota I. The mode of cerebello-thalamic relay transmission investigated with intracellular recording from cells of the ventrolateral nucleus of cat's thalamus. Exp Brain Res. 1970;10(2):121–139. doi: 10.1007/BF00234726. [DOI] [PubMed] [Google Scholar]


