Abstract
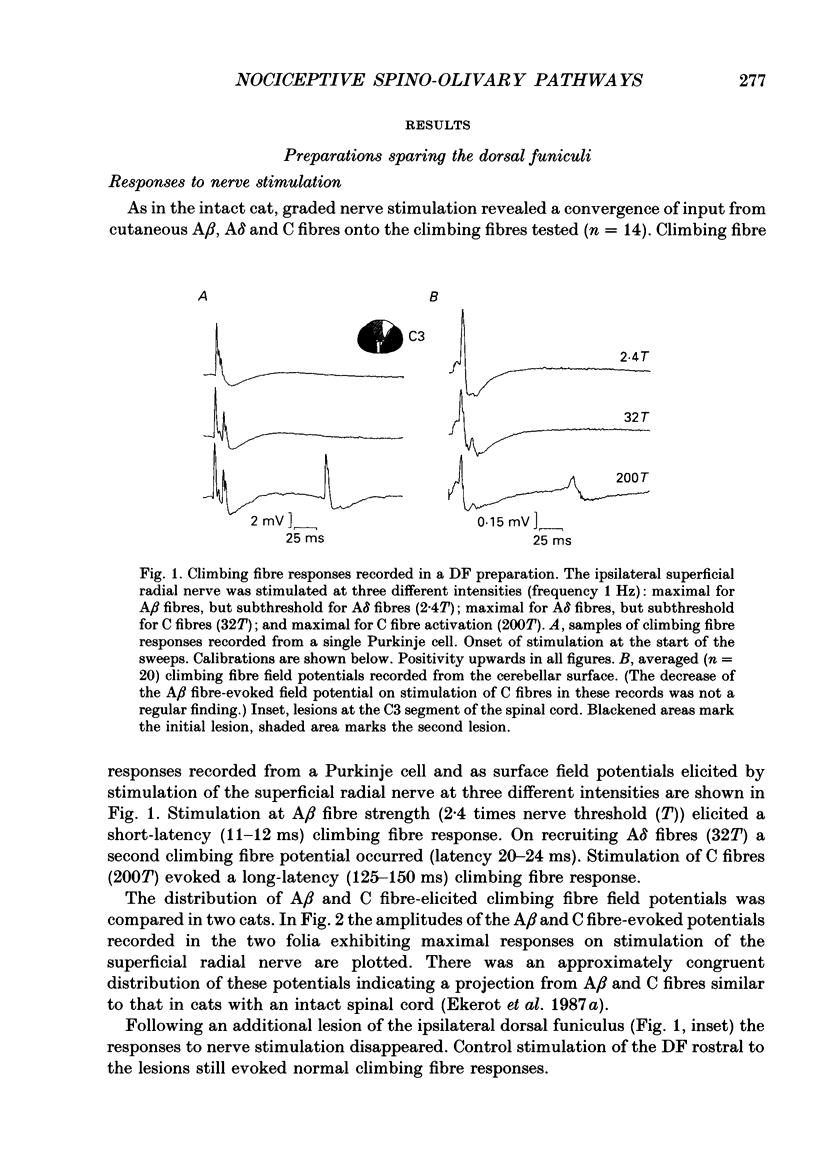
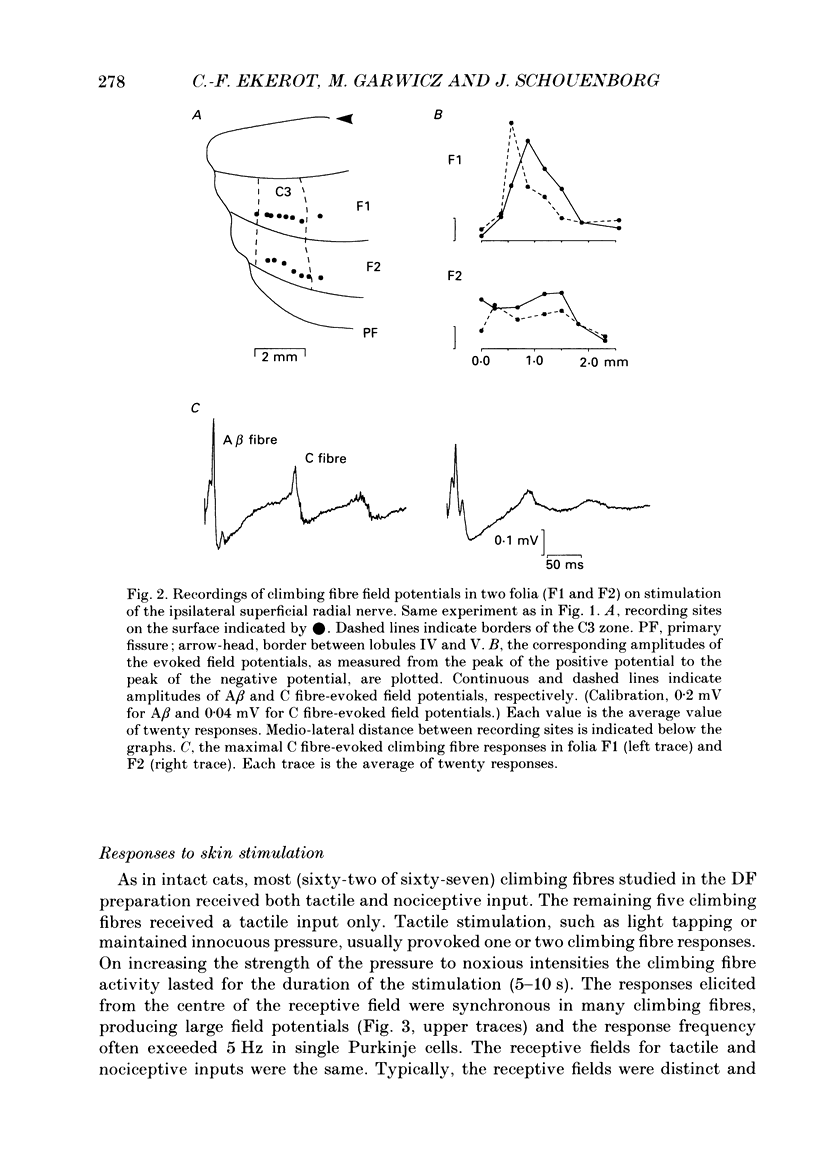
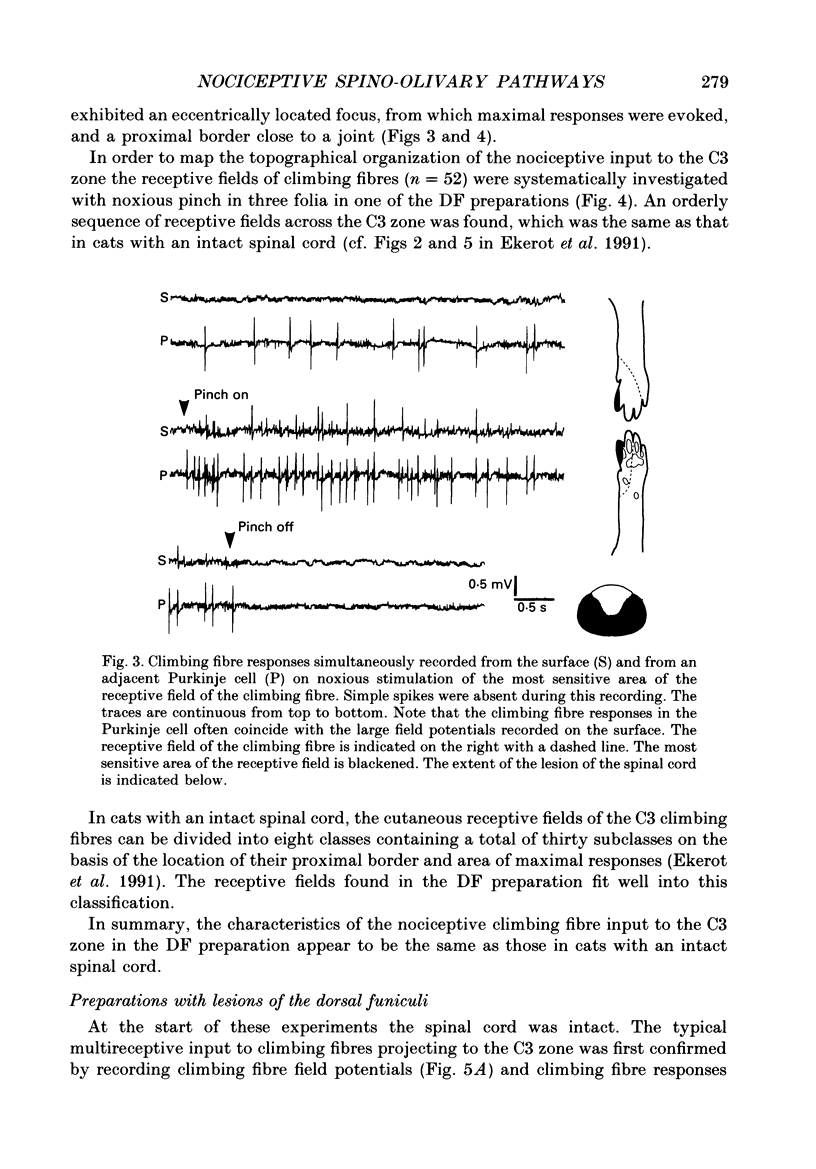
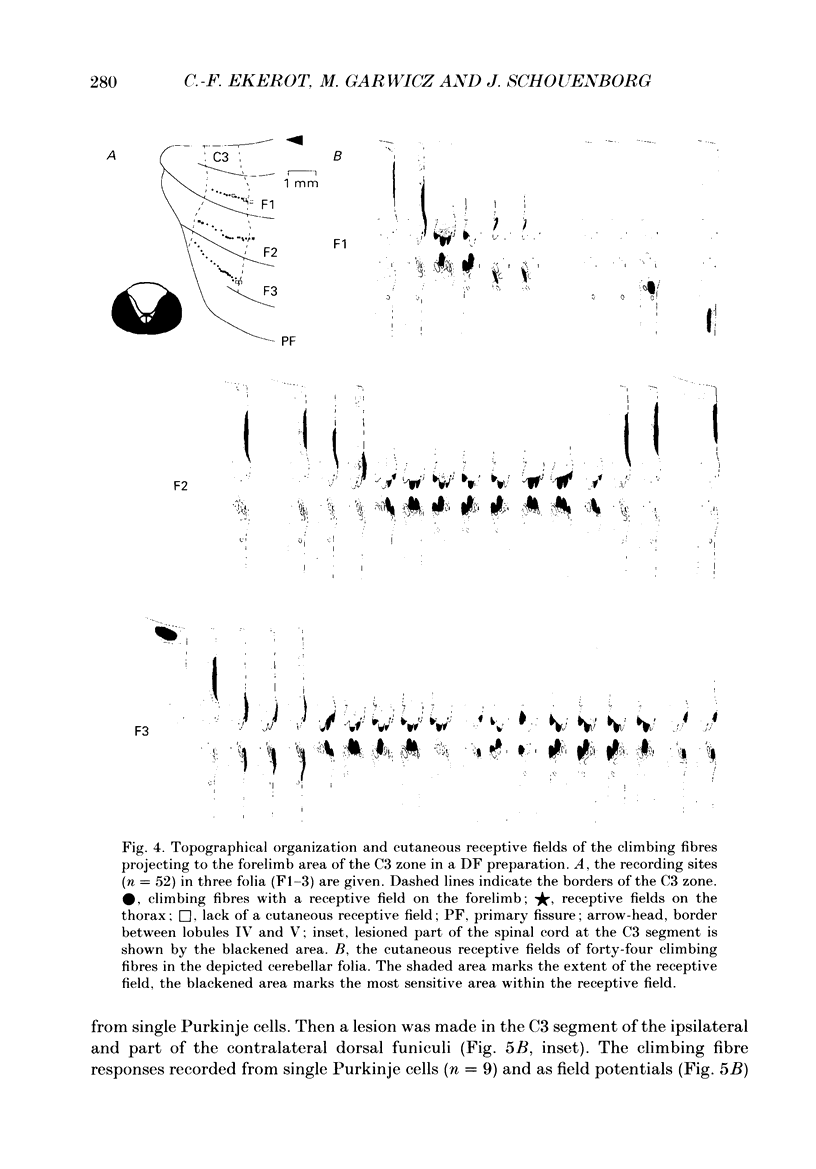
1. The location in the spinal cord of the pathway mediating cutaneous nociceptive C fibre input to climbing fibres projecting to the forelimb area of the C3 zone in the cerebellar anterior lobe was investigated in pentobarbitone-anaesthetized cats. Lesions of the spinal cord at the segmental level of C3 sparing the dorsal funiculi (DF preparation) or lesions of the ipsilateral and part of the contralateral dorsal funiculi were made. 2. In the DF preparation, the cutaneous input to climbing fibres projecting to the C3 zone was the same as in cats with an intact spinal cord. Also, the topography of tactile and nociceptive receptive fields and the distribution of A- and C fibre-evoked climbing fibre field potentials was similar to that in cats with an intact spinal cord. 3. In cats with an initially intact spinal cord the cutaneous nociceptive C fibre input and the topographically well organized tactile input to the C3 climbing fibres disappeared following a lesion of the ipsilateral and part of the contralateral dorsal funiculi. Following this lesion the receptive fields of the climbing fibres became indistinct and only irregular responses were evoked on skin stimulation. 4. It is concluded that the cutaneous nociceptive C fibre input from the forelimb to climbing fibres projecting to the C3 zone is mediated by the ipsilateral dorsal funiculus. Since cutaneous C fibres terminate exclusively in the spinal cord close to their entrance zone the postsynaptic dorsal column pathway must be part of this spino-olivocerebellar pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Nishikawa N., Lu G. W., Hoffert M. J., Dubner R. The morphology of dorsal column postsynaptic spinomedullary neurons in the cat. J Comp Neurol. 1984 Apr 20;224(4):568–578. doi: 10.1002/cne.902240406. [DOI] [PubMed] [Google Scholar]
- Berkley K. J., Budell R. J., Blomqvist A., Bull M. Output systems of the dorsal column nuclei in the cat. Brain Res. 1986 Sep;396(3):199–225. doi: 10.1016/0165-0173(86)90012-3. [DOI] [PubMed] [Google Scholar]
- Boesten A. J., Voogd J. Projections of the dorsal column nuclei and the spinal cord on the inferior olive in the cat. J Comp Neurol. 1975 May 15;161(2):215–237. doi: 10.1002/cne.901610206. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol. 1983 Apr;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbesson S. O. A connection between the dorsal column nuclei and the dorsal accessory alive. Brain Res. 1968 May;8(2):393–397. doi: 10.1016/0006-8993(68)90062-0. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Garwicz M., Schouenborg J. Topography and nociceptive receptive fields of climbing fibres projecting to the cerebellar anterior lobe in the cat. J Physiol. 1991 Sep;441:257–274. doi: 10.1113/jphysiol.1991.sp018750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Gustavsson P., Oscarsson O., Schouenborg J. Climbing fibres projecting to cat cerebellar anterior lobe activated by cutaneous A and C fibres. J Physiol. 1987 May;386:529–538. doi: 10.1113/jphysiol.1987.sp016549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. Branching of olivary axons to innervate pairs of sagittal zones in the cerebellar anterior lobe of the cat. Exp Brain Res. 1982;48(2):185–198. doi: 10.1007/BF00237214. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979 Jul 2;36(2):201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Oscarsson O., Schouenborg J. Stimulation of cat cutaneous nociceptive C fibres causing tonic and synchronous activity in climbing fibres. J Physiol. 1987 May;386:539–546. doi: 10.1113/jphysiol.1987.sp016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevoldson T. P., Gordon G. Postsynaptic dorsal column neurons in the cat: a study with retrograde transport of horseradish peroxidase. Exp Brain Res. 1989;75(3):611–620. doi: 10.1007/BF00249912. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Voogd J., Freedman S. L. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol. 1979 Feb 1;183(3):551–601. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. J Physiol. 1969 Aug;203(3):611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G. W., Bennett G. J., Nishikawa N., Hoffert M. J., Dubner R. Extra- and intracellular recordings from dorsal column postsynaptic spinomedullary neurons in the cat. Exp Neurol. 1983 Nov;82(2):456–477. doi: 10.1016/0014-4886(83)90417-x. [DOI] [PubMed] [Google Scholar]
- Molander C., Grant G. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience. 1986 Sep;19(1):297–312. doi: 10.1016/0306-4522(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Noble R., Riddell J. S. Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J Physiol. 1988 Feb;396:497–513. doi: 10.1113/jphysiol.1988.sp016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969 Jan;200(1):129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustioni A., Kaufman A. B. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res. 1977 Jan 18;27(1):1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the cuneate nucleus in the brachial dorsal funiculus of the cat. Brain Res. 1974 Jul 26;75(2):247–259. doi: 10.1016/0006-8993(74)90745-8. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Kalliomäki J. Functional organization of the nociceptive withdrawal reflexes. I. Activation of hindlimb muscles in the rat. Exp Brain Res. 1990;83(1):67–78. doi: 10.1007/BF00232194. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Lee C. L., Perl E. R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986 Oct 17;234(4774):358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]



