Abstract
Purpose
The clinicopathological or genetic features related to the prognosis of mucinous adenocarcinoma are unknown because of its rarity. The clinicopathological or targetable features were investigated for better management of patients with mucinous adenocarcinoma of the lung.
Methods
We comprehensively evaluated the clinicopathological and genetic features of 60 completely resected mucinous lung adenocarcinomas. Targetable genetic variants were explored using nCounter and polymerase chain reaction, PD-L1 and TTF-1 expression were evaluated using immunohistochemistry. We analyzed the prognostic impact using the Kaplan–Meier method and log-rank test.
Results
Of the 60 enrolled patients, 13 (21.7%) had adenocarcinoma in situ/minimally invasive adenocarcinoma, and 47 (78.3%) had invasive mucinous adenocarcinoma (IMA). Fifteen patients (25%) showed a pneumonic appearance on computed tomography (CT). CD74-NRG1 fusion, EGFR mutations, and BRAF mutation were detected in three (5%), four (6.7%), and one (1.7%) patient(s), respectively. KRAS mutations were detected in 31 patients (51.7%). Two patients (3.5%) showed immunoreactivity for PD-L1. No in situ or minimally invasive cases recurred. IMA patients with pneumonic appearance had significantly worse recurrence-free survival (RFS) and overall survival (OS) (p < 0.001). Furthermore, IMA patients harboring KRAS mutations had worse RFS (p = 0.211). Multivariate analysis revealed that radiological pneumonic appearance was significantly associated with lower RFS (p < 0.003) and OS (p = 0.012). KRAS mutations served as an unfavorable status for RFS (p = 0.043).
Conclusion
Mucinous adenocarcinoma had a low frequency of targetable genetic variants and PD-L1 immunoreactivity; however, KRAS mutations were frequent. Pneumonic appearance on CT imaging and KRAS mutations were clinicopathological features associated with a worse prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03609-3.
Keywords: Mucinous adenocarcinoma, nCounter, NRG1, PD-L1, KRAS
Introduction
Invasive mucinous adenocarcinoma (IMA) of the lung is a variant subtype of lung adenocarcinoma that has a low frequency (Travis et al. 2011). The prognosis of IMA varies widely (Russell et al. 2011; Warth et al. 2012; Yoshizawa et al. 2013; Shim et al. 2015; Watanabe et al. 2015; Lee et al. 2016), and a mucinous component can be present in non-invasive or minimally invasive adenocarcinoma. Previous studies report that the unique characteristics of IMA include pneumonia-like radiographic appearance and high frequency of KRAS G12D or G12V mutations (Shim et al. 2015; Trombetta et al. 2018). In addition to its natural history, the relationships between clinicopathological characteristics, genetic profile, and prognosis in patients with mucinous adenocarcinoma are little known because of the rarity of this type of adenocarcinoma. For further understanding of the biological profile and better clinical management, we investigated this relationship through a comprehensive analysis of targetable or non-targetable genetic/protein profiles and a review of the clinicopathological features of adenocarcinoma with mucinous products.
Materials and methods
Study design
We retrospectively reviewed cases of mucinous adenocarcinoma resected between April 1997 and December 2018 at Hiroshima University Hospital. Clinicopathological data were collected from medical records, and radiological findings were reviewed on preoperative computed tomography (CT). Cases presenting with tumor masses as solitary nodules and accompanying consolidation on CT image were classified as solitary and pneumonic type, respectively (Fig. 1). Pathological diagnoses were performed by two pathologists according to the World Health Organization 2015 classification (Travis et al. 2015). Patients diagnosed with mucinous adenocarcinoma who underwent resection before 2015 were reviewed for this study. The staging was performed as per the International Association for the Study of Lung Cancer (IASLC) 8th TNM classification (Goldstraw et al. 2016). Genetic variants were assessed using mutation-targeted polymerase chain reaction (PCR) assays or nCounter. Positive results in nCounter were validated using fluorescent in situ hybridization (FISH) or immunohistochemistry (IHC). As non-genetic therapeutic profiles or unique features of mucinous adenocarcinoma, the expressions of PD-L1 and TTF-1 were estimated using IHC. Cases without available tissue samples or wherein the patient died within 90-day post-operation due to causes other than lung cancer, were excluded from the analysis. Cases after preoperative chemotherapy or chemoradiotherapy were also excluded. Informed consent was obtained from the patients to stock tissues for research. The study protocol was approved by the institutional review board at Hiroshima University (no. E–1546).
Fig. 1.
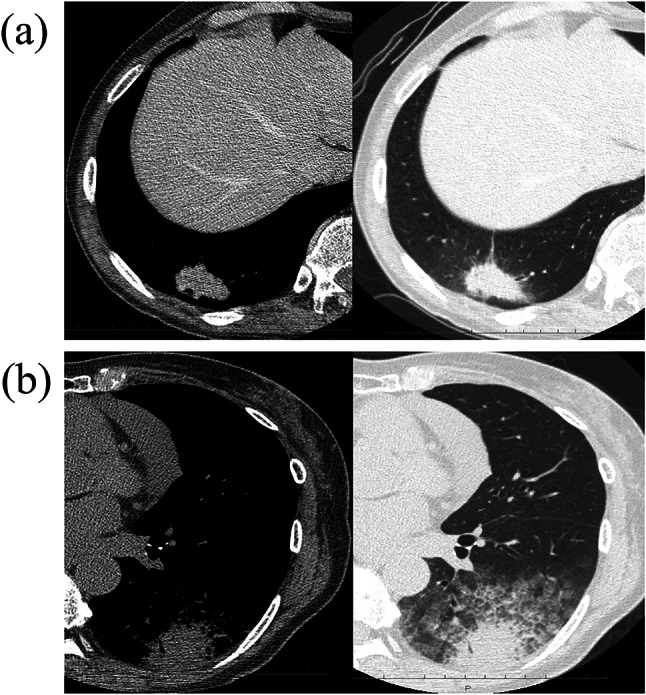
Representative computed tomography images of invasive mucinous adenocarcinoma cases. Mediastinal and lung window computed tomography images of mucinous adenocarcinoma with solitary appearance (a) and pneumonic appearance (b)
Comprehensive evaluation of targetable or representative genetic variations
The nCounter Elements system utilizes a non-enzymatic protocol based on digital color-coded barcoded probes and enables simultaneous counting of multiple gene fusion events in a single tube. In our study, total RNA was directly hybridized with a custom-designed multiplexed mixture of biotinylated capture tags and fluorescently labeled reporter probes located upstream (Elements Chemistry) complementary to ALK, ROS1, RET, NTRK1, NTRK2, NTRK3, METΔ14, and NRG1 target sequences. The mixture was designed and synthesized by NanoString Technologies Inc (Seattle, WA). The nCounter codeset used allowed for the detection of gene fusions based on pairs of molecular-barcoding junction probes designed to bind to specific fusion transcripts. Detailed sequence information for the NRG1 fusions target regions is provided in Online Resource 1. The codeset also contained probes for housekeeping genes (ACTB, GAPDH, PSMC4, and MRPL19), positive, and negative controls. All processes of hybridization, capture, cleanup, and digital data acquisition were performed using nCounter Prep Station™ and Digital Analyzer™ (NanoString Technologies) according to the manufacturer’s instructions. Reporter counts were collected using the nSolver analysis software version 2.6 and normalized as described in two steps, using the negative controls and the housekeeping genes as reference (Reguart et al. 2017). The average normalized data of the 56 evaluable samples included in the study plus two standard deviations (SD) were used to define the cut-off for each specific fusion. Other targetable variants, mutations in EGFR and BRAF were evaluated using real-time PCR as previously described (Ito et al. 2018,2019). Although KRAS somatic mutations were not targetable in the clinical setting, they were also assessed as frequently detected variants in mucinous adenocarcinoma cases and potential druggable targets. KRAS mutations were detected via direct sequencing PCR assay. The sequences of primers for KRAS are described in Online Resource 2. Regarding EGFR/KRAS/BRAF mutations detection, each primer and probe was validated to detect mutations precisely using customed gene fragments with or without mutation (gBlocks, Integrated DNA Technologies).
Validation of the results of nCounter
We validated positive results obtained via nCounter using FISH or IHC. FISH for the detection of NRG1 translocations was performed on 3-μm-thick Formalin-Fixed Paraffin-Embedded (FFPE) tissue sections using ZytoLight SPEC NRG1 Dual Color Break Apart Probe (Z-2028–20; ZytoVision, Bremerhaven, Germany) according to the manufacturer’s instructions. At least 50 tumor cells per sample were counted, and cases were considered positive if they contained at least 15% of tumor cells with either split red and green signals or single red signals corresponding to either translocations or losses of the 5′ end, respectively. Positive immunoreactivity of phosphorylated HER3 was also indicative of the presence of NRG1 fusion, as previously described (Trombetta et al. 2018; Drilon et al. 2018). IHC was performed on 3-μm whole-tumor sections using Phospho-HER3/ErbB3 (Tyr1289) (21D3) antibody (4791; Cell Signaling Technology, Danvers, MA, USA; 1:50 dilution) on a BenchMark ULTRA automated tissue staining system (Ventana Medical Systems, Tucson, Arizona, USA). Cases presenting membrane or cytoplasmic staining in ≥ 10% of tumor cells were considered positive.
Immunohistochemistry for PD-L1 and TTF-1 expression
IHC for PD-L1 (205,921; Abcam, Cambridge, MA, USA; 1:400 dilution) and TTF-1 (NCL-L-TTF-1; Leica Biosystems, Buffalo Grove, IL, USA; 1:50 dilution) was performed using FFPE tissue samples. Tissues sliced into 4-µm-thick sections were stained using an automatic IHC device (Roche Ventana BenchMark ULTRA Slide Stainer; Roche Diagnostics, Florham Park, NJ, USA). PD-L1 expression was estimated as positive if the cell membranes were stained in at least 1% of tumor cells. Positive expression of PD-L1 was further subclassified if stained cells exceeded 50% of tumor cells (> 50%) or did not (1–50%). TTF-1 was evaluated as positive if the nucleus was stained. Positive TTF-1 expression was subdivided according to the ratio of positive cells into positive (> 50%) or focally positive (1–50%).
Statistical analysis
Recurrence-free survival (RFS) and overall survival (OS) were determined in the prognostic analysis. RFS was defined as the period from the day of operation to the date of recurrence or death from any cause. OS was defined as the period from the day of operation to the date of death from any cause. The RFS and OS were calculated using the Kaplan–Meier method, and the differences were analyzed using the log-rank test. The significance of frequencies was evaluated using the Chi-squared test or Yates’ Chi-squared test. Patient ages were compared as continuous variables using the Mann–Whitney U tests. The impact of each variable on the RFS or OS was evaluated using univariate and multivariate analyses with the Cox proportional hazards model. All statistical analyses were performed using JMP version 14.2.0 (SAS Institute Inc., Cary, NC, USA). Two-tailed test items with p value of less than 0.05 were regarded as significant.
Results
Clinicopathological features
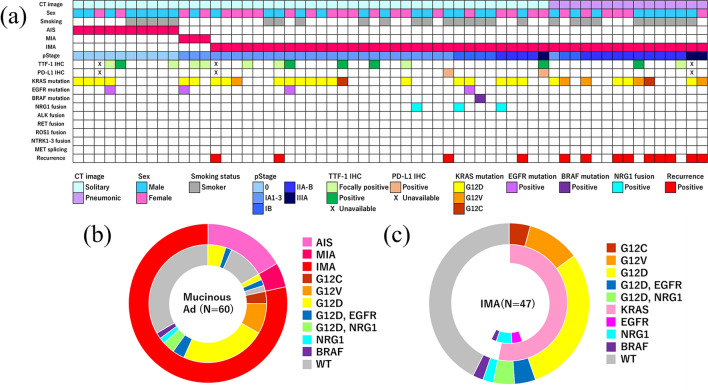
We reviewed a total of 63 mucinous adenocarcinoma cases. Three cases were excluded because of the unavailability of samples (one case) or patient death from another type of cancer within 90-day post-operation (two cases). The clinicopathological and targetable features of the remaining 60 patients included in the study are shown in Table 1. The median age of the patients was 71 years (range 45–86) and 53.3% (32/60) were male. Twenty-eight of the 60 patients (46.7%) were ex- or current smokers. The median follow-up duration was 55 months (range 1–148). Twenty-five percent (15/60) of patients showed features characteristic of pneumonia on CT scans. Ten and three patients were diagnosed with mucinous adenocarcinoma in situ and minimally invasive mucinous adenocarcinoma, respectively. All patients with in situ and minimally invasive status showed solitary appearance on CT scans and no recurrence. Among IMA cases, 31.9% (15/47) showed pneumonic appearance on CT scans and 27.7% (13/47) cases relapsed. Sixteen IMA patients received adjuvant chemotherapy with various regimens and treatment terms. However, the relapse ratios in patients, with or without adjuvant chemotherapy, were similar (25% vs. 29%, respectively). The adjuvant chemotherapies were not obviously effective.
Table 1.
Clinicopathological and targetable features of the included mucinous adenocarcinoma cases (n = 60)
| Clinicopathological characteristics | Number of cases |
|---|---|
| Age, years | |
| Median (range) | 71 (45–86) |
| Interquartile range | 65.0–75.0 |
| Sex, n (%) | |
| Male/female | 32 (53.3)/28 (46.7) |
| Smoking status, n (%) | |
| Never smoker | 32 (53.3) |
| Ex- or current smoker | 28 (46.7) |
| Radiological feature, n (%) | |
| Solitary type | 45 (75.0) |
| Pneumonic type | 15 (25.0) |
| Surgical procedure, n (%) | |
| Pneumonectomy | 1 (1.7) |
| Lobectomy | 40 (66.7) |
| Segmentectomy | 14 (23.3) |
| Wedge resection | 5 (8.3) |
| Invasive status, n (%) | |
| In situ | 10 (16.7) |
| Minimally invasive | 3 (5.0) |
| Invasive | 47 (78.3) |
| Genetic variant status, n (%) | |
| EGFR mutation | 4 (6.7) |
| BRAF mutation | 1 (1.7) |
| KRAS mutation | 31 (51.7) |
| G12D/V/C | 24 (40.0)/5 (8.3)/2 (3.3) |
| CD74-NRG1 fusion | 3 (5.0) |
| Wild type | 27 (45.0) |
| Immunoreactivity*, n (%) | |
| TTF-1 positive/focally positive | 6 (10.5)/8 (14.0) |
| PD-L1 positive | 2 (3.5) |
| Lymphovascular invasion, n (%) | 4 (6.7) |
| Nodal metastasis, n (%) | |
| N0/N1/N2 | 58 (96.7)/1 (1.7)/1 (1.7) |
| pStage, n (%) | |
| 0 | 10 (16.7) |
| IA1/IA2/IA3 | 9 (15.0)/12 (20.0)/6 (10.0) |
| IB | 6 (10.0) |
| IIA/IIB | 3 (5.0)/11 (18.3) |
| IIIA | 3 (5.0) |
| Recurrence, n (%) | 13 (21.7) |
AIS adenocarcinoma in situ, MIA minimally invasive adenocarcinoma, SUV standard uptake value
*Estimated among 57 available cases
Comprehensive profile of genetic variants
The nCounter detected CD74-NRG1 fusion in three IMA cases (Online Resource 3). Positive results in nCounter were validated using FISH or IHC (Fig. 2). No targetable fusion or splicing was detected in ALK, RET, ROS1, NTRK1-3, or MET. Targetable EGFR and BRAF mutations were detected in four and one case, respectively. KRAS mutations (G12D, G12V, and G12C) were detected in twenty four, five, and two cases, respectively. KRAS mutations in IMA were unrelated to any clinicopathological features (Table 2). All patients with EGFR mutations harbored KRAS G12D mutation Overall, targetable variants by clinically available drugs were detected only in EGFR, BRAF, and NRG1. The frequencies of targetable variants in all mucinous adenocarcinoma and IMA cases were 13.3% (8/60) and 12.8% (6/47), respectively.
Fig. 2.
FISH and IHC images validating CD74-NRG1 fusion. FISH suggested a split signal pattern (red and green arrows) or 3′isolated signal pattern (green arrow) of NRG1. Yellow arrow suggested a fused signal pattern (negative signal) (a). IHC showed immunoreactivity of phosphorylated HER3 in the membrane and/or cytoplasm of tumor cells. The representative area was marked with a red square and enlarged (b). FISH fluorescent in situ hybridization, IHC immunohistochemistry
Table 2.
Comparison of clinicopathological features between KRAS mutation positive and negative invasive mucinous adenocarcinoma cases (n = 47)
| Clinicopathological characteristics |
KRAS mutation status | p value | |
|---|---|---|---|
| Positive (n = 25) |
Negative (n = 22) |
||
| Age, years | |||
| Median (range) | 71.0 (45–86) | 70.5 (59–81) | 0.349 |
| Interquartile range | 62.5–75.5 | 65.0–76.3 | |
| Sex, n (%) | |||
| Male | 10 (40.0) | 13 (59.1) | 0.311 |
| Female | 15 (60.0) | 9 (40.9) | |
| Smoking status, n (%) | |||
| Never smoker | 15 (60.0) | 9 (40.9) | 0.311 |
| Ex- or current smoker | 10 (40.0) | 13 (59.9) | |
| Radiological feature, n (%) | |||
| Solitary | 16 (64.0) | 16 (72.3) | 0.744 |
| Pneumonic | 9 (36.0) | 6 (27.3) | |
| Surgical procedure, n (%) | |||
| Pneumonectomy | 0 (0.0) | 1 (4.6) | 0.948 |
| Lobectomy | 21 (84.0) | 17 (77.2) | 0.831 |
| Segmentectomy | 4 (16.0) | 3 (13.6) | 0.854 |
| Wedge resection | 0 (0.0) | 1 (4.6) | 0.948 |
| Genetic variant status, n (%) | |||
| EGFR mutation | 2 (8.7) | 0 (0.0) | 0.489 |
| BRAF mutation | 0 (0.0) | 1 (4.5) | 0.982 |
| NRG1 fusion | 2 (8.7) | 1 (4.5) | 0.968 |
| Wild type | 19 (82.6) | 20 (91.0) | 0.704 |
| Immunoreactivity, n (%) | |||
| TTF-1* | |||
| Negative | 18 (80.1) | 18 (19.2) | 0.941 |
| Positive/focally positive | 3 (9.5)/2 (9.5) | 2 (12.5)/2 (8.3) | |
| PD-L1* | |||
| Negative | 23 (100) | 20 (90.9) | 0.450 |
| Positive | 0 (0.0) | 2 (9.1) | |
| Lymphovascular invasion, n (%) | |||
| Negative | 23 (92.0) | 20 (91.0) | 0.696 |
| Positive | 2 (8.0) | 2 (9.0) | |
| Nodal metastasis, n (%) | |||
| Negative | 24 (96.0) | 21 (95.8) | 0.528 |
| Positive | 1 (4.0) | 1 (4.2) | |
| pStage, n (%) | |||
| IA1/IA2/IA3 | 11 (44.0) | 13 (59.1) | 0.301 |
| IB | 3 (12.0) | 4 (18.2) | 0.854 |
| IIA/IIB | 9 (36.0) | 4 (18.2) | 0.300 |
| IIIA | 2 (8.0) | 1 (4.5) | 0.909 |
| Recurrence, n (%) | |||
| Negative | 15 (60.0) | 19 (82.4) | 0.091 |
| Positive | 10 (40.0) | 3 (13.6) | |
AIS adenocarcinoma in situ, MIA minimally invasive adenocarcinoma, SUV standard uptake value
*Estimated among 54 available cases
TTF-1 and PD-L1 protein expression
Samples were unavailable for IHC analysis in three cases. TTF-1 tests yielded positive or focally positive results in 10.5% (6/57) and 14.0% (8/57) cases, respectively. PD-L1 tests yielded positive results in 3.6% (2/55) of cases. No case showed strong immunoreactivity (> 50% staining) to PD-L1. The patient clinicopathological profile, genetic variants, and results of IHC are shown in Fig. 3.
Fig. 3.
Clinicopathological features and distribution of genetic variations. The heatmap shows the clinicopathological features, results of genetic estimation or IHC, and recurrence status among 60 mucinous adenocarcinoma cases (a). Pie chart showing the distribution of genetic variations in all cases of mucinous adenocarcinoma (b) and IMA (c). Ad, adenocarcinoma; AIS, adenocarcinoma in situ; IMA invasive mucinous adenocarcinoma, MIA minimally invasive adenocarcinoma, WT wild type
Prognostic impact of clinical features and genetic variants
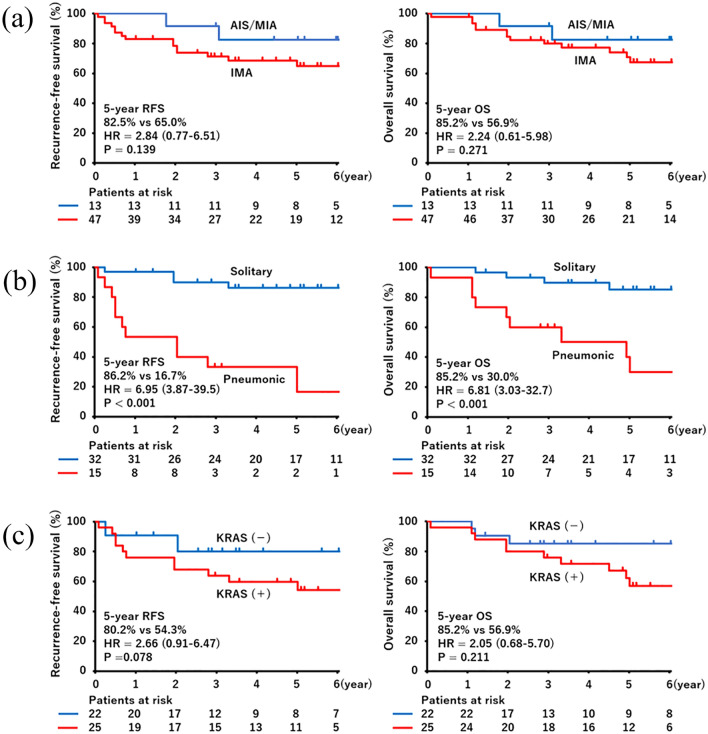
All mucinous adenocarcinoma in situ and minimally invasive mucinous adenocarcinoma patients showed solitary appearance on CT scans and no recurrence. Thirteen patients with IMA showed recurrence, four with the solitary type and nine with the pneumonic type. The pulmonary region was the site of recurrence in all relapsed cases. Two cases were accompanied by relapse at distant sites (bone or liver). Even after non-IMA patients were excluded from the analysis, the RFS was worse in patients with the pneumonic type than in those with solitary type (5-year RFS 16.7% vs. 86.2%, respectively; hazard ratio [HR] = 6.95, 95% confidence interval [CI]: 3.87–39.5, p < 0.001) among IMA. The OS was also worse in the pneumonic type (5-year OS 30.0% vs. 85.2%, respectively; HR = 6.81, 95% CI 3.03–32.7, p < 0.001). IMA patients harboring KRAS mutations had worse RFS than those without KRAS mutation (5-year RFS 54.3% vs. 80.2%, respectively; HR = 2.66, 95% CI 0.91–6.47, p = 0.078). The OS was also worse in IMA patients harboring KRAS mutations (5-year OS 56.9% vs. 85.2%, respectively; HR = 2.05, 95% CI 0.68–5.70, p = 0.211). Multivariate Cox proportional hazards analysis of IMA revealed that radiological features characteristic of pneumonia were significantly related to a worse RFS (HR = 7.080; 95% CI 1.943–25.805, p = 0.003) and OS (HR = 4.844, 95% CI 1.389–16.894, p = 0.012), and KRAS mutation was an unfavorable status for RFS (HR = 3.300; 95% CI 1.039–10.481, p = 0.043), but not for OS (HR = 2.475; 95% CI 0.702–8.730, p = 0.159) (Table 3).
Table 3.
Univariate and multivariate analyses of recurrence-free survival and overall survival in invasive mucinous adenocarcinoma cases (n = 47)
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Recurrence-free survival | ||||
| Sex (male) |
2.547 (0.871–7.454) |
0.0877 |
1.346 (0.239–7.571) |
0.736 |
| Age |
1.033 (0.972–1.107) |
0.317 |
0.997 (0.941–1.060) |
0.931 |
| Ex- or current smoking |
3.181 (1.001–10.046) |
0.049 |
1.378 (0.201–9.474) |
0.744 |
|
Radiological feature (pneumonic type) |
7.830 (2.647–23.164) |
< 0.001 |
7.080 (1.943–25.805) |
0.003 |
|
KRAS mutation status (positive) |
2.756 (0.886–8.571) |
0.080 |
3.300 (1.039–10.481) |
0.043 |
| Overall survival | ||||
| Sex (male) |
2.514 (0.443–1.417) |
0.126 |
0.837 (0.127–5.510) |
0.854 |
| Age |
1.022 (0.957–1.101) |
0.549 |
1.001 (0.935–1.076) |
0.986 |
| Ex- or current smoking |
4.226 (1.152–15.499) |
0.030 |
4.344 (0.555–38.989) |
0.162 |
|
Radiological feature (pneumonic type) |
6.582 (2.002–21.641) |
0.002 |
4.844 (1.389–16.894) |
0.012 |
|
KRAS mutation status (positive) |
1.934 (0.589–6.348) |
0.278 |
2.475 (0.702–8.730) |
0.159 |
CI confidence interval, HR hazard ratio
Discussion
Our comprehensive analysis revealed that the frequency of the genetic variants, which are targetable by clinically available drug were low in mucinous adenocarcinoma of the lung (13.3%, 8/60) and IMA (12.8%, 6/47). NRG1 fusion is a promising form of targetable fusion (Cheema et al. 2017; Gay et al. 2017; Drilon et al. 2018). However, its clinical utility is not confirmed at present. In addition, although KRAS mutations were frequently detected, the drug targeting these mutations are not available in clinical setting. EGFR mutations were detected more frequently among in situ or minimally invasive cases than in IMA cases (15.4%, 2/13 vs. 4.3%, 2/47) and the total frequency of EGFR mutation was low even in the Asian adenocarcinoma cohort (6.7%, 4/60).
Furthermore, only 4.3% (2/47) of cases were positive for PD-L1 on IHC. These results imply that molecular-targeting or immune therapy will be little used in IMA cases. Identifying the clinicopathological features related to prognosis or potential targetable genetic variants will be useful to understand the natural course and provide better clinical management procedure in mucinous adenocarcinoma of the lung.
The frequency of IMA is low (2.27–9.8% (Russell et al. 2011; Yoshizawa et al. 2013; Lee et al. 2016; Takahashi et al. 2018). Mucinous components may accompany not only invasive adenocarcinoma but also non- or minimally invasive cases. A previous study suggested that in situ or minimally invasive cases did not recur among patients with mucinous adenocarcinoma (Watanabe et al. 2015). The malignant grade of IMA is controversial and the reported OS varies widely from 20.0 to 90% (Russell et al. 2011; Warth et al. 2012; Yoshizawa et al. 2013; Shim et al. 2015; Watanabe et al. 2015; Lee et al. 2016). Because of its rarity, it is challenging to collect IMA cases of similar stages, follow-up durations, or treatment. The prognosis may depend on the cohort. To refine the prognosis of IMA, radiological features are useful (Watanabe et al. 2015; Lee et al. 2016). In our study, in situ or minimally invasive cases were free from recurrence (two patients died from interstitial pneumonia or bile duct cancer) and the 5-year RFS and OS in patients with IMA were 65.0% and 70.3%, respectively (Fig. 4a). All in situ and minimally invasive cases showed solitary appearance on CT scans. As previously reported (Watanabe et al. 2015; Lee et al. 2016), the solitary type of IMA showed a better prognosis than the pneumonic type (Fig. 4b). It stems from the difference in staging. Radiological features highly corresponded to pStage; 85.7% (30/35) of solitary type cases were pStage IA1–IB, whereas 80.0% (12/15) of pneumonic type were pStage IIA–III in our study. Therefore, radiological features are related to prognosis.
Fig. 4.
Survival curves according to CT image and/or KRAS G12D status. RFS and OS curves by histological subtype (a), and in 47 IMA cases according to radiological feature (b) or KRAS G12D/V status (c). AIS adenocarcinoma in situ, CT computed tomography, IMA invasive mucinous adenocarcinoma, MIA minimally invasive adenocarcinoma, OS overall survival, RFS recurrence-free survival
In addition to the radiological features, we suggested that the genetic profile can also be useful for predicting prognosis. Cases harboring KRAS mutations were more aggressive than those without them (Fig. 4c). The frequency of KRAS mutations in mucinous adenocarcinoma has been reported to be 63–75% (Shim et al. 2015; Watanabe et al. 2015). G12D and G12V were the most common KRAS mutations in mucinous adenocarcinoma (Shim et al. 2015). Maeda et al. reported that G12D and negative TTF-1 status were associated with tumorigenesis in adenocarcinoma with mucin (Maeda et al. 2012). Another group reported that lymphatic invasion is likely to be present among IMA cases harboring KRAS mutations (Watanabe et al. 2015); This tendency was confirmed in our study and the frequency of PD-L1 and TTF-1 immunoreactivity was low as previously described (Guo et al. 2017). Several studies have shown that KRAS mutation is related to a worse prognosis (Izar et al. 2014; Kneuertz et al. 2020), suggesting that KRAS drives higher malignant potential as an oncogenic variant. Although there was no significance because of the low number of cases, patients harboring KRAS mutations showed inferior PFS and OS in our study. KRAS G12D/G12V mutation might play a major role in tumorigenesis and may be useful as a prognostic predictor in IMA. Studies including a large number of cases aimed at estimating KRAS status are warranted for further understanding of mucinous adenocarcinoma cases.
Our study has some limitations. The number of included cases was small. We collected data on 60 cases resected over 21 years. In the long term, the pathological diagnosis and treatment of choice for lung cancer have changed. The clinical management and devices for follow-up have also progressed. Therefore, considering all cases uniformly as resected cases might be inappropriate in such a long-term study. Multi-center analysis is warranted to overcome this challenge. Several cases harbored co-existent driver variants: KRAS/EGFR and KRAS/NRG1. In previous reports, NRG1 fusion was assessed among cases without KRAS mutation (Nakaoku et al. 2014; Shim et al. 2015), and other forms of fusion or mutation were not explored in KRAS-mutant cases (Jinesh et al. 2018) because driver variants are fundamentally mutually exclusive. However, co-existence of onco-driver genes can be detected in a high-sensitive comprehensive analysis (Kim et al. 2016; Yoda et al. 2018; Cho et al. 2020). Although KRAS G12C is a potentially targetable variant (Caruso 2019), there is no clinical evidence that KRAS G12D/G12V mutations are also onco-driver variants as therapeutic targets in lung adenocarcinoma. Analysis of newly discovered targetable variants may reveal other candidates as onco-driver variants or another role of G12D/G12V in IMA cases.
In conclusion, targeted therapy or immune-check point inhibitors will be of little use in mucinous adenocarcinoma of the lung due to the low frequency of targetable genetic variants or PD-L1 expression. Even when accompanied by a mucinous component, cases with in situ or minimally invasive status do not relapse. However, invasive mucinous adenocarcinoma is characterized by radiological pneumonic features and high frequency of KRAS G12D/G12V mutations, which are related to worse prognosis. Estimating the radiological and KRAS mutation status are important for better understanding of the malignant potential and clinical management of this rare type of tumor.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was conducted in part at the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University, Hiroshima, Japan. The authors thank Editage (www.editage.jp) for English language editing.
Author contributions
Conceptualization and Data Curation: DU, MI, YaT, and YM. Formal analysis: DU, MI, AGC, CA, and MMV. Investigation: DU, MI, AGC, RRL, APR, CA, KK, KA, and YuT. Supervision: MO and RR. Visualization: DU, MI, RRL, and MMV. Writing: all authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data is original from our study. No data from public or shared database was utilized.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study protocol was approved by the institutional review board at Hiroshima University (no. E–1546).
Consent to participate
Informed Consent was obtained from patients to stock tissues for research.
Consent for publication
Informed Consent was obtained from patients to for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Caruso C (2019) AMG 510 first to inhibit “Undruggable” KRAS. Cancer Discov 9:988–989 [DOI] [PubMed] [Google Scholar]
- Cheema PK, Doherty M, Tsao MS (2017) A case of invasive mucinous pulmonary adenocarcinoma with a CD74-NRG1 fusion protein targeted with afatinib. J Thorac Oncol 12:e200–e202. 10.1016/j.jtho.2017.07.033 [DOI] [PubMed] [Google Scholar]
- Cho JH, Lim SH, An HJ, Kim KH, Park KU, Kang EJ, Choi YH, Ahn MS, Lee MH, Sun JM, Lee SH (2020) Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09). J Clin Oncol 10(38):488–495. 10.1200/JCO.19.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon A, Somwar R, Mangatt BP, Edgren H, Desmeules P, Ruusulehto A, Smith RS, Delasos L, Vojnic M, Plodkowski AJ, Sabari J (2018) Response to ERBB3-directed targeted therapy in NRG1 \-rearranged cancers. Cancer Discov 8:686–695. 10.1158/2159-8290.CD-17-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay ND, Wang Y, Beadling C, Warrick A, Neff T, Corless CL, Tolba K (2017) Durable response to afatinib in lung adenocarcinoma harboring NRG1 gene fusions. N Thorac Oncol 12:e107–e110. 10.1016/j.jtho.2017.04.025 [DOI] [PubMed] [Google Scholar]
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, Rami-Porta R (2016) The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 11:39–51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, Karns R, Warth A, Fink-Baldauf IM, Nagayasu T, Naomoto Y (2017) Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med 9:462–481. 10.15252/emmm.201606711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Miyata Y, Kushitani K, Yoshiya T, Kai Y, Tsutani Y, Mimura T, Konishi K, Takeshima Y, Okada M (2018) Increased risk of recurrence in resected EGFR-positive pN0M0 invasive lung adenocarcinoma. Thorac Cancer 9:1594–1602. 10.1111/1759-7714.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Miyata Y, Hirano S, Kimura S, Irisuna F, Ikeda K, Kushitani K, Kishi N, Tsutani Y, Takeshima Y, Okada M (2019) Synchronicity of genetic variants between primary sites and metastatic lymph nodes, and prognostic impact in nodal metastatic lung adenocarcinoma. J Cancer Res Clin Oncol 145:2325–2333. 10.1007/s00432-019-02978-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izar B, Zhou H, Heist RS, Azzoli CG, Muzikansky A, Scribner EE, Bernardo LA, Dias-Santagata D, Iafrate AJ, Lanuti M (2014) The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncol 9:1363–1369. 10.1097/JTO.0000000000000266 [DOI] [PubMed] [Google Scholar]
- Jinesh GG, Sambandam V, Vijayaraghavan S, Balaji K, Mukherjee S (2018) Molecular genetics and cellular events of K-Ras-driven tumorigenesis. Oncogene 37:839–846. 10.1038/onc.2017.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Cho EN, Park HS, Hong JY, Lim S, Youn JP, Hwang SY, Chang YS (2016) Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 17:237–245. 10.1080/15384047.2016.1139235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneuertz PJ, Carbone DP, D’Souza DM, Shilo K, Abdel-Rasoul M, Zhao W, Williams TM, Jones D, Merritt RE (2020) Prognostic value and therapeutic implications of expanded molecular testing for resected early stage lung adenocarcinoma. Lung Cancer 143:60–66. 10.1016/j.lungcan.2020.03.012 [DOI] [PubMed] [Google Scholar]
- Lee HY, Cha MJ, Lee KS, Lee HY, Kwon OJ, Choi JY, Kim HK, Choi YS, Kim J, Shim YM (2016) Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J Thorac Oncol 11:1064–1073. 10.1016/j.jtho.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tsuchiya T, Hao TDH, Xu Y, Mucenski ML, Du L, Keiser AR, Fukazawa T, Naomoto Y, Nagayasu T (2012) Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J Clin Invest 122:4388–4400. 10.1172/JCI64048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoku T, Tsuta K, Ichikawa H, Shiraishi K, Sakamoto H, Enari M, Furuta K, Shimada Y, Ogiwara H, Watanabe SI, Nokihara H (2014) Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res 20:3087–3093. 10.1158/1078-0432.CCR-14-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguart N, Teixido C, Gimenez-Capitan A, Pare L, Galvan P, Viteri S, Rodriguez S, Peg V, Aldeguer E, Vinolas N, Remon J (2017) Identification of ALK, ROS1, and RET fusions by a multiplexed mRNA-based assay in formalin-fixed, paraffin-embedded samples from advanced non-small-cell lung cancer patients. Clin Chem 63:751–760. 10.1373/clinchem.2016.265314 [DOI] [PubMed] [Google Scholar]
- Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA (2011) Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 6:1496–1504. 10.1097/JTO.0b013e318221f701 [DOI] [PubMed] [Google Scholar]
- Shim HS, Kenudson M, Zheng Z, Cha YJ, Ho QH, Onozato M, Le LP, Heist RS, Iafrate AJ (2015) Uniquegenetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol 10:1156–1162. 10.1097/JTO.0000000000000579 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Eguchi T, Kameda K, Lu S, Vaghjiani RG, Tan KS, Travis WD, Jones DR, Adusumilli PS (2018) Histologic subtyping in pathologic stage I-IIA lung adenocarcinoma provides risk-based stratification for surveillance. Oncotarget 9:35742–35751. 10.18632/oncotarget.26285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K (2011) International Association for the Study of Lung cancer/American Thoracic society/European Respiratory Society International multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K (2015) WHO Panel. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10:1243–1260. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- Trombetta D, Graziano P, Scarpa A, Sparaneo A, Rossi G, Rossi A, Di Maio M, Antonello D, Mafficini A, Fabrizio FP, Manzorra MC (2018) Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by Phospho-ErbB3 expression. Oncotarget 9:9661–9671. 10.18632/oncotarget.23800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A, Muley T, Meister M et al (2012) The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 30:1438–1446. 10.1200/JCO.2011.37.2185 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saito H, Yokose T, Sakuma Y, Murakami S, Kondo T, Oshita F, Ito H, Nakayama H, Yamada K, Iwazaki M (2015) Relation between thin-section computed tomography and clinical findings of mucinous adenocarcinoma. Ann Thorac Surg 9:975–981. 10.1016/j.athoracsur.2014.10.065 [DOI] [PubMed] [Google Scholar]
- Yoda S, Lin JJ, Lawrence MS, Burke BJ, Friboulet L, Langenbucher A, Dardaei L, Prutisto-Chang K, Dagogo-Jack I, Timofeevski S, Hubbeling H (2018) Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov 8:714–729. 10.1158/2159-8290.CD-17-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD (2013) Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 8:52–61. 10.1097/JTO.0b013e3182769aa8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is original from our study. No data from public or shared database was utilized.