Abstract
1. Patch clamp recording techniques have been used to investigate the block by amiloride of the mechanosensitive cation-selective channel in frog (Xenopus laevis) oocytes. 2. Cell-attached and outside-out patch recording configurations were employed to study the differences in block produced when amiloride was present at either the extracellular (external) or intracellular (internal) membrane face. 3. External amiloride causes a highly voltage-dependent 'flickery' block of single mechanosensitive channel currents in which inward mechanosensitive current recorded at negative potentials is reduced in amplitude but outward mechanosensitive current recorded at positive potentials is almost unaffected. 4. At -100 mV the apparent dissociation constant (Kd) for external amiloride block is 0.5 mM. The extracellular concentration dependence of amiloride block yields a Hill coefficient equal to 2, inconsistent with a single site blocking stoichiometry. 5. The shapes of current-voltage relationships measured in different external amiloride concentrations also indicate deviations from a simple channel plug model in which a single blocking cation is driven into the channel by the membrane potential. 6. Internal amiloride causes a voltage-independent 'flickery' block of mechanosensitive channel currents which equally reduces both inward and outward mechanosensitive currents. 7. The present data indicate that a minimum of two amiloride binding sites are necessary to predict external amiloride block. A model involving a voltage-dependent conformational change with subsequent voltage-independent co-operative binding of two amiloride molecules is found to explain the data. 8. The relevance of the present actions of amiloride on mechanosensitive channels is discussed in relation to reports of amiloride-inhibitable cation flux pathways involved in a number of basic physiological functions including mechanosensitivity of sensory cells, volume regulation and fertilization.
Full text
PDF



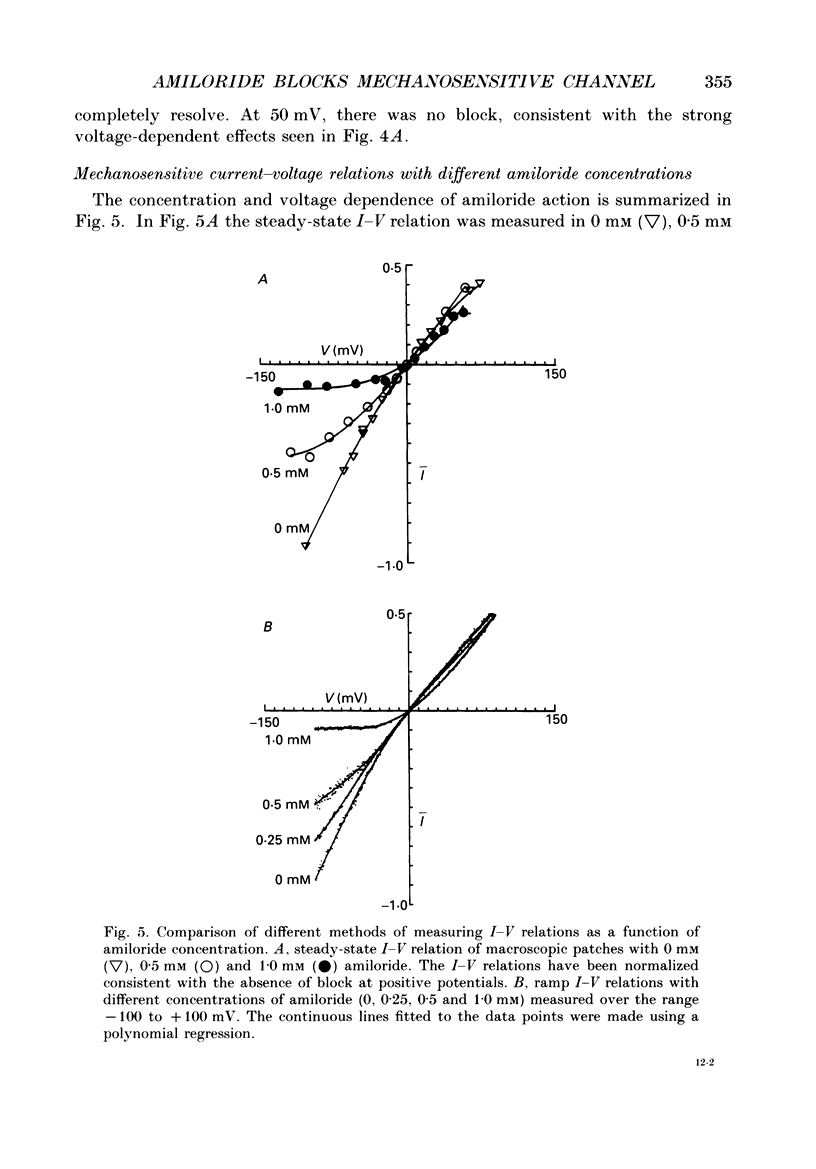
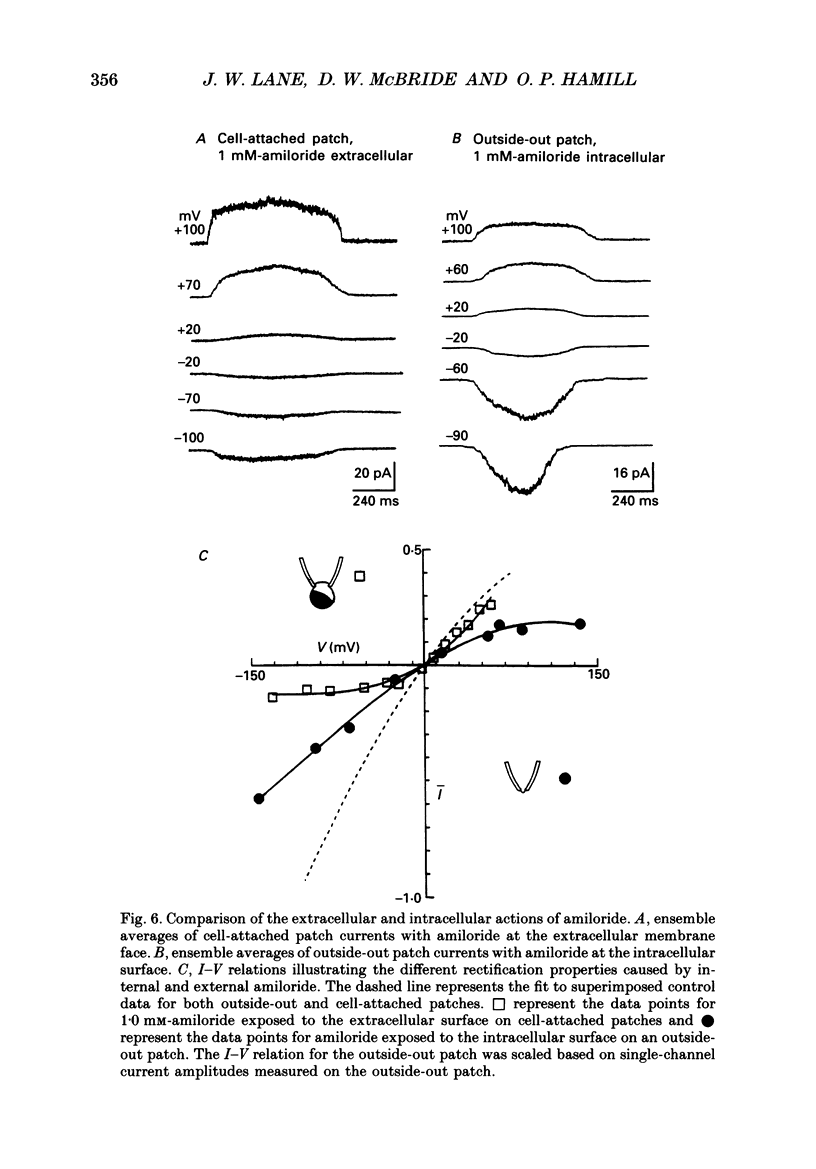



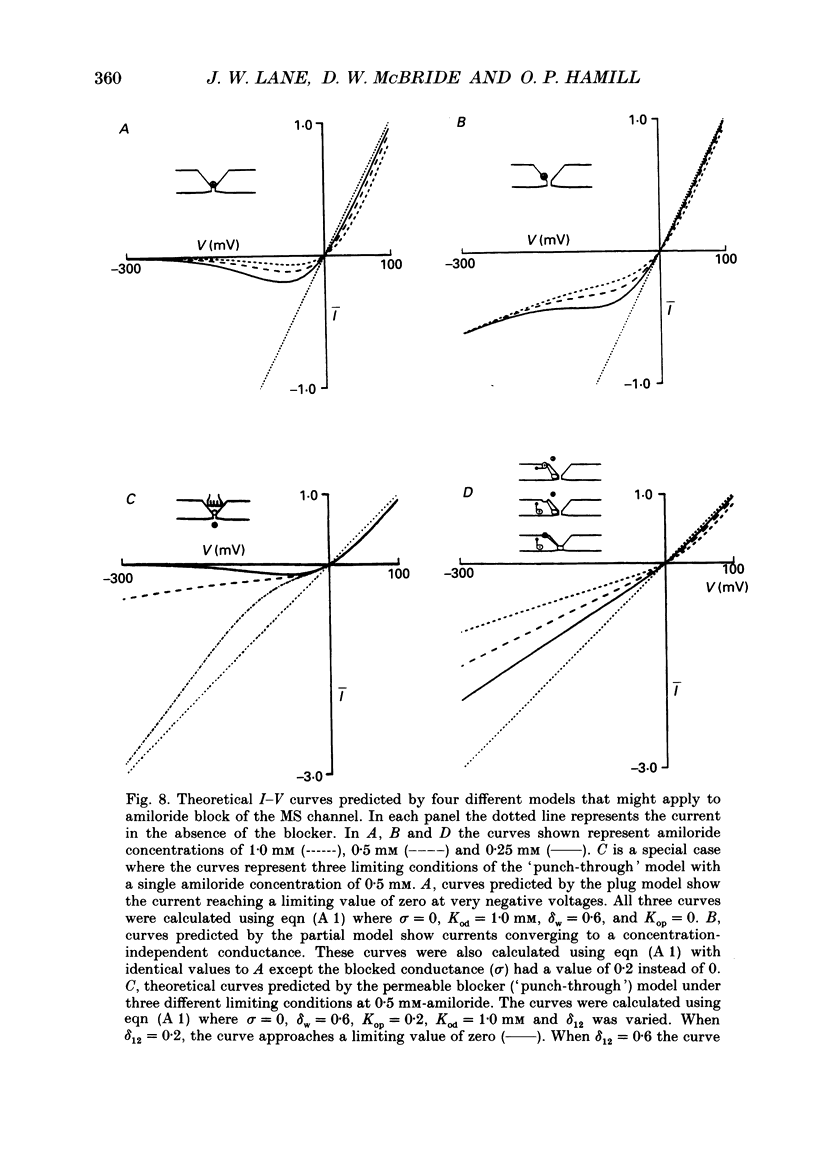

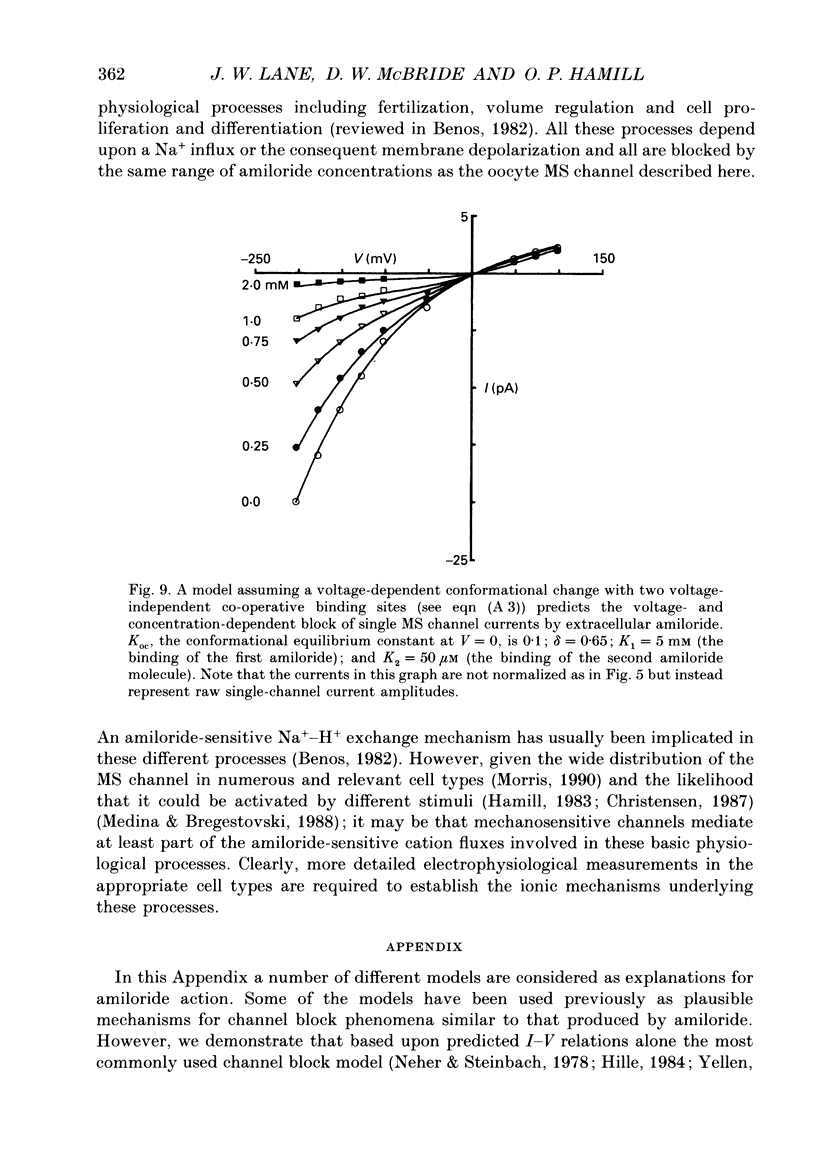




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Datyner N. B., Gintant G. A., Cohen I. S. Microprocessor controlled trituration device for the dissociation of cardiac and other tissues. Pflugers Arch. 1985 Jan;403(1):105–108. doi: 10.1007/BF00583289. [DOI] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Mechanotransducer ion channels in chick skeletal muscle: the effects of extracellular pH. J Physiol. 1985 Jun;363:119–134. doi: 10.1113/jphysiol.1985.sp015699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jørgensen F., Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J Physiol. 1988 Sep;403:577–588. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina I. R., Bregestovski P. D. Stretch-activated ion channels modulate the resting membrane potential during early embryogenesis. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):95–102. doi: 10.1098/rspb.1988.0064. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G. Voltage-dependent block by amiloride and other monovalent cations of apical Na channels in the toad urinary bladder. J Membr Biol. 1984;80(2):153–165. doi: 10.1007/BF01868771. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Taglietti V., Toselli M. A study of stretch-activated channels in the membrane of frog oocytes: interactions with Ca2+ ions. J Physiol. 1988 Dec;407:311–328. doi: 10.1113/jphysiol.1988.sp017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


