Abstract
Purpose
Transport and Golgi organization protein 1 (TANGO) promotes angiogenesis and lymphangiogenesis in oral squamous cell carcinoma (OSCC). To elucidate the underlying mechanisms, this study aims to identify and characterize elements downstream of TANGO that mediate its involvement in OSCC.
Methods
In this study, microarray analysis compared gene expression between control and TANGO-repressed HSC3 cells. Protein expression in 213 OSCC tissue samples was analyzed immunohistochemically.
Results
TANGO repression decreased or increased expression of Mucin 20 (MUC20) and small proline-rich protein 1B (SPRR1B), respectively. MUC20 increased the growth and invasiveness of OSCC cells via altered matrix metalloproteinase (MMP)-2 and E-cadherin expression and c-met phosphorylation. MUC20 induced angiogenesis and lymphangiogenesis by activating vascular endothelial growth factors A and C. In well-differentiated OSCC, SPRR1B expression was high (P = 0.0091) and correlated with keratinization markers and promoted proliferation by inducing mitogen-activated protein kinase p38 phosphorylation. MUC20 expression correlated significantly with clinical stage (P = 0.0024), lymph node metastasis (P = 0.0036), and number of blood and lymph vessels (P < 0.0001). MUC20-expressing cases had a significantly worse prognosis than non-expressing cases (P < 0.0001).
Conclusion
MUC20 and SPRR1B located downstream of TANGO may be useful molecular markers for OSCC.
Keywords: TANGO, MUC20, SPRR1B, Oral cancer
Introduction
Oral squamous cell carcinoma (OSCC), including lip cancer, was newly diagnosed in 354,864 patients and caused 177,384 deaths worldwide in 2018, accounting for approximately 2% of all malignancies and 1.9% of cancer deaths(Bray et al. 2018). The incidence of OSCC is particularly high in Papua New Guinea (42.6 per 100,000 persons/year), Pakistan (24.4), Bangladesh (18.9), and India (18.2), with mortality rates of 17.8, 17.3, 12.4, and 11.1, respectively (Miranda-Filho and Bray 2020). Despite the remarkable progress in cancer treatment that has been afforded by advances in molecular biology, the prognosis of OSCC has scarcely improved in the past few decades, and the five-year survival rate remains at around 50%. Therefore, early detection and diagnosis are critical for improving the prognosis of OSCC.
The melanoma inhibitory activity (MIA) genes encode a family of secreted proteins including MIA, MIA2, transport and Golgi organization protein 1 (TANGO), and otoraplin (Schmidt et al. 2013). These highly homologous family members possess a highly conserved Src homology 3 (SH3)-like domain and a hydrophobic N-terminal secretory signal sequence (Riechers and Bosserhoff 2014; Schmidt et al. 2013). TANGO, also known as MIA3, plays a crucial role in the secretion of numerous collagens from the endoplasmic reticulum of chondrocytes, fibroblasts, and endothelial cells (ECs) (Wilson et al. 2011). TANGO is known to act as a tumor suppressor in melanoma (Arndt and Bosserhoff 2006), hepatoma (Arndt and Bosserhoff 2007), and colon cancer (CRC) (Arndt and Bosserhoff 2007; Gao et al. 2017; Schubert et al. 2017). In addition, TANGO has been observed to be involved in tumor progression, nodal metastasis, and platelet-derived growth factor beta- (PDGF-β) and neuropilin2 (NRP2)-mediated lymphangiogenesis and angiogenesis in OSCC (Sasahira et al. 2014b, 2018). However, many questions still remain regarding the function of TANGO in cancer.
This study investigates two candidate TANGO-related signaling factors: mucin 20 (MUC20) and small proline-rich protein 1B (SPRR1B). MUC20 is a heavy glycoprotein associated with poor prognosis in endometrial cancer (Chen et al. 2013), ovarian cancer (Chen et al. 2016), CRC (Xiao et al. 2013), esophageal SCC (Wang et al. 2015), and pancreatic cancer (Chen et al. 2018). In CRC, MUC20 increases the suppression of E-cadherin and the activation of matrix metalloproteinase (MMP)-2 and facilitates cancer cell growth and invasiveness (Xiao et al. 2013). Interaction between MUC20 and c-met (MET) induces MET phosphorylation and promotes pancreatic cancer cell proliferation and invasion (Chen et al. 2018). The expression of MUC20 also correlates with the degree of histological differentiation and clinical stage of cervical SCC (Kong et al. 2017). SPRR1B, the second candidate, is known as a marker for squamous cell differentiation, and its expression is high in the tongue (Fujimoto et al. 1997) and in the squamous metaplasia of the respiratory epithelium (Reddy et al. 2003). SPRR1B expression is reportedly lower in gastric cancer (Hippo et al. 2001) and lung adenocarcinoma (Ludovini et al. 2016) and higher in well-differentiated OSCC-derived stem-like cells, and it promotes the proliferation of OSCC cells (Michifuri et al. 2013). Nonetheless, the role of MUC20 and SPRR1B in OSCC remains unclear. This study aims to clarify the relationship between these proteins and TANGO in OSCC.
Materials and methods
Cell culture
HSC3 and HSC4 cells, derived from poorly and well-differentiated OSCC, respectively, were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank, Osaka, Japan (Momose et al. 1989). It was previously confirmed that the expression of TANGO was higher in HSC3 cells than in HSC4 cells (Sasahira et al. 2014b). The cells were authenticated by the JCRB via a short tandem repeat analysis. Primary human umbilical vein endothelial cells (HUVEC) and human dermal lymphatic microvascular endothelial cells (HDLMVEC) were purchased from Cell Applications (San Diego, CA, USA). OSCC, HUVEC, and HDLMVEC cell lines were maintained in Dulbecco’s modified Eagle medium (Wako Pure Chemical, Osaka, Japan), a human EC growth medium (Cell Applications), and a microvascular EC growth medium (Cell Applications) supplemented with a 10% fetal bovine serum (Nichirei Biosciences, Tokyo, Japan), respectively, under conditions of 5% CO2 in air at 37 °C. Cells were treated with 5 nM recombinant human TANGO (Novus Biologicals, Centennial, CO, USA) or 0.5 µg/mL of an anti-TANGO/MIA3 antibody (LifeSpan BioSciences, Seattle, WA, USA; catalog number LS-B4210) for 48 h under the conditions of 5% CO2 in air at 37 °C, followed by an in vitro analysis. It has been confirmed that this antibody and recombinant protein of the MIA gene family neutralize the secreted TANGO or act via the receptor present on the cell membrane, respectively (Kurihara-Shimomura et al. 2020; Kurihara et al. 2013; Sasahira et al. 2014b, 2016).
Microarray analysis
We performed a microarray analysis of control HSC3 cells and TANGO-repressed HSC3 cells. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Venlo, Limburg, The Netherlands), and their purity was determined via standard spectrophotometric methods. A CodeLink Human Whole Genome Bioarray (Applied Microarrays, Tempe, AZ, USA) containing 54,841 probes was used to compare the gene expression profiles of the cell lines. The data analysis was performed using the Microarray Data Analysis Tool Ver3.2 (Filgen, Nagoya, Japan). The microarray data have been registered in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) and can be accessed using the GEO Series accession number GSE80347.
Transient transfection
Cells were treated with small interfering RNA (siRNA) to inhibit gene expression. FlexiTube siRNA for MIA3/TANGO (identification number: Hs_MIA3_1), MUC20 (identification number: Hs_MUC20_7), and SPRR1B (identification number: Hs_SPRR1B_2) were purchased from QIAGEN, and AllStars Negative Control siRNA (Qiagen) was used as a control. MUC20 and SPRR1B cDNA were amplified using a polymerase chain reaction (PCR) and sub-cloned into pcDNA3.3 (Thermo Fisher Scientific, Waltham, MA, USA). According to the manufacturer’s instructions, 5 nmol of siRNA, MUC20 cDNA, and SPRR1B cDNA was transfected into cells using Lipofectamine 3000 (Thermo Fisher Scientific).
Enzyme-linked immunosorbent assay
Proteins were extracted from cell lysates and culture supernatants using M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific). An enzyme-linked immunosorbent assay was used to measure the protein concentrations of MMP-2 (Abcam, Cambridge, UK), E-cadherin (Takara Bio, Otsu, Japan), involucrin (LifeSpan BioSciences), keratin 1 (MyBioSource, San Diego, CA, USA), phosphorylated MET (Cell Signaling Technology Japan, Tokyo, Japan), vascular endothelial growth factor (VEGF)-A (R&D Systems, Minneapolis, MN, USA), VEGF-C (R&D Systems), and phosphorylated mitogen-activated protein kinase (MAPK) p38 (RayBiotech, Peachtree Corners, GA, USA).
Immunoblotting
Whole-cell lysates were obtained with the Mammalian Protein Extraction Reagent (M-PER, Thermo Fisher Scientific), and 50 µg of lysate was separated by electrophoresis on a 12.5% SDS-PAGE gel and electro-transferred to a polyvinylidene fluoride membrane (Novus Biologicals). The membrane was incubated with anti-TANGO/MIA3 (LifeSpan BioSciences; 1:5000), anti-MUC20 (Abnova; 1:2000), and anti-SPRR1B antibodies (LifeSpan BioSciences; 1:4000) followed by peroxidase-conjugated IgG (Medical & Biological Laboratories, Nagoya, Japan). Immune complexes were visualized using the ECL Western Blotting Detection System (GE Healthcare, Amersham, UK). An anti-GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:5000) was used as an internal control.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted using the RNeasy Mini kit (Qiagen), and cDNA was synthesized with the ReverTra Ace qRT kit (Toyobo, Osaka, Japan), using 1 μg of total RNA as a template. qRT-PCR was performed using a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) with TaqMan Fast Universal PCR Master Mix (Thermo Fisher Scientific), and gene expression levels were quantified using the relative standard curve method. Gene expression was determined using the TaqMan Gene Expression assay for MIA3/TANGO (identification number: Hs01558382_m1), MUC20 (Hs00416321_m1), SPRR1B (Hs00234164_m1), MMP2 (Hs01548727_m1), and glyceraldehyde-3-phosphate dehydrogenase (Hs02786624_g1) (Thermo Fisher Scientific).
Cell growth, migration, and invasion assays
A cell growth assay was performed using the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). Moreover, cell migration was evaluated by Cvtoselect Cell Migration Assay kit (Cell Biolabs, San Diego, CA, USA). A modified Boyden chamber assay using BD BioCoat cell culture inserts coated with type IV collagen (BD Biosciences, Bedford, MA, USA) was performed as previously described to assess in vitro invasion (Sasahira et al. 2014b).
Endothelial cell proliferation and interactions with OSCC cells and tube formation assay
To generate a conditioned medium, negative siRNA or MUC20 siRNA-treated HSC3 cells were incubated at 37 °C. The medium of the sub-confluent HSC3 cells was changed to an Opti-MEM reduced-serum medium (Thermo Fisher Scientific), and the conditioned medium was collected after further culturing for 24 h. ECs were seeded at a density of 2000 cells per well in 96-well tissue culture plates in a medium or a conditioned medium and incubated for 48 h at 37 °C. The anti-TANGO/MIA3 antibody (LifeSpan BioSciences) was added at a concentration of 0.5 µg/mL to cells cultured in the conditioned medium, incubated for 2 h, and treated with negative siRNA. Interactions between HSC3 and ECs were assessed using a CytoSelect Tumor Transendothelial Migration Assay system (Cell Biolabs). Briefly, a suspension of fluorescently labeled OSCC cells was cultured with a monolayer of ECs and lysed in a lysis buffer. Migrated cells were measured using the monochromator-based Multiskan GO microplate spectrophotometer (Thermo Fischer Scientific). Tube formation ability was evaluated using the Angiogenesis kit (PromoCell, Heidelberg, Germany) as instructed by the supplier. After 18 h, the capillary tube structure was captured using an inverted phase contrast microscope (Olympus, Tokyo, Japam) in a 40 × magnification, and the relative tubal length was quantified by Image J software.
Tumor specimens
Fresh-frozen samples from 30 patients with primary OSCC (19 males; median age, 67.9 years; age range 54–81 years) and 10 samples of dysplasia (5 males; median age, 46.8 years; age range 41–68 years) and nontumor oral mucosa (7 males; median age, 44.3 years; age range 33–61 years) were utilized for gene expression. Frozen specimens of 10 colorectal cancer (CRC) (8 males; median age, 63.8 years; age range 47–77 years) and cervical SCC (median age, 46.6 years; age range 32–60 years) were also used for gene expression analysis. In total, 213 formalin-fixed, paraffin-embedded samples from patients with OSCC (median age, 66.1 years; age range 45–89 years) were prepared for an immuno-histochemical analysis. All specimens were collected preoperatively from untreated patients who were chosen at random from the Nara Medical University Hospital, Kashihara, Nara, Japan. The tumor stage and histological grade were determined according to the Union for International Cancer Control’s TNM classification system, 8th edition (O'Sullivan 2017) and the World Health Organization’s criteria (Sloan et al. 2017), respectively. Medical records were obtained from the patient database managed by the hospital. Written informed consent for using the tissue samples was obtained from the patients. All experiments with human samples were performed according to the Declaration of Helsinki and were approved by the Ethical Committee of Nara Medical University (approval number 719).
Immunohistochemistry
Consecutive 3-µm sections were cut from each block, and the immuno-histochemical analysis was performed using the EnVision + DualLink system (DAKO, Carpinteria, CA, USA). The antigen retrieval was performed by a microwave treatment (95 °C) in a citrate buffer (pH 6.0) for 45 min. Each section was pretreated with 3% H2O2–methanol to block endogenous peroxidase activity and then blocked in a 10% skim milk solution (Morinaga Milk, Tokyo, Japan) for 20 min. The anti-MUC20 (Abnova, Taipei, Taiwan; 1:100) and anti-SPRR1B antibodies (LifeSpan BioSciences; 1:150) were added as the primary antibodies, incubated for 2 h, and then incubated with the secondary antibody for 30 min at room temperature. The specimens were color-developed with a diaminobenzidine (DAB) solution (Dako, Carpinteria, CA, USA) and counterstained with Meyer’s hematoxylin (Sakura Finetek, Tokyo, Japan).
The immunostaining of MUC20 and SPRR1B was quantified using the proportional and intensity score, as reported previously, with a score of 5 or higher considered positive for expression (Shimomura et al. 2019). The micro-vessel density (MVD) and lymphatic vessel density (LVD) were measured in specimens that were immuno-positive for CD34 (Dako) and LYVE-1 (Dako), respectively. To quantify MVD or LVD, five maximum vessel density fields were selected from around the tumor cells (the “hot spot”) and examined under a microscope at a 200-fold magnification; the results are reported as the average of the five fields.
Statistical analysis
A statistical analysis was performed using Fisher’s exact test, Student’s t test, Welch’s t test, and the Mann–Whitney U test. Disease-free survival (DFS) was analyzed using the Kaplan–Meier method and compared between groups using the log-rank test. All statistical analyses were conducted using JMP13 (SAS Institute, Cary, NC, USA), and P < 0.05 was considered statistically significant.
Results
Identification of genes up- or downregulated by TANGO in HSC3 cells
To identify genes that had their expressions regulated by TANGO, we performed a cDNA microarray analysis. Data with a low reliability were omitted. The expression levels of many genes were increased or decreased by TANGO in OSCC cells. The top 10 genes that showed a higher or lower expression in OSCC cells with TANGO knockdown than in control cells are summarized in Table 1. Of these genes, MUC20, which had an increased expression, and SPRR1B, which had a decreased expression, were investigated in detail because their roles in OSCC are controversial.
Table 1.
The 10 most upregulated or downregulated genes except TANGO in TANGO knockdown OSCC cells
| Downregulated | Upregulated | ||
|---|---|---|---|
| Gene | Fold (TANGO knockdown/control) | Gene | Fold (TANGO knockdown/control) |
| MAGEA4 | 0.008 | SPRR1B | 128.303 |
| MUC20 | 0.009 | H19 | 70.814 |
| MMP7 | 0.028 | SPINK5 | 64.955 |
| SLC47A2 | 0.039 | SOX21 | 57.521 |
| ARHGDIB | 0.059 | ID3 | 31.753 |
| KRT14 | 0.063 | GSTA4 | 25.978 |
| ESM1 | 0.066 | AQP3 | 23.050 |
| GPRC5A | 0.068 | TMCO5B | 22.226 |
| CTSS | 0.072 | SPRR1A | 21.833 |
| SRPX2 | 0.080 | HSPB1 | 21.262 |
Expression of MUC20 and SPRR1B in OSCC cells
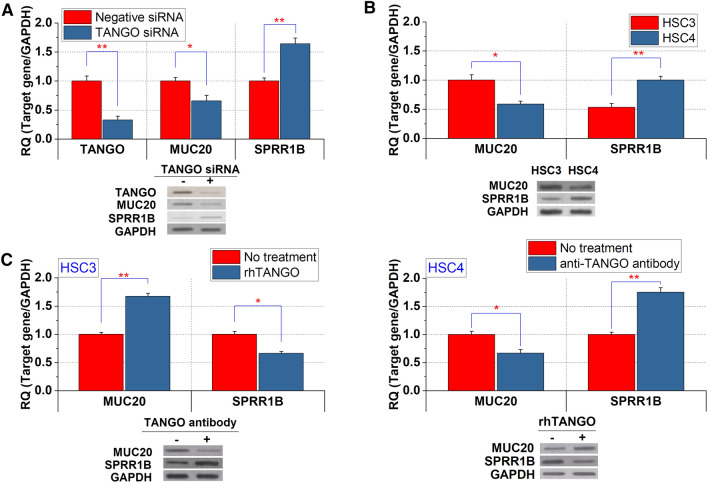
The downregulation of TANGO and MUC20 and the upregulation of SPRR1B were observed in HSC3 cells with TANGO siRNA treatment (Fig. 1a). Our previous study showed that TANGO is overexpressed in HSC3 cells (poorly differentiated) compared to HSC4 cells (well differentiated) (Sasahira et al. 2014b). Similarly, the expression levels of MUC20 were higher in HSC3 cells than in HSC4 cells (Fig. 1b). By contrast, the expression of SPRR1B was higher in HSC4 cells than in HSC3 cells (Fig. 1b).
Fig. 1.
Expression and regulation of MUC20 and SPRR1B in OSCC cells. a Expression of TANGO, MUC20, and SPRR1B in control HSC3 cells and HSC3 cells with the downregulation of TANGO. b Differential expression of MUC20 and SPRR1B in HSC3 and HSC4 cells. c Expression levels of MUC20 and SPRR1B by anti-TANGO antibody or rhTANGO treatment in OSCC cells. *P < 0.01; **P < 0.001
In addition, the expression levels of MUC20 and SPRR1B were increased and/or decreased under recombinant protein or antibody treatment for TANGO in OSCC cells (Fig. 1d). As TANGO is a secreted protein (Schmidt et al. 2013; Wilson et al. 2011), these results suggest that TANGO regulates the expression levels of MUC20 and SPRR1B in an autocrine/paracrine manner in OSCC cells.
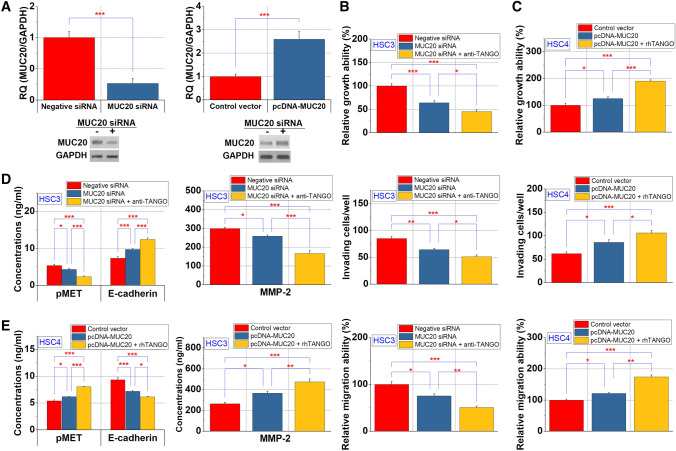
Effect of MUC20 on cell proliferation and invasion in OSCC cells
We then investigated the effect of MUC20 on the proliferation and invasive potential of OSCC cells (Fig. 2a). The proliferation, migration and invasion abilities were suppressed in HSC3 cells with downregulated MUC20 (Fig. 2b) and increased in HSC4 cells overexpressing MUC20 (Fig. 2c). The effects of both HSC3 cells and HSC4 cells were facilitated by TANGO (Fig. 2b, c). In addition, we observed that MUC20 downregulation resulted in a decreased MET phosphorylation in cell lysates as well as a decreased secretion of MMP-2 and an increased secretion of soluble E-cadherin into culture supernatants. These results were also observed upon the anti-TANGO antibody treatment of HSC3 cells (Fig. 2d). Moreover, we observed that MUC20 regulates MET phosphorylation and the secretion of MMP2 and soluble E-cadherin in HSC4 cells under the influence of TANGO (Fig. 2e).
Fig. 2.
The effect of MUC20 on growth, invasion and migration of OSCC cells. a Expression change by knockdown or increase in MUC20. b, c Changes in OSCC cell proliferation, invasion and migration by MUC20 in HSC3 cells (b) and HSC4 cells (c). Altered MMP-2 and E-cadherin secretion and MET phosphorylation levels by MUC20 in HSC3 (d) and HSC4 cells (e). *P < 0.05; **P < 0.01; ***P < 0.001
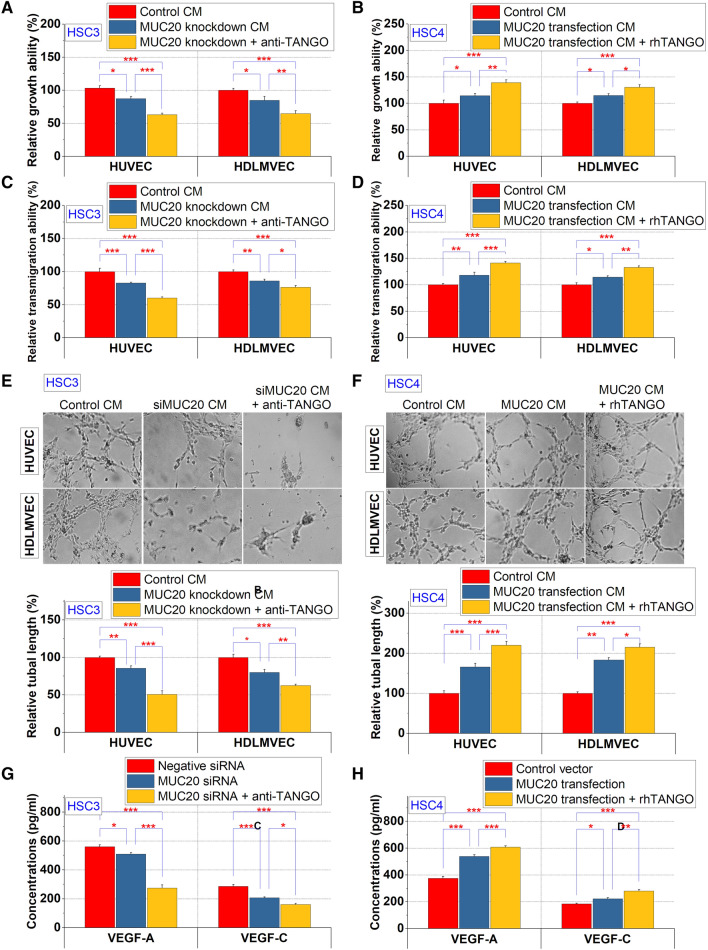
Effect of MUC20 on angiogenesis in OSCC cells
Since MET promotes tumor angiogenesis and lymphangiogenesis via VEGF-A activation (Matsumura et al. 2013; Swoboda et al. 2012), we investigated the angiogenic and lymphangiogenic potential of MUC20 in OSCC cells. The conditioned medium of MUC20 knockdown HSC3 cells suppressed the proliferation of HUVEC and HDLMVEC cells, and these effects were augmented by combining this treatment with TANGO suppression (Fig. 3a). By contrast, the conditioned medium from MUC20-overexpressing HSC4 cells promoted the proliferation of ECs, and the administration of rhTANGO further increased this effect (Fig. 3b). Moreover, the transmigration ability of OSCC cells to ECs was inhibited and promoted by the downregulation and upregulation of MUC20, respectively, and these effects were potentiated by treatment with the anti-TANGO antibody or rhTANGO (Fig. 3c, d). Further, it was revealed that MUC20 in HSC3 (Fig. 3e) cells and HSC4 cells (Fig. 3f) regulates the tube formation ability of endothelial cells by interacting with TANGO. The secretion levels of VEGF-A and VEGF-C were reduced in HSC3 cells with MUC20 siRNA, and those levels were further decreased by the anti-TANGO antibody treatment (Fig. 3g). The inverse result was also observed in MUC20-overexpressing HSC4 cells with rhTANGO treatment (Fig. 3h).
Fig. 3.
Regulation of angiogenic and lymphangiogenic potential of OSCC cells by MUC20. a, b Altered endothelial cell proliferation induced by treatment with conditioned medium from MUC20 siRNA-treated HSC3 cells (a) and MUC20 transfected HSC4 cells (b). c, d Transmigration of endothelial cells to HSC3 cells that suppressed MUC20 expression (c) or HSC4 cells that overexpressed MUC20 (D). e, f Effect of MUC20 derived from HSC3 cells (e) and HSC4 cells (f) on tube formation ability of endothelial cells. g, h Alterations in VEGF-A and VEGF-C secretion levels by MUC20 in HSC3 (g) and HSC4 cells (h). *P < 0.05; *P < 0.01; ***P < 0.001
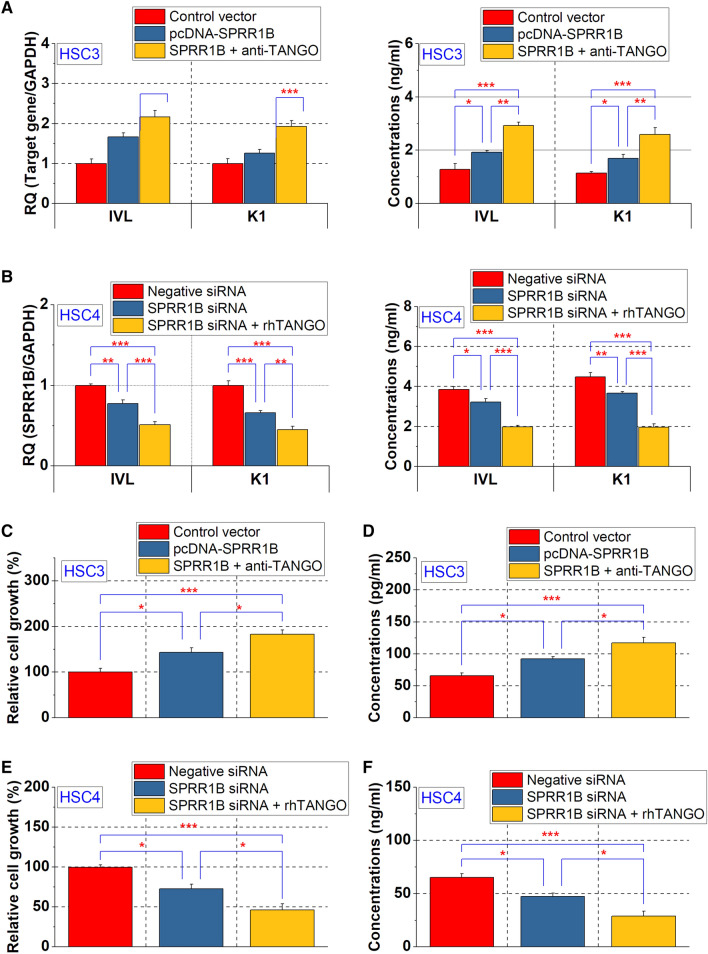
Role of SPRR1B in OSCC cell differentiation
Because SPRR1B was more highly expressed in well-differentiated OSCC cells (Fig. 1c), we next investigated the relationship between SPRR1B and cell differentiation. The keratinocyte differentiation markers involucrin and keratin 1 were used as indicators of OSCC cell differentiation (Naher et al. 2012). In HSC3 cells, the expression levels of involucrin and keratin 1 in cell lysates were increased by a transfection with SPRR1B, and their expression was further elevated by the anti-TANGO antibody treatment (Fig. 4a), the expression of involucrin and keratin 1 was decreased in HSC4 cells with SPPRR1B knockdown, and those levels were further attenuated by rhTANGO (Fig. 4b).
Fig. 4.
Functions of SPRR1B in OSCC cells. Altered expression of keratinocyte differentiation markers induced by SPRR1B in HSC3 and HSC4 cells
Effect of SPRR1B on OSCC cell proliferation
SPRR1B has been reported to promote the proliferation of well-differentiated OSCC cells (Michifuri et al. 2013). As the MAPK p38 signaling pathway is essential for keratinocyte growth (Li et al. 2001), we investigated the effect of SPRR1B on the proliferation and phosphorylation of MAPK p 38 of well-differentiated and poorly differentiated OSCC cells. Cell proliferation and MAPK p38 phosphorylation were elevated in HSC3 cells that had been transfected with SPRR1B, and those levels were increased by a combined treatment with anti-TANGO antibody (Fig. 4c, e). Conversely, the knockdown of SPRR1B in HSC4 cells decreased cell proliferation and MAPK p38 phosphorylation, and this effect was further increased by a treatment with rhTANGO. (Fig. 4d, f). These results suggest that SPRR1B may promote cell differentiation and MAPK p38 phosphorylation-mediated cell proliferation in differentiated OSCC.
MUC20 and SPRR1B expression in human OSCC specimens
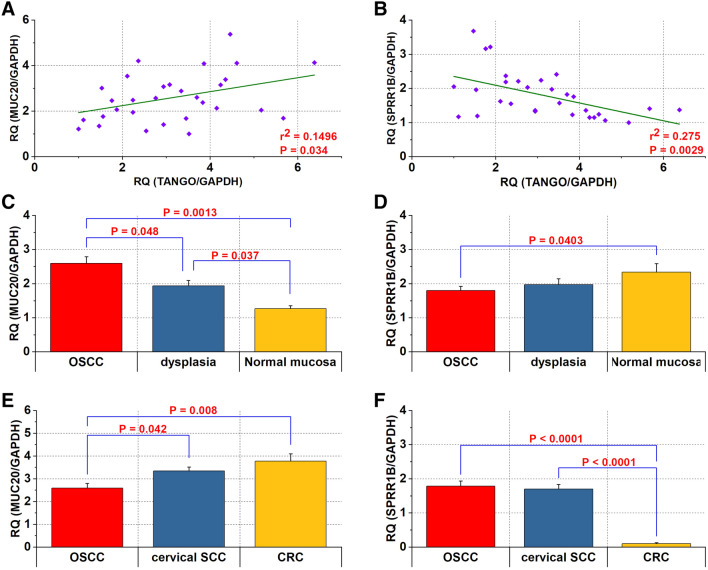
qRT-PCR revealed that the expression levels of MUC20 correlated significantly with TANGO expression in OSCC (P = 0.034) (Fig. 5a). On the other hand, expression levels of SPRR1B and TANGO correlated inversely in OSCC tissues (P = 0.0029) (Fig. 5b). Although MUC20 expression levels were significantly higher in OSCC than in dysplasia (P = 0.048) and normal mucosa adjacent to cancer (P = 0.0013) (Fig. 5c), those of SPRR1B were significantly higher in adjacent normal mucosa than in OSCC tissues (P = 0.0403) (Fig. 5d). We then confirmed that expression of MUC20 was higher in cervical SCC (P = 0.042) and CRC (P = 0.008) than in OSCC (Fig. 5e).
Fig. 5.
Expression of MUC20 and SPRR1B in OSCC samples. a, b Relationship between the expression levels of TANGO and MUC20 (a) or SPRR1B (b) in OSCC. c, d Differential expression of MUC20 (c) and SPRR1B (d) in OSCC, dysplasia and normal mucosa. e, f Comparison of MUC20 (e) and SPRR1B (f) in OSCC, cervical SCC and CRC
On the other hand, SPRR1B was significantly higher in OSCC (P < 0.0001) and cervical SCC (P < 0.0001) than in CRC (Fig. 5f).
Relationship between MUC20 or SPRR1B expression and clinicopathological characteristics in OSCC
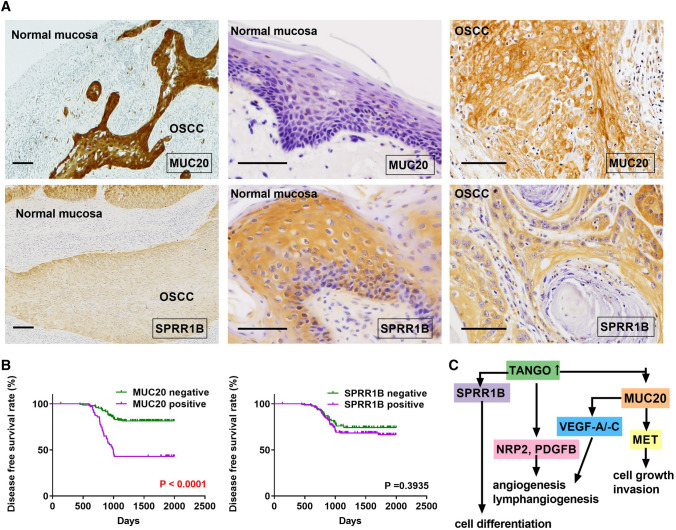
In normal oral mucosa, immunostaining was negative or weak for MUC20 and extremely strong for SPRR1B (Fig. 6a). The immuno-histochemical analysis of 213 OSCC specimens revealed that MUC20 was mainly positive for the cell membrane, whereas SPRR1B was expressed in the cytoplasm. The positivity rates of MUC20 and SPRR1B were 30.1% (64/213) and 63.9% (136/213), respectively (Fig. 6a). The relationship between MUC20 or SPRR1B expression and clinicopathologic characteristics is shown in Table 2. MUC20 expression correlated significantly with clinical stage (P = 0.0036). MUC20 expression was also found in 41.9% (36/86) of the nodal metastasis cases and was significantly higher than that in non-metastatic cases (22.1%; 28/127) (P = 0.0024). Moreover, the MVD (P < 0.0001) and LVD (P < 0.0001) of MUC20-positive cases were also significantly higher than those of negative cases. SPRR1B positivity was 71.6% in well-differentiated OSCC but 53.3% in those with moderate to poorly differentiated OSCC (P = 0.0091). No other relationship was found between MUC20 or SPRR1B expression and clinicopathological parameters.
Fig. 6.
Immunostaining of MUC20 and SPRR1B in OSCC specimens. a Expression of MUC20 and SPRR1B in normal mucosa and OSCC. b Disease-free survival curve of MUC20-positive and SPRR1B-positive cases. c Schema of TANGO-related signals in OSCC
Table 2.
Relationship between MUC20 or SPRR1B expression and clinicopathological parameters
| Parameters | MUC20 | SPRR1B | ||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| Gender | ||||
| Male | 94 | 38 | 46 | 86 |
| Female | 55 | 26 | 31 | 50 |
| P value | 0.6458 | 0.6605 | ||
| Age | ||||
| < −65 | 53 | 25 | 24 | 54 |
| > 65 | 96 | 39 | 53 | 82 |
| P value | 0.6443 | 0.2384 | ||
| Site | ||||
| Tongue | 94 | 43 | 46 | 91 |
| Other | 55 | 21 | 31 | 45 |
| P value | 0.6408 | 0.3017 | ||
| Histological differentiationa | ||||
| Well | 85 | 38 | 35 | 88 |
| Mod, por | 64 | 26 | 42 | 48 |
| P value | 0.7649 | 0.0091 | ||
| T classification | ||||
| T1–T2 | 81 | 27 | 44 | 64 |
| T3–T4 | 68 | 37 | 33 | 72 |
| P value | 0.1347 | 0.1991 | ||
| Clinical stage | ||||
| I–II | 70 | 16 | 37 | 49 |
| IIII–IV | 79 | 48 | 40 | 87 |
| P value | 0.0036 | 0.1097 | ||
| Tumor size | ||||
| < –4 cm | 87 | 35 | 48 | 74 |
| > 4 cm | 62 | 29 | 29 | 62 |
| P value | 0.6521 | 0.3131 | ||
| Invasive depth | ||||
| < − 10 mm | 92 | 33 | 50 | 75 |
| > 10 mm | 57 | 31 | 27 | 61 |
| P value | 0.1753 | 0.1930 | ||
| Nodal metastasis | ||||
| Negative | 99 | 28 | 48 | 79 |
| Positive | 50 | 36 | 29 | 57 |
| P value | 0.0021 | 0.5643 | ||
| MVDb | 14.665 ± 5.983 | 20.011 ± 4.695 | 15.857 ± 5.147 | 16.506 ± 6.628 |
| P value | < 0.0001 | 0.4593 | ||
| LVDc | 12.145 ± 5.152 | 17.506 ± 4.148 | 13.638 ± 5.121 | 13.823 ± 5.647 |
| P value | < 0.0001 | 0.8214 | ||
Relationship between expression of MUC20 or SPRR1B and parameters were calculated by Fisher’s exact test or student’s t test. T classification and clinical stage were classified according to the TNM classification
aHistological differentiation: Well, well-differentiated squamous cell carcinoma; Mod, moderately differentiated squamous cell carcinoma; Por, poorly differentiated squamous cell carcinoma
bMVD means micro-vessel density
cLVD means lympho vessel density
Disease-free survival according to MUC20/SPRR1B expression
During the follow-up period, local and/or nodal recurrence was observed in 64 of 213 patients. Therefore, we determined the DFS curve of OSCC cases with MUC2 or SPRR1B expression. A Kaplan–Meier analysis showed that patients with MUC20 expression were significantly associated with a shorter DFS as compared to those without MUC20 expression (P < 0.0001) (Fig. 6b). No statistically significant association was observed between SPRR1B expression and prognosis (P = 0.3935) (Fig. 6b).
Discussion
TANGO has been suggested as a suppressor of the invasion and migration of cancer cells in malignant melanoma (Arndt and Bosserhoff 2006) and HCC (Arndt and Bosserhoff 2007). In CRC, the expression of TANGO is reduced in comparison to normal mucosa (Arndt and Bosserhoff 2007; Schubert et al. 2017), and the downregulation of TANGO promotes poor prognoses (Gao et al. 2017). Interestingly, TANGO expression levels are reported to be higher in precancerous serrated lesions and colon adenomas than in CRC (Schubert et al. 2017). We also confirmed that TANGO promotes OSCC by inducing lymphangiogenesis and angiogenesis through the activation of PDGFB and NRP2 (Sasahira et al. 2014b). Accordingly, the role of TANGO in each tumor is unclear. Our microarray analysis identified MUC20 and SPRR1B as novel genes that are up- or downregulated by TANGO, respectively. In vitro studies revealed that MUC20 and SPRR1B expression may be autocrine- or paracrine-regulated by TANGO in OSCC. Furthermore, the expression levels of TANGO in OSCC specimens were correlated positively with MUC20 expression and negatively with SPRR1B expression. Thus, we propose that MUC20 and SPRR1B are newly recognized downstream signaling factors of TANGO in OSCC. In this study, no interaction between MUC20 and SPRR1B was confirmed in OSCC.
MUC20 is a membrane-bound protein belonging to the mucin family and is expressed in the colon, endometrium, liver, lung, middle ear, cornea, conjunctiva, placenta, and prostate (Higuchi et al. 2004b; Woodward and Argüeso 2014). MUC20 acts as a tumor promoter in several cancers (Chen et al. 2013, 2016, 2018; Kong et al. 2017; Wang et al. 2015; Xiao et al. 2013); we observed herein that MUC20 expression in OSCC correlated significantly with the clinical stage of oral cancer, lymph node metastasis, and prognosis. Previous reports have shown that MUC20 promotes CRC cell invasiveness by decreasing E-cadherin expression and activating MMP-2 (Xiao et al. 2013). Cancer invasion requires a decrease in E-cadherin to reduce epithelial binding as well as an increase in cancer cell motility and in MMP-2 expression to destroy the basement membrane and stroma (Sasahira and Kirita 2018). We also found that MUC20 potentiates the growth and invasiveness of OSCC cells by increasing MMP-2 secretion and decreasing E-cadherin expression and MET phosphorylation. In addition, MUC20 has been observed to increase cell proliferation by activating MET signaling in pancreatic cancer (Chen et al. 2018); the results that we obtained confirmed that the proliferation of OSCC cells was increased by MUC20.
Angiogenesis and lymphangiogenesis are essential processes in cancer metastasis, and their protagonists comprise a variety of nascent factors, including the VEGF family members (Sasahira and Kirita 2018; Sasahira et al. 2014a). Here, we observed that MUC20-positive cases had significantly more angiolymphatic vessels than did MUC20-negative cases. Thus, we propose that MUC20 contributes to the induction of angiogenesis and lymphangiogenesis by activating VEGF-A and VEGF-C. While MUC1 is known to induce angiogenesis (Kitamoto et al. 2013), to our knowledge, no study reports that MUC20 induces tumor angiogenesis or lymphangiogenesis. Although we previously reported that TANGO promotes angiogenesis and lymphangiogenesis via PDGFB and NRP2 (Sasahira et al. 2014b), our current results indicate that TANGO also stimulates neogenesis by activating VEGF family members via MUC20 signaling in OSCC. MUC20 is reported to be upregulated by starvation, hypoxia, and an acidic microenvironment in pancreatic cancer cells (Chen et al. 2018). Hypoxia is well known to activate hypoxia-inducible factor-1 (HIF-1) and to induce VEGF-A and VEGF-C-mediated angiogenesis and lymphangiogenesis in cancer (Kitamoto et al. 2013; Morfoisse et al. 2014). Under hypoxic conditions, HIF1 may upregulate MUC20, leading to the activation of VEGF-related signaling to induce angiogenesis and lymphangiogenesis in OSCC.
The molecular mechanism underlying the involvement of MUC20 in OSCC remains unclear. Although MUC20 has been shown to suppress MET signaling in chronic glomerulonephritis (Higuchi et al. 2004a), Chen et al. report that the interaction between MUC20 and MET increases the growth and invasion ability of pancreatic cancer cells (Chen et al. 2018). Our results are generally consistent with the latter report. These conflicting results may result from differences in the activity of MUC20 between normal and cancer cells; however, further research is required. While MUC20 may contribute to the invasiveness of OSCC cells, its relationship to the epithelial–mesenchymal transition remains unclear. The involvement of MUC20 in the acquisition of paclitaxel resistance in esophageal SCC has been shown (Shen et al. 2016), but the relationship between MUC20 expression and drug resistance in OSCC is not well understood. Additional investigations are required to understand the molecular details of the function of MUC20.
SPRR1B is a differentiation marker of squamous epithelium (Fujimoto et al. 1997; Reddy et al. 2003). Although SPRR1B is downregulated in gastric cancer (Hippo et al. 2001) and lung adenocarcinoma (Ludovini et al. 2016), its overexpression has been observed in the squamous metaplasia of the bronchial epithelium (Reddy et al. 2003) as well as in normal skin, tongue mucosa, the verrucous carcinoma of the skin, and the well-differentiated SCC of the skin and esophagus (Fujimoto et al. 1997). Since previous studies have shown that SPRR1B promotes the growth of well-differentiated OSCC cells (Michifuri et al. 2013), we also examined the function of SPRR1B in well-differentiated HSC4 cells and poorly differentiated HSC3 cells. We observed that SPRR1B contributes to elevated proliferative activity in HSC4 cells by phosphorylating MAPK p38. In clinical samples, SPRR1B was expressed in well-differentiated OSCC as well as in normal oral mucosa. SPRR1B was more highly expressed in HSC4 cells than in HSC3 cells, and its expression correlated with the expression levels of the keratinocyte markers involucrin and keratin 1. These markers are specific for keratinocytes located in the granular layer and play a pivotal role in the terminal differentiation of keratinocytes (Cho et al. 1996). Thus, SPRR1B signaling may be responsible for the final differentiation of OSCC cells under the regulation of TANGO. Moreover, because poorly differentiated OSCC has few well-differentiated keratinocytes (Kitamura et al. 2012), the transfection of SPRR1B into poorly differentiated HSC3 cells may increase the degree of differentiation, resulting in an increased proliferation ability via the phosphorylation of MAPK p38. Together, these results suggest that SPRR1B regulates the terminal differentiation of OSCC and cell growth in differentiated OSCC.
Several questions still remain regarding the role of SPRR1B in OSCC. In the present study, SPRR1B expression was not associated with clinical outcomes. Generally, histological differentiation is considered to have a minimal relationship with prognosis in OSCC (Sloan et al. 2017). A large-scale study using more samples is needed to clarify the role of SPRR1B in OSCC. Activator protein 1 (AP1) is reported to act on the promoter region of SPRR1B in bronchial epithelial cells and Clara cells to regulate SPRR1B expression (Patterson et al. 2001; Reddy et al. 2002, 2003). In OSCC, SPRR1B expression may be regulated negatively by autocrine and/or paracrine mechanisms by TANGO, but a SPRR1B promoter analysis was not included in this study. Moreover, the role of SPRR1B in poorly differentiated cancer remains unclear. Further detailed analyses are essential to determine the molecular mechanism underlying the function of SPRR1B in poorly differentiated OSCC.
In conclusion, this study identified MUC20 and SPRR1B as downstream genes of TANGO, as summarized in Fig. 6c. MUC20 induces OSCC proliferation, invasion, angiogenesis, and lymphangiogenesis. We also found that SPRR1B is involved in OSCC cell differentiation. These findings suggest that MUC20 and SPRR1B may be attractive targets for anti-angiogenic and lymphangiogenic therapy and differentiation therapy, respectively, in OSCC. Further studies will explore the utility of MUC20 and SPRR1B as diagnostic and therapeutic markers in OSCC.
Author contributions
Conception and design; TS. Acquisition of data; TS, MKS, HS. Analysis and interpretation of data; TS, MKS, HS. Writing, review, and/or revision of the manuscript; TS, AKB, TK.
Funding
This study was funded by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science, Japan (Grant No. 17K11621).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Medical Ethics Committee of Nara Medical University and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arndt S, Bosserhoff AK (2006) TANGO is a tumor suppressor of malignant melanoma. Int J Cancer 119:2812–2820. 10.1002/ijc.22242 [DOI] [PubMed] [Google Scholar]
- Arndt S, Bosserhoff AK (2007) Reduced expression of TANGO in colon and hepatocellular carcinomas. Oncol Rep 18:885–891 [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Chen CH et al (2013) MUC20 overexpression predicts poor prognosis and enhances EGF-induced malignant phenotypes via activation of the EGFR-STAT3 pathway in endometrial cancer. Gynecol Oncol 128:560–567. 10.1016/j.ygyno.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Chen CH et al (2016) MUC20 promotes aggressive phenotypes of epithelial ovarian cancer cells via activation of the integrin β1 pathway. Gynecol Oncol 140:131–137. 10.1016/j.ygyno.2015.11.025 [DOI] [PubMed] [Google Scholar]
- Chen ST, Kuo TC, Liao YY, Lin MC, Tien YW, Huang MC (2018) Silencing of MUC20 suppresses the malignant character of pancreatic ductal adenocarcinoma cells through inhibition of the HGF/MET pathway. Oncogene 37:6041–6053. 10.1038/s41388-018-0403-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Son YS, Lee DY, Chung EK, Hur KC, Hong SI, Fuchs E (1996) Calcipotriol (MC 903), a synthetic derivative of vitamin D3 stimulates differentiation of squamous carcinoma cell line in the raft culture. Anticancer Res 16:337–347 [PubMed] [Google Scholar]
- Fujimoto W, Nakanishi G, Arata J, Jetten AM (1997) Differential expression of human cornifin alpha and beta in squamous differentiating epithelial tissues and several skin lesions. J Invest Dermatol 108:200–204. 10.1111/1523-1747.ep12334240 [DOI] [PubMed] [Google Scholar]
- Gao H, Cong X, Zhou J, Guan M (2017) MicroRNA-222 influences migration and invasion through MIA3 in colorectal cancer. Cancer Cell Int 17:78. 10.1186/s12935-017-0447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T et al (2004a) MUC20 suppresses the hepatocyte growth factor-induced Grb2-Ras pathway by binding to a multifunctional docking site of met. Mol Cell Biol 24:7456–7468. 10.1128/mcb.24.17.7456-7468.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T et al (2004b) Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem 279:1968–1979. 10.1074/jbc.M304558200 [DOI] [PubMed] [Google Scholar]
- Hippo Y et al (2001) Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res 61:889–895 [PubMed] [Google Scholar]
- Kitamoto S, Yokoyama S, Higashi M, Yamada N, Takao S, Yonezawa S (2013) MUC1 enhances hypoxia-driven angiogenesis through the regulation of multiple proangiogenic factors. Oncogene 32:4614–4621. 10.1038/onc.2012.478 [DOI] [PubMed] [Google Scholar]
- Kitamura R et al (2012) Association of cytokeratin 17 expression with differentiation in oral squamous cell carcinoma. J Cancer Res Clin Oncol 138:1299–1310. 10.1007/s00432-012-1202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Ding LJ, Wang ZX (2017) Mucin expression profile of benign and malignant cervical tissues and correlation with clinical-pathologic parameters. Eur J Gynaecol Oncol 38:350–355 [PubMed] [Google Scholar]
- Kurihara M et al (2013) Protumoral roles of melanoma inhibitory activity 2 in oral squamous cell carcinoma. Br J Cancer 108:1460–1469. 10.1038/bjc.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara-Shimomura M, Sasahira T, Shimomura H, Bosserhoff AK, Kirita T (2020) Mast cell chymase promotes angiogenesis and lymphangiogenesis mediated by activation of melanoma inhibitory activity gene family members in oral squamous cell carcinoma. Int J Oncol 56:1093–1100. 10.3892/ijo.2020.4996 [DOI] [PubMed] [Google Scholar]
- Li W et al (2001) The p38-MAPK/SAPK pathway is required for human keratinocyte migration on dermal collagen. J Invest Dermatol 117:1601–1611. 10.1046/j.0022-202x.2001.01608.x [DOI] [PubMed] [Google Scholar]
- Ludovini V et al (2016) Gene identification for risk of relapse in stage I lung adenocarcinoma patients: a combined methodology of gene expression profiling and computational gene network analysis. Oncotarget 7:30561–30574. 10.18632/oncotarget.8723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura A et al (2013) HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int J Oncol 42:535–542. 10.3892/ijo.2012.1728 [DOI] [PubMed] [Google Scholar]
- Michifuri Y et al (2013) Small proline-rich protein-1B is overexpressed in human oral squamous cell cancer stem-like cells and is related to their growth through activation of MAP kinase signal. Biochem Biophys Res Commun 439:96–102. 10.1016/j.bbrc.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Miranda-Filho A, Bray F (2020) Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol 102:104551. 10.1016/j.oraloncology.2019.104551 [DOI] [PubMed] [Google Scholar]
- Momose F, Araida T, Negishi A, Ichijo H, Shioda S, Sasaki S (1989) Variant sublines with different metastatic potentials selected in nude mice from human oral squamous cell carcinomas. J Oral Pathol Med 18:391–395. 10.1111/j.1600-0714.1989.tb01570.x [DOI] [PubMed] [Google Scholar]
- Morfoisse F et al (2014) Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1α-independent translation-mediated mechanism. Cell Rep 6:155–167. 10.1016/j.celrep.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Naher L et al (2012) STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma. Int J Oncol 41:1577–1586. 10.3892/ijo.2012.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan B (2017) Head and neck tumours. In: Brierley JD, Gospodarowicz MK, Wittekind C (eds) UICC TNM classification of malignant tumours, 8 th edn. Wiley-Blackwell, Hoboken, pp 17–54 [Google Scholar]
- Patterson T, Vuong H, Liaw YS, Wu R, Kalvakolanu DV, Reddy SP (2001) Mechanism of repression of squamous differentiation marker, SPRR1B, in malignant bronchial epithelial cells: role of critical TRE-sites and its transacting factors. Oncogene 20:634–644. 10.1038/sj.onc.1204134 [DOI] [PubMed] [Google Scholar]
- Reddy SP, Adiseshaiah P, Shapiro P, Vuong H (2002) BMK1 (ERK5) regulates squamous differentiation marker SPRR1B transcription in Clara-like H441 cells. Am J Respir Cell Mol Biol 27:64–70. 10.1165/ajrcmb.27.1.20020003oc [DOI] [PubMed] [Google Scholar]
- Reddy SP, Vuong H, Adiseshaiah P (2003) Interplay between proximal and distal promoter elements is required for squamous differentiation marker induction in the bronchial epithelium: role for ESE-1, Sp1, and AP-1 proteins. J Biol Chem 278:21378–21387. 10.1074/jbc.M212258200 [DOI] [PubMed] [Google Scholar]
- Riechers A, Bosserhoff AK (2014) Melanoma inhibitory activity in melanoma diagnostics and therapy - a small protein is looming large. Exp Dermatol 23:12–14. 10.1111/exd.12281 [DOI] [PubMed] [Google Scholar]
- Sasahira T, Kirita T (2018) Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int J Mol Sci. 10.3390/ijms19082413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahira T, Kirita T, Kuniyasu H (2014a) Update of molecular pathobiology in oral cancer: a review. Int J Clin Oncol 19:431–436. 10.1007/s10147-014-0684-4 [DOI] [PubMed] [Google Scholar]
- Sasahira T et al (2014b) Transport and Golgi organisation protein 1 is a novel tumour progressive factor in oral squamous cell carcinoma. Eur J Cancer 50:2142–2151. 10.1016/j.ejca.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Sasahira T, Nishiguchi Y, Fujiwara R, Kurihara M, Kirita T, Bosserhoff AK, Kuniyasu H (2016) Storkhead box 2 and melanoma inhibitory activity promote oral squamous cell carcinoma progression. Oncotarget 7:26751–26764. 10.18632/oncotarget.8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahira T, Bosserhoff AK, Kirita T (2018) The importance of melanoma inhibitory activity gene family in the tumor progression of oral cancer. Pathol Int 68:278–286. 10.1111/pin.12672 [DOI] [PubMed] [Google Scholar]
- Schmidt J, Riechers A, Bosserhoff AK (2013) MIA–a new target protein for malignant melanoma therapy. Histol Histopathol 28:421–426. 10.14670/hh-28.421 [DOI] [PubMed] [Google Scholar]
- Schubert SA et al (2017) Evidence for genetic association between chromosome 1q loci and predisposition to colorectal neoplasia. Br J Cancer 117:1215–1223. 10.1038/bjc.2017.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LY, Wang H, Dong B, Yan WP, Lin Y, Shi Q, Chen KN (2016) Possible prediction of the response of esophageal squamous cell carcinoma to neoadjuvant chemotherapy based on gene expression profiling. Oncotarget 7:4531–4541. 10.18632/oncotarget.6554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura H, Sasahira T, Nakashima C, Kurihara-Shimomura M, Kirita T (2019) Non-SMC condensin I complex subunit H (NCAPH) is associated with lymphangiogenesis and drug resistance in oral squamous cell carcinoma. J Clin Med. 10.3390/jcm9010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan P et al (2017) Malignant surface epithelial tumors. In: El-Naggar AK, Chan JKC, Rubin Grandis J, Takata T, Slootweg PJ (eds) WHO classification of head and neck tumours, 4th edn. World Health Organization, Lyon, pp 17–54 [Google Scholar]
- Swoboda A, Schanab O, Tauber S, Bilban M, Berger W, Petzelbauer P, Mikula M (2012) MET expression in melanoma correlates with a lymphangiogenic phenotype. Hum Mol Genet 21:3387–3396. 10.1093/hmg/dds171 [DOI] [PubMed] [Google Scholar]
- Wang H, Shen L, Lin Y, Shi Q, Yang Y, Chen K (2015) The expression and prognostic significance of Mucin 13 and Mucin 20 in esophageal squamous cell carcinoma. J Cancer Res Ther 11(Suppl 1):C74-79. 10.4103/0973-1482.163846 [DOI] [PubMed] [Google Scholar]
- Wilson DG et al (2011) Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol 193:935–951. 10.1083/jcb.201007162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AM, Argüeso P (2014) Expression analysis of the transmembrane mucin MUC20 in human corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci 55:6132–6138. 10.1167/iovs.14-15269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X et al (2013) Role of MUC20 overexpression as a predictor of recurrence and poor outcome in colorectal cancer. J Transl Med 11:151. 10.1186/1479-5876-11-151 [DOI] [PMC free article] [PubMed] [Google Scholar]