Abstract
Purpose
Mutations in BRAF are the most prominent activating mutations in melanoma and are increasingly recognized in other cancers. There is currently no accepted treatment regimen for patients with mutant BRAFK601N melanoma, and the study of melanoma driven by BRAF mutations at the 601 locus is lacking due to a paucity of cellular model systems. Therefore, we sought to better understand the treatment and clinical approach to patients with mutant BRAFK601N melanoma and subsequently develop a novel personalized oncology platform for rare or treatment-refractory cancers.
Methods
We developed and characterized the first patient-derived, naturally occurring BRAFK601N melanoma model, described herein as OHRI-MEL-13, and assessed efficacy using the Prestwick Chemical Library and select targeted therapeutics.
Results
OHRI-MEL-13 exhibits loss of heterozygosity of BRAF, closely mimics the original tumor’s gene expression profile, is tumorigenic in immune-deficient murine models, and is available for public accession through American Type Culture Collection. We present in silico modeling data, which illustrates the therapeutic failure of BRAFV600E-targeted therapies in BRAFK601N mutants. Our platform elucidated a unique role for MEK inhibition with cobimetinib, which resulted in short-term clinical success by reducing the metastatic burden.
Conclusion
Our model of BRAFK601N-activated melanoma was developed, thoroughly characterized, and made available for public accession. This model served to demonstrate the feasibility of a novel personalized oncology platform that could be optimized at an institutional level for rare variant or treatment-refractory cancers. We also demonstrate the clinical utility of monotherapy MEK inhibition in a case of BRAFK601N melanoma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03545-2.
Keywords: BRAFK601N, Model development, Personalized oncology, Pipeline, Drug discovery
Background
The growth signal-mediating MAP kinase (MAPK) pathway is known to be activated in a large proportion of human cancers. BRAF is one component of the MAPK pathway that is mutated in approximately 8% of human cancers and in most malignant melanomas as well as several other cancer types. This group most notably includes papillary thyroid carcinoma, colorectal carcinoma, non-small cell lung cancer, some forms of leukemia, and less frequently multiple myeloma, low-grade serous ovarian cancers, and others (Tol et al. 2009; Leonetti et al. 2018; Grisham et al. 2013; Kebebew et al. 2007; Davies et al. 2002; Lohr et al. 2014; Schadendorf et al. 2018). The presence of a BRAF mutation is generally a negative prognostic factor, however, paradoxically, the presence of BRAF mutations in low-grade serous ovarian cancers has been identified as a positive prognostic indicator (Grisham et al. 2013; Gershenson et al. 2015). Mutated BRAF proteins commonly lead to a constitutively activated MAPK pathway due to an increase in its serine/threonine kinase activity (Davies et al. 2002). The most common BRAF mutation is a valine-to-glutamic acid substitution at codon 600 (V600E); however, dozens of other specific mutations have been observed. The frequency of the BRAFV600E mutation in melanoma ranges 38–89% with significant cohort variability (Davies et al. 2002; Greaves et al. 2013; Si et al. 2012; Krauthammer et al. 2015). Molecular and translational research has largely focused on the inhibition of this specific activating mutation and/or various factors within this pathway. There are many models derived from BRAFV600E-activated tumors which have led to the development of targeted pharmaceuticals and the downstream translation of these drugs into a standard treatment for BRAFV600E/K melanomas.
The continued advancement of targeted therapeutics is driven by impressive clinical effects, including the rapid reduction in tumor size that can be seen along with rapid symptomatic improvement and a low risk of serious systemic toxicity (Long et al. 2016; Ascierto et al. 2016). In the case of BRAFV600E-targeted inhibition, there tends to be an initial robust clinical response that is commonly followed by the development of treatment resistance in cells expressing wild-type (wt) BRAF (Halaban et al. 2010; Poulikakos et al. 2010). Clinicians can therapeutically target further downstream with MEK inhibitors, which can be used concurrently with BRAF inhibition (Larkin et al. 2014; Long et al. 2014, 2015; Robert et al. 2015). However, the treatment of non-V600E/K BRAF-mutant cancers has proven more challenging in part because of a less robust understanding of the mechanistic implications of such mutations stemming from the paucity of appropriate experimental models.
One site of non-V600E BRAF mutations occurs at codon 601, which encodes lysine in the wt protein. Missense mutations at this position account for approximately 3.5% of non-V600 BRAF mutants amongst various cancers in the publicly accessible database cBioPortal for Cancer Genomics (http://cbioportal.org/msk-impact) (Cerami et al. 2012; Gao et al. 2013). Most commonly, K601 BRAF missense mutations are observed in melanoma; however, such mutations have also been described in colorectal adenocarcinoma, prostate adenocarcinoma, lung adenocarcinoma, uterine leiomyosarcoma, thyroid cancer, salivary duct carcinoma, intrahepatic cholangiocarcinoma, invasive breast carcinoma, and T-cell lymphoma (Cerami et al. 2012; Gao et al. 2013; Zehir et al. 2017). One oncogenic variant is that of BRAFK601N, which confers a gain-of-function substitution resulting in increased MEK and ERK phosphorylation (Lohr et al. 2014), an increase in cell proliferation, survival, and metastasis (Roberts and Der 2007). MEK inhibitors, such as trametinib and cobimetinib, may have therapeutic efficacy in cases with BRAFK601 mutations (Dahlman et al. 2012; Marconcini et al. 2017; Bowyer et al. 2014; Kim et al. 2013; Menzer et al. 2019). However, recent data suggest that this may not be the case (Johnson et al. 2020). While the utility of BRAF and MEK inhibition in BRAFV600E/K melanomas is well established and supported clinically, the approach to non-V600E BRAF-mutant melanomas using this class of therapy is not well established. This gap in clinical knowledge is driven by a fundamental limitation of basic knowledge focused on non-V600E BRAF-mutant melanomas, which is limited by the lack of appropriate experimental models. Unfortunately, no targeted therapies for these variants currently exist.
Commercially available melanoma cell lines with non-V600E BRAF mutations are difficult to come by. Indeed, of the 9 melanoma cell lines in the NCI-60 panel, 8 are BRAFV600E mutants and one represents wtBRAF. Likewise, all 41 melanoma cell lines analyzed from the American Type Culture Collection (ATCC; as of May 2020) (31 with known BRAF status) contain BRAF mutations only at the V600 (n = 23) and V599 (n = 1) loci, or are wt (n = 7). Interestingly, to our knowledge, there is only one established cancer cell line that expresses a BRAFK601N mutation—the multiple myeloma cell line U266, which is known to carry a duplication of the q32q34 region of chromosome 7 in which BRAF is located and thus carries the likelihood that the BRAF allele is duplicated (Lode et al. 2013; Drexler and Matsuo 2000; Nilsson et al. 1970). As a result of the lack of BRAFK601N experimental models, and the complete lack of such melanoma models, the laboratory study of these cancers has not been possible thus far. These facts make the basic understanding of the molecular interactions between targeted therapies and individual disease nearly impossible for scientists and physicians as we seek to improve therapeutic outcomes for all genetic variants and all individual patients.
Recently, there has been an emergence of functional genomic methodology that aims to characterize the phenotypic characteristics of cancer variants of unknown significance. This has taken the form of more than a dozen computational approaches (Porta-Pardo et al. 2017), and even more recently in assays that can study minimal functional readouts in vitro from large numbers of selected genetic variants (Ng et al. 2018; Kohsaka et al. 2017; Kim et al. 2016). Despite these advances, none of these tools have yet been translated for clinical utility, and it is likely that a lower throughput approach might be necessary to gain greater functional perspective before downstream translation. Novel pipelines that may be utilized to translate basic laboratory findings to clinical practice are needed. One possible approach is the screening of large drug libraries of FDA-approved agents, such as the Prestwick Chemical Library for efficacy on patient-derived, and therefore personalized, models of disease (Coleman et al. 2016). Cancers, such as melanoma, represent a clinical context in which such pipelines could be explored because patients often present in the early stages of disease up to several years before metastases may develop. In this study, we have generated, characterized, and made available for public accession a BRAFK601N model of melanoma, performed a drug screen using the Prestwick Chemical Library that identified various novel agents as potential therapeutics in melanoma, and we have additionally demonstrated a clinical response of BRAFK601N melanoma to monotherapy MEK inhibition.
Methods
Establishing a BRAFK601N-activated melanoma model for experimental use
OHRI-MEL-13 was derived from an excised focus of melanoma that had metastasized to the patient’s left pelvic lymph nodes. The surgery was performed at The Ottawa Hospital General Campus and tissue was taken following the receipt of full informed consent from the patient according to the Ottawa Health Science Network Research Ethics Board protocol number 20120559-01. Primary culture was established following scalpel-mediated homogenization of the tumor specimen (Fig. 1a) in the presence of Roswell Park Memorial Institute (RPMI)-1640 medium (Corning, Manassas, USA) supplemented with 10% fetal bovine serum, 100 µg/mL Gentamicin (Gibco—Life Technologies, Grand Island, USA), 0.1 mg/mL Normocin™ (InvivoGen, San Diego, USA), and 1× Antibiotic–Antimycotic (Gibco—ThermoFisher Scientific, Toronto, Canada). The homogenate was then filtered through a 70 μm nylon mesh cell strainer (ThermoFisher Scientific, Toronto, Canada). Homogenate was maintained in 5% CO2 with periodically refreshed media until sufficient cellular proliferation occurred for experimental purposes and cryopreservation.
Fig. 1.
Deriving and characterizing an in vitro and in vivo model of naturally occurring BRAFK601N melanoma. a OHRI-MEL-13 was derived from an excised pelvic lymph node containing metastatic melanoma from a treatment-naïve individual. b Histological analysis of this specimen demonstrates loosely cohesive melanoma cells with a central focus of degenerating tumor cells. Original magnification × 200. Scale bar = 200 µm. c A representative photomicrograph of OHRI-MEL-13 cell growth from a limiting dilution colony-formation assay demonstrates the presence of in vitro colonies (scale bar = 250 µm), which can be quantified d to determine the representative colony-formation efficiency of this model in vitro. OHRI-MEL-13 cells were observed to be tumorigenic in both NOD/SCID e, f and nude g, h mice. Histological analysis of tumors from these animals was performed and the representative photomicrographs presented, original magnification ×200 (NOD/SCID = F; nude = H) were observed to be histologically indistinguishable from the original specimen. Scale bars = 200 µm. Transcriptomic profiling was performed on tissue from the original specimen as part of a standard clinical protocol as well as on OHRI-MEL-13 cells that had been maintained in vitro; this model was found to possess a lysine-to-asparagine activating mutation at codon 601 of BRAF (BRAFK601N; schematized in I), and does not contain a BRAFV600E mutation. A full gene expression analysis of 55 genes was performed on both the original tumor specimen as well as on cultured OHRI-MEL-13 cells, which demonstrates an in vitro model that is similar to the original specimen with respect to gene expression across a comprehensive panel of custom cancer-relevant genes j. Error bars represent standard deviation between all data sets (n = 3)
Basic characterization of OHRI-MEL-13 was performed initially at the RNA and protein levels. Reverse transcriptase PCR was performed using primers targeting the MelanA (TAAGGAAGGTGTCCTGTGC; AGAGACACTTTGCTGTCCCG), Tyrosinase (GGAAGAATGCTCCTGGCTGT; GGCTACAGACAATCTGCCAAG), and S100A1 (TTGGCCATCTGTCCAGAACC; TGTCCACAGCATCCACATCC) genes, respectively (Additional file 1). As part of routine clinical protocols, the original tumor specimen was studied histologically by a pathologist certified by the Royal College of Physicians and Surgeons of Canada, and some of the remainder of the tissue block was used to access tissue for molecular studies.
Cell lines and culture
The SK-MEL 28 cell line was obtained from the ATCC (RRID:CVCL_0526) and the UACC 62 (RRID:CVCL_1780) cell line was obtained from the National Cancer Institute (Bethesda, MD). Both were maintained according to distributor’s instructions. All cells were tested regularly for the absence of mycoplasma and were free of any contaminants throughout the duration of the experiments herein.
Public accession of OHRI-MEL-13
Early passage, cryopreserved OHRI-MEL-13 cells were submitted to the ATCC for public accession under the terms of ATCC Material Deposit Agreement 2017-MDA-00039A1 between the Ottawa Hospital Research Institute and ATCC. This model is now publically accessible as part of the ATCC General Collection (ATCC® CRL-3427™).
Plating efficiency and colony-formation assays
OHRI-MEL-13 cells were plated at increasing cell densities in 96-well format (n = 20/plating density). Following growth of well-defined colonies, the number of colonies per well was quantified and colony formation was calculated using the equation: Plating efficiency (%) = (No. colonies formed/No. cells seeded) × 100.
Animal husbandry and in vivo tumorigenicity
All experiments were performed in accordance with the University of Ottawa’s Animal Care and Veterinary Service guidelines and followed established ethics protocols. Both NOD/SCID (n = 5) and nude mice (n = 5) were implanted subcutaneously with 2 × 107 OHRI-MEL-13 cells that had been cultured as previously described. Tumors were observed to be palpable at approximately 20 days post-implantation, and tumors presented in Fig. 1 E and G were harvested following mouse sacrifice at 50 days and 38 days post-implantation, respectively. Histologic examination of tumors derived in vivo was performed by the same Royal College-certified pathologist that diagnosed the original clinical specimen and comparative analyses were performed at the time of study.
DNA extraction and next-generation sequencing
DNA was isolated from the original tumor specimen from unstained 10 μm sections of formalin-fixed, paraffin-embedded (FFPE) tumor blocks. Samples were deparaffinized in mineral oil and genomic DNA was isolated using the QIAamp FFPE Tissue Kit (Qiagen). NGS was performed using the Ion Torrent Ampliseq CHPv2 and analyzed according to clinical protocols at the Ottawa Hospital.
DNA was isolated from OHRI-MEL-13 cells using the Blood and Cell Culture DNA Mini Kit (Qiagen). NGS was performed using the Ion Torrent Ampliseq CHPv2 and OHRI-MEL-13 was proven to exhibit complete loss of heterozygosity within codon 601 of BRAF, with 100% of reads demonstrating a point mutation causing a lysine to asparagine substitution at this locus.
RNA extraction and transcriptomic profiling
OHRI-MEL-13-derived total RNA was extracted from cultured cells using TRIzol reagent (Thermo Fisher) following manufacturer’s instructions. Original tumor-derived total RNA was extracted from unstained sections of FFPE tumor with the RecoverAll Total Nucleic Acid Isolation Kit (Thermo Fisher) following microdissection with the Avenio Millisect (an automated tissue dissection instrument that allows for precise isolation of tumor). RNA quantity was determined using the Qubit RNA HS assay (Thermo Fisher).
Transcriptional readouts were assessed using the Nanostring nCounter instrument according to manufacturer’s recommendations. RNA (100 ng input) was analyzed using a custom codeset of 55 probes (reported in Additional file 2). Raw RNA counts were exported from the nSolver Analysis Software (Nanostring) and normalized using the geometric mean of 14 housekeeping genes.
The relative difference in expression between original tumor and OHRI-MEL-13 was defined as significant when greater than 1 order of magnitude separated the relative expression between original tumor and OHRI-MEL-13 when that gene had a relative expression of over 50 in the original tumor specimen.
BRAF and MEK targeted therapy assays
OHRI-MEL-13 and SK-MEL 28 cells were seeded in multi-well plate format and cultured to desired density before beginning experiments. Vemurafenib (Selleckchem, Houston, TX; Cat. # S1267), trametinib (Selleckchem; Cat. # S2673), and cobimetinib (Selleckchem; Cat. # S8041) were utilized according to manufacturer’s instructions at the stated concentrations. After treatment for 48 h, the cells were either (1) harvested for counting using a Vi-Cell (Beckman-Coulter, Brea, CA), and/or (2) overlayed with alamarBlue® metabolic dye (Thermo Fisher) until a visible indicator change occurred, at which point relative fluorescence was read using a Fluoroskan Ascent® FL (Thermo Fisher) system with an excitation filter of 540–570 nm and an emission filter of 580–610 nm. Crystal Violet assays were performed to visualize direct cytotoxicity by adding Crystal Violet solution to wells of interest for 10 min per well before aspirating off excess solution and washing wells thoroughly with water.
Molecular dynamics and potential of mean force analysis of BRAF binding to vemurafenib
As structures of BRAF protein consistently lack at least some density in the loop region associated with the V600 and K601 residues, a complete wt model was generated from the most complete structure (PDB: 5VAM) by modelling in the missing loop residues (606–609) using MODELLER (version 9, salilab.org/modeller/) with bound ligands and waters removed. The V600E and K601N mutants were then generated from the wt structure using MODELLER. The bound conformation of vemurafenib was taken from the PDB 5JSM (the chemical linker was manually removed) and modelled into the same binding sites of wt, V600E, and K601N by aligning the protein backbone of the binding site from 5JSM with the previously generated models using VMD (www.ks.uiuc.edu/Research/vmd/).
To prepare the structures for molecular dynamics (MD), the proteins were solvated in a TIP3P water box with 80 Å sides (giving at least 14 Å of water between periodic protein images), and solvated with 150 mM NaCl to neutralize charge using the LEaP program from the AMBER suite (http://ambermd.org/). The protein was parameterized using the AMBER FF14SB force field with charges from the same, whereas vemurafenib was parameterized using the GAFF2 force field and partial charges derived using the RED server (upjv.q4md-forcefieldtools.org/REDServer-Development/) using Gaussian C.01. Umbrella sampling simulations were performed as previously reported (Cui et al. 2017).
Screening OHRI-MEL-13 using the Prestwick Chemical Library
OHRI-MEL-13 cells were cultured as previously described and seeded in 96-well format at a density of 40,000 cells/well in 100uL total volume for high-throughput screening purposes. Assay plates were incubated at 37 °C, 5% CO2 overnight. Plates were then treated with the Prestwick Chemical Library (Prestwick Chemical, Illkirch, France) of approved pharmaceutical compounds (n = 3). Library drugs were dispensed to the central 10 columns of the assay plates using a Biotek Precision Pipetting System to a final concentration of 1 μM in treated wells. Cobimetinib (1 μM, Selleckchem, Houston, USA) was used as a positive control for this screening platform and 0.1% dimethyl sulfoxide (vehicle control, Fisher Scientific, USA) as a negative control. Treated plates were then incubated for 72 h at 37 °C, 5% CO2 (Additional file 3).
Following this incubation, assay plates equilibrated to room temperature for 30 min in staggered intervals to maintain 72 h between drug treatment and reading of relative cellular metabolic activity levels. The effect of library drugs on cell viability was assayed using resazurin metabolic dye (Sigma-Aldrich, St. Louis, USA). Treated cells were incubated with resazurin for 1.5 h at 37 °C, 5% CO2 prior to fluorescence readout (indicating metabolized resazurin and metabolically active cells) using a Biotek Synergy Mx Microplate Reader. The ability of library compounds to hinder OHRI-MEL-13 metabolic activity was assessed by normalizing fluorescent values to those of cells treated with negative control to determine the relative metabolic activity of each assay well. These relative values were subsequently compared to those obtained from cobimetinib-treated wells to determine how treatment with these FDA-approved compounds resulted in OHRI-MEL-13 cytotoxicity relative to our positive control. The full data from these assays is presented in Additional file 5.
Selected cytotoxic drugs from this assay, including cobimetinib, were used for further validation. OHRI-MEL-13 cells were plated in 96-well format and treated with digoxin, proscalliridin A, gemcitabine, docetaxel, doxorubicin, mitoxantrone, and cobimetinib at 1, 0.5, 0.25, and 0.125 μM (n = 6). Treatment duration and resazurin treatment were performed as described above. Statistical analysis was performed using a two-way ANOVA of the data followed by multiple comparisons post-hoc testing.
Statistical analysis
Cytotoxicity and cell viability experiments following vemurafenib treatment were analyzed using one-way analysis of variance tests with post hoc analyses comparing each variable against a normalized control group (DMSO). There were four replicates in each of these assays.
Similar MEK inhibition (cobimetinib and trametinib) assays were normalized to DMSO control (n = 18) with replicates (n = 6) within each treatment concentration group. Ordinary one-way analysis of variance tests were used for statistical analysis and comparisons were made to the respective DMSO control groups.
Prestwick screen validation studies were repeated in replicates (n = 6) and analyzed using two-way analysis of variance tests. Tukey multiple comparisons tests were used to compare group means with one another. The statistical results highlighted in Fig. 3b represent the results of studying the mean metabolic activity following treatment at various concentrations of cobimetinib with the means of the same using other agents. Denoted with asterisks are selected respective results of post hoc Tukey analyses for relevant compounds. The results presented herein are representative of further biological replicates and additional supportive experiments.
Fig. 3.
Large scale drug screen of BRAFK601N melanoma identifies MEK inhibition as an effective therapeutic approach. a The Prestwick Chemical Library was utilized as a screening tool on OHRI-MEL-13 and the 25 most efficacious agents are highlighted. b Using selected drugs from the initial screen to validate performance, along with the MEK inhibitor cobimetinib, post-treatment metabolic activity is observed across several selected treatment concentrations. The MEK inhibitor cobimetinib proved to be more efficacious as a possible cytotoxic agent in OHRI-MEL-13 in this in vitro setting. c, d The patient from whom OHRI-MEL-13 was originally derived was treated with cobimetinib and strong interval improvements in metastatic disease are observed at Treatment Day 154 in both hepatic c, and pulmonary d sites. Metastatic sites are highlighted by yellow arrows. Error bars represent the standard deviation between all data sets (a; n = 3) and standard error of the mean between all data sets (b; n = 6), respectively. Statistical analyses were performed using a two-way ANOVA followed by multiple comparison post-hoc tests, ns = not significant, **P < 0.01, ****P < 0.0001
All statistical analyses were performed using GraphPad Prism 7 software.
Results
Developing and characterizing a naturally occurring model of BRAFK601N melanoma
A 59-year-old male patient with no family history of melanoma and Fitzpatrick Type I–II skin presented with a palpable lymph node in the left inguinal chain approximately nine months following the excision of a primary melanoma (Breslow thickness 4.9 mm) on the left lower extremity (full case vignette presented below). With the exception of the initial surgery, this patient was treatment naïve. The initial ultrasound-guided biopsy of the palpable lymph node demonstrated extensive replacement of nodal architecture with malignant epithelioid neoplasm compatible with metastatic melanoma. The excised lymph node measured approximately 4 cm in diameter (Fig. 1a) and was histologically confirmed to demonstrate tumor architecture consistent with that of metastatic melanoma, a representative image of which is presented in Fig. 1b and demonstrates loosely cohesive melanoma cells with a central focus of degenerating tumor cells. Following informed consent, a small sample from this specimen was obtained and a primary tumor cell line was generated. This novel cell line expressed the selected diagnostic markers MelanA, Tyrosinase, and S100 by RT-PCR as did the commercially available melanoma cell lines SK-MEL 28 and UACC 62 used as experimental controls (Additional file 1).
Following the establishment of in vitro growth of cells derived from the biopsy specimen, the line named OHRI-MEL-13 demonstrated colony formation, the morphology of which can be observed in the representative photomicrograph in Fig. 1c. There was an in vitro doubling time of 42 h, and a colony plating efficiency of approximately 2.5% (Fig. 1d). The cultured cells were noted to form tumors in both non-obese diabetic/severe combined immunodeficient (NOD/SCID) and CD-1 nude mice, and the tumors established in these animals were assessed by the original diagnosing pathologist to be histologically indistinguishable from the patient-derived specimen (Fig. 1e–h).
DNA was extracted from the FFPE block made from the biopsy from which OHRI-MEL-13 was derived. Targeted next-generation sequencing (NGS) demonstrated a BRAFK601N mutation in the kinase domain (Fig. 1I) with loss of heterozygosity. This specimen does not possess the common V600E mutation. The gene expression of 55 genes in a previously established custom panel was studied (Fig. 1j) in the specimen (n = 1) isolated from the FFPE block as well as OHRI-MEL-13 cells from in vitro culture (n = 3). No significant difference in the relative expression was seen for 46 genes. No genes were observed to be upregulated in OHRI-MEL-13 when compared to the biopsy. The relative expression was found to be significantly downregulated in OHRI-MEL-13 compared to the original biopsy for CD74, SPP1, AHNAK, S100A4, S100A9, MGP, CXCL14, GJA1, and CRABP2. The full dataset generated from these assays can be observed in Additional file 2.
BRAF and MEK inhibition in a BRAFK601N model of melanoma
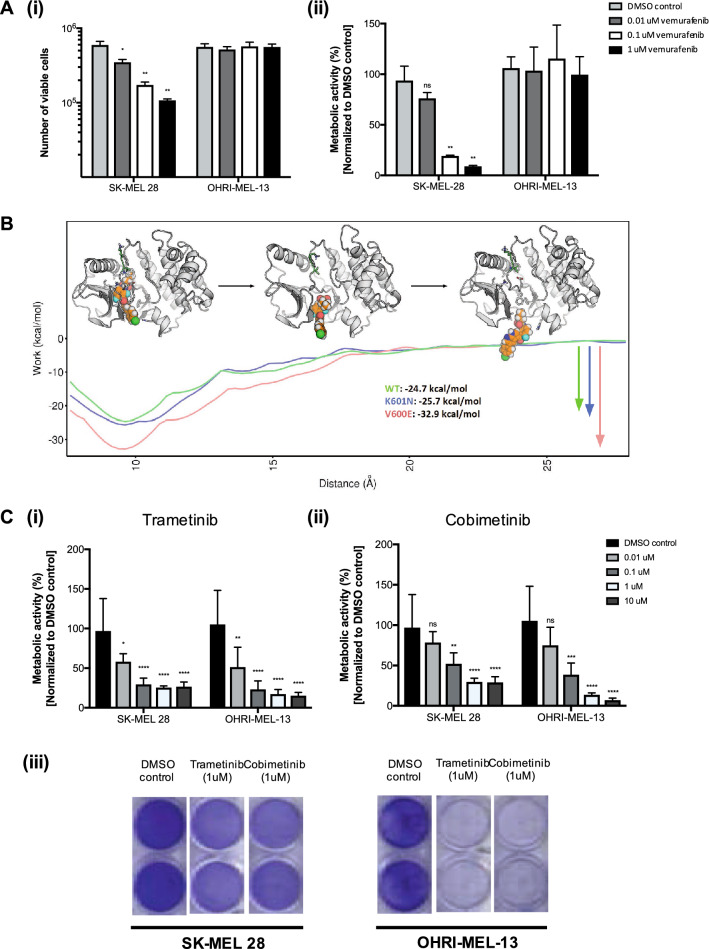
OHRI-MEL-13 was cultured in parallel with SK-MEL 28, a well-characterized melanoma cell line known to bear the BRAFV600E mutation in the presence of increasing concentrations of the BRAF inhibitor vemurafenib. In a concentration-dependent manner, vemurafenib was observed to be cytotoxic to SK-MEL 28 cells while non-cytotoxic to OHRI-MEL 13 cells. (Fig. 2a i, ii).
Fig. 2.
Selective BRAF inhibition of BRAFK601N-activated melanoma is not efficacious, while downstream MEK inhibition has therapeutic potential. a Targeted BRAF inhibition is observed to be cytotoxic in a concentration-dependent manner in SK-MEL-28, a BRAFV600E-activated melanoma model, while not efficacious in OHRI-MEL-13, a BRAFK601N-activated melanoma model as shown by cell quantification (i) and metabolic activity using the alamarBlue® assay (ii). b Enhanced sampling molecular dynamics is used to estimate the work needed to pull the vemurafenib molecule from the binding site of wt BRAF (green), BRAF-K601N (blue), and BRAF-V600E (red) proteins, showing that wt and K601N have similar binding affinity, while the binding of vemurafenib to the V600E variant is much stronger. Structures embedded in this figure illustrate representative molecular dynamics snapshots as vemurafenib is pulled from the wt protein, showing distances between the center of the drug molecule and center of the binding site (left-to-right) of 9, 16, and 23 Å, respectively, approximately corresponding to their positions along the x-axis. c Downstream MEK inhibition using trametinib (i) or cobimetinib (ii) was observed to be efficacious in OHRI-MEL-13, slightly more so than in SK-MEL-28 as measured by metabolic activity using the alamarBlue® assay. Remaining cellular metabolic activity following treatment with each respective drug can be observed (i, ii) as well as the cell viability following treatment as assessed by Crystal Violet staining (iii). Statistical analyses were performed using one-way ANOVAs followed by multiple comparison post-hoc tests, ns = not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Enhanced sampling molecular dynamics studies demonstrated that the binding affinities between vemurafenib and wtBRAF and BRAF-K601N were similar (-24.7 vs -25.7 kcal/mol, respectively); while the energy required to achieve dissociation of vemurafenib from BRAF-V600E was observed to be -32.9 kcal/mol (Fig. 2b). Structural dynamics simulations of interactions between vemurafenib and wtBRAF, BRAF-K601N, and BRAF-V600E proteins predict that differences could be explained (or modeled) by changes in a highly dynamic loop that forms between domains within the BRAF protein. As is represented in Additional file 4, the dynamic loop in the wtBRAF and BRAF-K601N proteins is formed by residues 596–615 and is partially structured by a series of hydrogen bonds between backbone carbonyl oxygens and the arginine sidechain at position 575. In the BRAF-V600E protein, by contrast, these hydrogen bonds are replaced by a salt-bridge that forms between the glutamate introduced at position 600 and the arginine at position 575, which causes the dynamic loop to adopt a more open conformation, and therefore improved protein-drug binding (Additional file 4). Therefore, the lack of toxicity of vemurafenib in OHRI-MEL-13 can be attributed to closed loop orientation of the K601N mutant protein, which reduces protein–drug binding. Our results suggest that BRAF inhibition with currently available BRAF-V600E inhibitors, such as vemurafenib, would not be successful due to the specificity of these drugs for BRAFV600E-activated cancers, which has been observed clinically (Fedor et al. 2019). As a result, we studied the efficacy of the MEK inhibitors trametinib and cobimetinib on OHRI-MEL-13. Both MEK inhibitors were cytotoxic to SK-MEL 28 and OHRI-MEL-13 in a concentration-dependent manner (Fig. 2c), and because there was a trend towards greater cytotoxicity of cobimetinib on OHRI-MEL-13 at higher concentrations, it was selected as a MEK inhibitor agent for further study.
Screening of large drug library in BRAFK601N melanoma
Cardiac glycosides and anthracyclines were the most effective drugs at killing OHRI-MEL-13 from the Prestwick Chemical Library, a 1200-compound small molecular library composed of FDA-approved compounds with clinical utility. The top hits were determined by cytotoxicity, which was measured as a function of post-treatment relative metabolic activity. This consisted of proscillaridin-A and digoxin, two cardiac glycosides (lanatoside C, and digitoxigenin were also found in the top 25 hits), and doxorubicin HCL and mitoxantrone, two anthracyclines. In addition, docetaxel and gemcitabine were of interest due to their well-studied mechanisms of action, frequent clinical use, and established safety profiles. The full results of our Prestwick Chemical Library screen on the BRAFK601N-activated melanoma cell line OHRI-MEL-13 can be observed in Additional file 5. These six compounds (Fig. 3a, highlighted in red) have well-studied molecular mechanisms of action, safety profiles, clinical accessibility and in vitro treatment resulted in a < 30% survival of OHRI-MEL-13 cells. Thus, they were selected for further comparative analysis alongside the MEK inhibitor cobimetinib.
In a subsequent and direct comparison, treatment of OHRI-MEL-13 with the MEK inhibitor cobimetinib was observed to be more efficacious than any of the other six selected compounds at all concentrations, and equal to docetaxel at 0.125 μM (Fig. 3b).
MEK inhibition in BRAFK601N melanoma–clinical correlation
Following a failed course of dual immune checkpoint inhibition, MEK inhibitor treatment with monotherapy cobimetinib was initiated. Treatment was initiated on “Treatment Day 0” (Fig. 3c,d) and continuously pursued for one month before becoming more sporadic over the next five months. On “Treatment Day 154”, the patient was observed to be significantly improved both clinically and radiographically (Fig. 3c,d). However, shortly thereafter, the patient presented with evidence of multiple brain lesions (data not shown) to which the patient succumbed to while pursuing an alternative therapeutic approach eight months after discontinuing cobimetinib treatment (detailed case vignette presented in Additional file 6).
Discussion and conclusion
BRAFK601N-activated melanoma is a rare genetic variant of melanoma about which little is currently known and for which no standard therapeutic approach exists. Despite the prevalence of BRAF-activated melanoma with mutations at codon 601 (representing approximately 3.5% of non-V600 BRAF-mutant melanomas), there is an extreme lack of experimental models that would allow for the effective study of such variants. As such, the clinical treatment of patients possessing such mutations is not well understood; however, anecdotal reports have suggested possibilities for treatment even across multiple cancer types (Wilson et al. 2014; Luk et al. 2015). In this study, we have established and comprehensively characterized the first patient-derived model of BRAFK601N melanoma, which is now available for public accession through the ATCC. As demonstrated in the data presented herein, this model effectively replicates in vitro, is tumorigenic in vivo, and tumors can be generated in both NOD/SCID and CD1 nude mice. Such tumors were observed to retain the unique histologic and genomic expression characteristics of the original specimen, and repeated NGS of this model has demonstrated the complete loss of heterozygosity in codon 601 of BRAF, suggesting that this is a pure BRAFK601N model with which effective future studies can be performed within the scientific community.
Interestingly, our comparative gene expression analyses demonstrated downregulation of nine genes in OHRI-MEL-13 compared to the original tumor from our pre-selected panel of 55 genes—CD74, SPP1, AHNAK, S100A4, S100A9, MGP, CXCL14, GJA1, and CRABP2. Each of these genes has been implicated on some level in oncogenesis or cancer progression. Although the findings regarding this specific gene set are interesting, it is important to understand that OHRI-MEL-13 has retained expression levels of approximately 85% of the endogenous genes studied and we believe it is therefore a highly representative model of the BRAFK601N melanoma from which it was derived.
Targeted therapy is commonly used for BRAFV600E/K-activated melanomas, which represents the most common genomic landscape seen clinically; however, the treatment approach to non-V600E BRAF-activated melanomas is unclear. This is especially true given the clinical adoption of immune checkpoint inhibition in recent years. However, if immune checkpoint inhibition fails, the clinician is then left with no options. To postulate whether the use of conventional targeted therapy may be efficacious in such cases, OHRI-MEL-13 cells were treated with vemurafenib, the BRAFV600E-specific inhibitor. As expected, this treatment demonstrated good concentration-dependent efficacy in BRAFV600E-activated cells with little-to-no therapeutic benefit in BRAFK601N-activated cells. However, our molecular dynamics simulations critically demonstrate the protein structural changes within the V600E and K601N mutant forms of BRAF proteins that cause differing responses to vemurafenib treatment. BRAF-V600E results in the formation of a salt-bridge anchored by the mutation-derived glutamate, which causes an open dynamic loop and significantly increased binding affinity between vemurafenib and the mutated protein compared to the wt version. Similar to wtBRAF, BRAF-K601N possesses a more closed dynamic loop that is partially structured by a series of hydrogen bonds, which lends itself to a significantly lower binding affinity with vemurafenib. This closed dynamic loop of BRAF-K601N compared to the open dynamic loop of BRAF-V600E explains the lack of response that was observed using BRAF inhibition in vitro in OHRI-MEL-13. Coupled with the fact that BRAFK601N is known to be an activating mutation, the decision was made to study downstream MEK inhibition with two independent agents on OHRI-MEL-13 cells, which resulted in a concentration-dependent cytotoxicity favoring cobimetinib. This simple result suggests that MEK inhibition may be effective in BRAFK601N-activated melanoma, and is likely to be more broadly representative of BRAF-activated melanomas with mutations at codon 601 since there is a lack of targeted therapies specifically designed for such cancers; however, this approach continues to provide an inhibitory effect within the activated pathway in question.
As we move more firmly towards an era of personalized oncology, there are many approaches and pipelines that may be utilized to best care for patients at the institutional level. In the present study, the use of the Prestwick Chemical Library in combination with our patient-derived in vitro model, helped to facilitate the understanding of treatment efficacy of a 1200-member panel of FDA-approved small molecules on this rare form of cancer with no established treatments. One interesting result from this screen was the clear emergence of several cardiac glycosides as potentially efficacious drugs for the treatment of this cancer. Notably, proscalliridin A and the clinically prolific digoxin were identified as the top two drugs in the screen. The idea that cardiac glycosides may be utilized in the treatment of some cancers first came to light in the 1980s as retrospective clinical studies began to demonstrate a prolonged survival of patients who were being treated for cancer with conventional chemotherapeutics concomitantly with their cardiac ailments (Kepp et al. 2012; Stenkvist et al. 1982, 1980, 1979). Mechanistically, this activity is due to Na+/K+ ATPase inhibition, making many types of cancer that are known to overexpress this solute pump exquisitely sensitive to cardiac glycosides (Kepp et al. 2012). From the perspective of contemporary anti-cancer therapeutics, it is also noteworthy that cardiac glycosides have been identified as causative agents of immunogenic cell death when used in combination with some classical chemotherapy agents (Menger et al. 2012). Interestingly, two recent reports have identified, both pre-clinically and clinically, the potential of combination cardiac glycoside treatment along with traditional targeted therapies in some melanomas (Eskiocak et al. 2016; Frankel et al. 2017). Taken together with the present data, this suggests that combination therapy using the MEK inhibitor cobimetinib plus a select cardiac glycoside, such as proscalliridin A or digoxin, may be a clinically reasonable approach in BRAFK601N melanoma or other codon 601-centric BRAF-activated melanomas; however, this is an area in which further study is absolutely required.
When the top performing molecules from the 1200-member panel of FDA-approved small molecules were validated along with the selected MEK inhibitor cobimetinib, it was interestingly noted that MEK inhibition demonstrated to be a more efficacious approach in vitro for OHRI-MEL-13. Initial success of cobimetinib was observed in controlling advancing metastatic disease in the liver, lung, and in transit metastases; however, the patient ultimately succumbed to advancing CNS lesions while pursuing an alternate therapeutic approach. Despite the poor clinical outcome ultimately, there is much to be learned from the treatment of BRAFK601N-activated melanoma using this approach, which is one that may warrant formal clinical investigation in the future.
Although the findings and tools generated from this study are interesting, it is necessary to acknowledge the fact that we have conducted this study on resources generated from one patient. As discussed above, the relative paucity of BRAFK601N-activated melanomas makes a significantly more highly powered study a logistical near impossibility, as did the complete lack of BRAFK601N melanoma models available for public accession. For this reason, the completion of this study at this point and making the public accession of this model possible is a crucial step as the community works to understand more about targeted therapy in all variants of melanoma, especially those with mutations at codon 601 of BRAF, and other cancers. With this first-of-its-kind cell line, further mechanistic studies are now possible to advance the translation of therapeutic options in these cases.
Furthermore, the widespread utility of such a pipeline could be a challenge at an institutional level; however, the approach (or aspects of the approach) described herein could be optimized as we work towards the delivery of truly personalized oncology. Optimization of such a pipeline could especially be of utility in rare variant cancers, such as the one presented herein, for which larger-scale bioinformatics-based predictive approaches have not been optimized. This pipeline could allow for a more rationalized approach to treating such cancers as the results from an FDA-approved library screen may point clinicians in the direction of a family of drugs that may have not been previously considered as a viable therapeutic option. One clear barrier that must be overcome is the reliable and timely generation of patient-derived in vitro models. In our hands, we were able to generate a patient-derived tumor line and perform screening using the Prestwick Library in 3 months. An institutionally-dedicated laboratory, however, would likely succeed in such an endeavor in a significantly shorter time frame as it has been known for many years that highly representative patient-derived in vitro models can be established from as little material as that obtained from a fine needle aspiration biopsy (Araujo et al. 1999; Riker et al. 1999). Alternatively, more recent developments in the high-throughput approach to personalized oncology use strictly genomics-based methodologies (Sho et al. 2017; Reaz et al. 2018; Pao and Fodor 2018), however, the effective development of patient-derived models may be necessary for a functional genomics approach especially if dealing with rare genetic variants or unique sets of mutations in genetically heterogeneous samples. In such cases, having a representative tumor-derived model on which genomics-based results can be confirmed at the pre-clinical level could be extremely powerful. We believe that these pipelines could be optimized at an institutional level for improved therapeutic outcomes of individuals with cancer, especially those with rare variant or treatment-refractory disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge technical assistance from those in the clinical histology and molecular genetics laboratories of the Ottawa Hospital. We also acknowledge the resourcefulness of Dr. Jim Dimitroulakos for managing the appropriate ethics protocols to obtain and utilize patient-derived specimens. Most importantly, we acknowledge the donation of tissue from all patients who contribute to our work, present and ongoing.
Abbreviations
- ATCC
American Type Culture Collection
- CNS
Central nervous system
- DNA
Deoxyribonucleic acid
- FDA
U.S. Food & Drug Administration
- FFPE
Formalin-fixed, paraffin-embedded
- MAPK
Mitogen-activated protein kinase
- MD
Molecular dynamics
- NCI
National Cancer Institute
- RNA
Ribonucleic acid
- RPMI
Roswell Park Memorial Institute
- WT
Wild type
Author contributions
BAK obtained specimen, created cell line, designed experiments, conducted some experiments, analyzed data, and wrote manuscript. BJL performed BRAF and MEK cytotoxicity studies. BJL and OV performed high-throughput drug screening and associated data analysis. AB and RAC performed enhanced sampling molecular dynamics studies and associated modeling. MEW, BM, CC, and JP assisted in technical capacities and helped execute selected experiments. BJL conducted and analyzed transcriptome analyses. BB performed histological analysis of both patient-derived and mouse specimens. CN performed surgeries. MO provided medical oncology care for patient and assessed medical progress. HLA, J-SD, CSI and JCB supervised all aspects of project and participated in manuscript writing. All authors were involved in manuscript editing.
Funding
The work performed herein was partially funded by a Terry Fox Research Institute, New Frontiers Program in Cancer grant number TFF-122868 and by a Canadian Institutes in Health Research Foundation Scheme grant number FDN-148428. BAK was funded by a Vanier Canada Graduate Scholarship. OV was supported by a CIHR Master’s Award. AB was funded by an NSERC postdoctoral fellowship. MEW was supported by an Ontario Graduate Scholarship.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests. OHRI-MEL-13 was licensed to the American Type Culture Collection (ATCC) by the Ottawa Hospital Research Institute according to the terms set forth in the ATCC Material Deposit Agreement.
Ethics approval
Experiments including mouse models were undertaken at the University of Ottawa Animal Care and Veterinary Services facility and approved by the local institutional ethics board.
Consent to participate
Full informed consent from the patient participant was obtained and approved according to the Ottawa Health Science Network Research Ethics Board protocol number 20120559-01.
Consent for publication
Consent for publication has been obtained according to the Ottawa Health Science Network Research Ethics Board protocol outlined above.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Araujo RW, Paiva V, Gartner F, Amendoeira I, Oliveira JM, Schmitt FC (1999) Fine needle aspiration as a tool to establish primary human breast cancer cultures in vitro. Acta Cytol 43:985–990 [DOI] [PubMed] [Google Scholar]
- Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandala M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Yan Y, Wongchenko M, Chang I, Hsu JJ, Koralek DO, Rooney I, Ribas A, Larkin J (2016) Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17:1248–1260 [DOI] [PubMed] [Google Scholar]
- Bowyer SE, Rao AD, Lyle M, Sandhu S, Long GV, McArthur GA, Raleigh JM, Hicks RJ, Millward M (2014) Activity of trametinib in K601E and L597Q BRAF mutation-positive metastatic melanoma. Melanoma Res 24:504–508 [DOI] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DT, Gray AL, Stephens CA, Scott ML, Cardelli JA (2016) Repurposed drug screen identifies cardiac glycosides as inhibitors of TGF-beta-induced cancer-associated fibroblast differentiation. Oncotarget 7:32200–32209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui DS, Broom A, McLeod MJ, Meiering EM, Holyoak T (2017) Asymmetric anchoring is required for efficient omega-loop opening and closing in cytosolic phosphoenolpyruvate carboxykinase. Biochemistry 56:2106–2115 [DOI] [PubMed] [Google Scholar]
- Dahlman KB, Xia J, Hutchinson K, Ng C, Hucks D, Jia P, Atefi M, Su Z, Branch S, Lyle PL, Hicks DJ, Bozon V, Glaspy JA, Rosen N, Solit DB, Netterville JL, Vnencak-Jones CL, Sosman JA, Ribas A, Zhao Z, Pao W (2012) BRAF(L597) mutations in melanoma are associated with sensitivity to MEK inhibitors. Cancer Discov 2:791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- Drexler HG, Matsuo Y (2000) Malignant hematopoietic cell lines: in vitro models for the study of multiple myeloma and plasma cell leukemia. Leuk Res 24:681–703 [DOI] [PubMed] [Google Scholar]
- Eskiocak U, Ramesh V, Gill JG, Zhao Z, Yuan SW, Wang M, Vandergriff T, Shackleton M, Quintana E, Johnson TM, DeBerardinis RJ, Morrison SJ (2016) Synergistic effects of ion transporter and MAP kinase pathway inhibitors in melanoma. Nat Commun 7:12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor V, Mikhail E, Svetlana M, Vladimir, and Evgeny. (2019) Lack of response to vemurafenib in melanoma carrying BRAF K601E mutation. Case Rep Oncol 12:339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AE, Eskiocak U, Gill JG, Yuan S, Ramesh V, Froehlich TW, Ahn C, Morrison SJ (2017) Digoxin plus trametinib therapy achieves disease control in Braf wild-type metastatic melanoma patients. Neoplasia 19:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenson DM, Sun CC, Wong KK (2015) Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br J Cancer 113:1254–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves WO, Verma S, Patel KP, Davies MA, Barkoh BA, Galbincea JM, Yao H, Lazar AJ, Aldape KD, Medeiros LJ, Luthra R (2013) Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn 15:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham RN, Iyer G, Garg K, Delair D, Hyman DM, Zhou Q, Iasonos A, Berger MF, Dao F, Spriggs DR, Levine DA, Aghajanian C, Solit DB (2013) BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 119:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y, Sznol M (2010) PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res 23:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Zhao F, Noel M, Riely GJ, Mitchell EP, Wright JJ, Chen HX, Gray RJ, Li S, McShane LM, Rubinstein LV, David Patton P, Williams M, Hamilton SR, Conley BA, Arteaga CL, Harris LN, O’Dwyer PJ, Chen AP, Flaherty KT (2020) Trametinib activity in patients with solid tumors and lymphomas harboring BRAF non-V600 mutations or fusions: results from NCI-MATCH (EAY131). Clin Cancer Res 26(8):1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh QY, Shibru D, Bastian B, Griffin A (2007) The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246:466–470; discussion 70–71 [DOI] [PMC free article] [PubMed]
- Kepp O, Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Sukkurwala AQ, Michaud M, Galluzzi L, Zitvogel L, Kroemer G (2012) Anticancer activity of cardiac glycosides: at the frontier between cell-autonomous and immunological effects. Oncoimmunology 1:1640–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, Fecher LA, Millward M, McArthur GA, Hwu P, Gonzalez R, Ott PA, Long GV, Gardner OS, Ouellet D, Xu Y, DeMarini DJ, Le NT, Patel K, Lewis KD (2013) Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 31:482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Ilic N, Shrestha Y, Zou L, Kamburov A, Zhu C, Yang X, Lubonja R, Tran N, Nguyen C, Lawrence MS, Piccioni F, Bagul M, Doench JG, Chouinard CR, Wu X, Hogstrom L, Natoli T, Tamayo P, Horn H, Corsello SM, Lage K, Root DE, Subramanian A, Golub TR, Getz G, Boehm JS, Hahn WC (2016) Systematic functional interrogation of rare cancer variants identifies oncogenic alleles. Cancer Discov 6:714–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, Shimada N, Takahashi K, Suzuki K, Takamochi K, Takahashi F, Mano H (2017) A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med 9(416):eaan6566 [DOI] [PubMed] [Google Scholar]
- Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, McCusker JP, Ma S, Cheng E, Straub R, Serin M, Bosenberg M, Ariyan S, Narayan D, Sznol M, Kluger HM, Mane S, Schlessinger J, Lifton RP, Halaban R (2015) Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet 47:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Sovak MA, Chang I, Choong N, Hack SP, McArthur GA, Ribas A (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371:1867–1876 [DOI] [PubMed] [Google Scholar]
- Leonetti A, Facchinetti F, Rossi G, Minari R, Conti A, Friboulet L, Tiseo M, Planchard D (2018) BRAF in non-small cell lung cancer (NSCLC): pickaxing another brick in the wall. Cancer Treat Rev 66:82–94 [DOI] [PubMed] [Google Scholar]
- Lode L, Moreau P, Menard A, Godon C, Touzeau C, Amiot M, Le Gouill S, Bene MC, Pellat-Deceunynck C (2013) Lack of BRAF V600E mutation in human myeloma cell lines established from myeloma patients with extramedullary disease. Blood Cancer J 3:e163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo P, Lawrence MS, Auclair D, Sougnez C, Knoechel B, Gould J, Saksena G, Cibulskis K, McKenna A, Chapman MA, Straussman R, Levy J, Perkins LM, Keats JJ, Schumacher SE, Rosenberg M, Consortium Multiple Myeloma Research, Getz G, Golub TR (2014) Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell 25:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandala M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin AM, Le N, Patel K, Flaherty K (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–1888 [DOI] [PubMed] [Google Scholar]
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion-Sileni V, Lebbe C, Mandala M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Swann S, Legos JJ, Jin F, Mookerjee B, Flaherty K (2015) Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444–451 [DOI] [PubMed] [Google Scholar]
- Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, Sosman JA, Hamid O, Schuchter L, Cebon J, Kefford RF, Lawrence D, Kudchadkar R, Burris HA 3rd, Falchook GS, Algazi A, Lewis K, Puzanov I, Ibrahim N, Sun P, Cunningham E, Kline AS, Del Buono H, McDowell DO, Patel K, Flaherty KT (2016) Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 34:871–878 [DOI] [PubMed] [Google Scholar]
- Luk PP, Yu B, Ng CC, Mercorella B, Selinger C, Lum T, Kao S, O’Toole SA, Cooper WA (2015) BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res 4:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconcini R, Galli L, Antonuzzo A, Bursi S, Roncella C, Fontanini G, Sensi E, Falcone A (2017) Metastatic BRAF K601E-mutated melanoma reaches complete response to MEK inhibitor trametinib administered for over 36 months. Exp Hematol Oncol 6:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, Schlemmer F, Sulpice E, Locher C, Gidrol X, Ghiringhelli F, Modjtahedi N, Galluzzi L, Andre F, Zitvogel L, Kepp O, Kroemer G (2012) Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 4:143ra99 [DOI] [PubMed] [Google Scholar]
- Menzer C, Menzies AM, Carlino MS, Reijers I, Groen EJ, Eigentler T, Jan WB, Groot De, Van Der Veldt AAM, Johnson DB, Meiss F, Schlaak M, Schilling B, Westgeest HM, Gutzmer R, Pföhler C, Meier F, Zimmer L, Suijkerbuijk KPM, Haalck T, Thoms K-M, Herbschleb K, Leichsenring J, Menzer A, Kopp-Schneider A, Long GV, Kefford R, Enk A, Blank CU, Hassel JC (2019) Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol 37:3142–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PK, Li J, Jeong KJ, Shao S, Chen H, Tsang YH, Sengupta S, Wang Z, Bhavana VH, Tran R, Soewito S, Minussi DC, Moreno D, Kong K, Dogruluk T, Lu H, Gao J, Tokheim C, Zhou DC, Johnson AM, Zeng J, Ip CKM, Ju Z, Wester M, Yu S, Li Y, Vellano CP, Schultz N, Karchin R, Ding L, Lu Y, Cheung LWT, Chen K, Shaw KR, Meric-Bernstam F, Scott KL, Yi S, Sahni N, Liang H, Mills GB (2018) Systematic functional annotation of somatic mutations in cancer. Cancer Cell 33:450-462e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K, Bennich H, Johansson SG, Ponten J (1970) Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol 7:477–489 [PMC free article] [PubMed] [Google Scholar]
- Pao W, Fodor I (2018) Catalyzing the field of precision oncology, one basket at a time. Nat Med 24:387–388 [DOI] [PubMed] [Google Scholar]
- Porta-Pardo E, Kamburov A, Tamborero D, Pons T, Grases D, Valencia A, Lopez-Bigas N, Getz G, Godzik A (2017) Comparison of algorithms for the detection of cancer drivers at subgene resolution. Nat Methods 14:782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N (2010) RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464:427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaz S, Tamkus D, Andrechek ER (2018) Using gene expression data to direct breast cancer therapy: evidence from a preclinical trial. J Mol Med (Berl) 96:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riker AI, Panelli MC, Kammula US, Wang E, Wunderlich J, Abati A, Fetsch P, Rosenberg SA, Marincola FM (1999) Development and characterization of melanoma cell lines established by fine-needle aspiration biopsy: advances in the monitoring of patients with metastatic melanoma. Cancer Detect Prev 23:387–396 [DOI] [PubMed]
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D (2015) Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30–39 [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291–3310 [DOI] [PubMed] [Google Scholar]
- Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A, Ugurel S (2018) Melanoma. Lancet 392:971–984 [DOI] [PubMed] [Google Scholar]
- Sho S, Court CM, Kim S, Braxton DR, Hou S, Muthusamy VR, Watson RR, Sedarat A, Tseng HR, Tomlinson JS (2017) Digital PCR improves mutation analysis in pancreas fine needle aspiration biopsy specimens. PLoS ONE 12:e0170897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si L, Kong Y, Xu X, Flaherty KT, Sheng X, Cui C, Chi Z, Li S, Mao L, Guo J (2012) Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer 48:94–100 [DOI] [PubMed] [Google Scholar]
- Stenkvist B, Bengtsson E, Eriksson O, Holmquist J, Nordin B, Westman-Naeser S (1979) Cardiac glycosides and breast cancer. Lancet 1:563 [DOI] [PubMed] [Google Scholar]
- Stenkvist B, Bengtsson E, Eklund G, Eriksson O, Holmquist J, Nordin B, Westman-Naeser S (1980) Evidence of a modifying influence of heart glucosides on the development of breast cancer. Anal Quant Cytol 2:49–54 [PubMed] [Google Scholar]
- Stenkvist B, Bengtsson E, Dahlqvist B, Eriksson O, Jarkrans T, Nordin B (1982) Cardiac glycosides and breast cancer, revisited. N Engl J Med 306:484 [PubMed] [Google Scholar]
- Tol J, Nagtegaal ID, Punt CJ (2009) BRAF mutation in metastatic colorectal cancer. N Engl J Med 361:98–99 [DOI] [PubMed] [Google Scholar]
- Wilson MA, Morrissette JJ, McGettigan S, Roth D, Elder D, Schuchter LM, Daber RD (2014) What you are missing could matter: a rare, complex BRAF mutation affecting codons 599, 600, and 601 uncovered by next generation sequencing. Cancer Genet 207:272–275 [DOI] [PubMed] [Google Scholar]
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF (2017) Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Not applicable.