Abstract
Purpose
Aging is closely related to the occurrence of many diseases, including cancer, and involves changes in the immune microenvironment. γδT cells are important components of resident lymphocytes in mucosal tissues. However, little is known about the effects that the aged lung has on γδT cells and their prognostic significance in non-small cell lung cancer.
Methods
In the current study, the expression of γδTCR and IL-17A was measured by immunohistochemistry in paraffin-embedded lung tissues from 168 patients with adenocarcinoma (LUAD) and 144 patients with squamous cell carcinoma (LUSC). Furthermore, gene transcription patterns in LUAD and LUSC tumors and normal controls were extracted from TCGA and GTEx databases and were analyzed.
Results
High frequency of γδT cells was observed in patients with LUAD and LUSC, whereas the levels of CD4 + T cells, CD8 + T cells and CD56 + cells were decreased. Elevated γδT cells in tumors were mainly IL-17A-releasing γδT17 cells, which were found to be enriched in aged patients. High γδT cell levels positively corelated with the overall survival (OS) of patients, especially the 5-year OS in the elderly. Further analysis of gene transcription patterns indicated that increased expression of LTBR, HES1, RORC, CCR6, IL1, and IL23A may contribute to the transformation of the tumor microenvironment in a manner conducive to γδT17 cell development and differentiation. Finally, gene analysis between different age groups revealed that the expression of CCR6 and IL7 in LUAD, as well as Hes1, IL7, and IL23A in LUSC, were remarkably higher in elderly (age ≥ 60 years) than in younger individuals (age < 60 years).
Conclusion
Our findings suggest that intrinsic alterations in the aging lung lead to γδT17 cell enrichment, which subsequently may exert anti-tumor effects in the elderly.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03742-z.
Keywords: Elderly, γδT cells, LUAD, LUSC, Overall survival
Introduction
The epidemiology and prevention of lung cancer have changed over the past few years owing to the advances in our understanding of its risk factors, genetics, development, and treatment strategies. Nonetheless, lung cancer remains the most frequently diagnosed cancer and the leading cause of cancer death among men and women globally (Bade 2020; Siegel et al. 2019). The incidence of lung cancer is closely associated with age. It has been reported that advanced lung cancer having a worse prognosis is more likely to occur in younger patients compared to older patients (Bourke et al. 1992; Chen et al. 2016). Furthermore, studies have revealed that the incidence rate of lung cancer peaks at ages 80–84 years in males and at 75–79 years in females in the United States; 85–89 years in males and 80–85 years in females in the United Kingdom; and 60–74 years for both in males and females in China, and then gradually decreases (Cheng et al. 2020), which underlines an essential link between the alterations of the aged lung microenvironment and the occurrence and development of lung cancer. The progressive deterioration of immune function is a prominent feature of aging, along with reduced development and function of innate immune cells including neutrophils, macrophages, monocytes, dendritic cells, and natural killer (NK) cells, and acquired immune cells such as CD4 + T, CD8 + T, and B cells (Shaw et al. 2013; Solana et al. 2012; Nikolich-Zugich 2018). These changes lead to an impairment in efficient immune responses and increase the susceptibility to infectious diseases and cancer in the elderly (Raynor et al. 2012).
We previously demonstrated that the number of lung-resident γδT cells significantly increased together with altered gene expression in older mice (aged 20–24 months) versus younger mice (aged 10–16 weeks), and markedly controlled lung melanoma by improving survival via interleukin (IL)-17A release in older mice (Cheng et al. 2020). Compared to the 1–5% of total T cells constitutively circulating in the peripheral blood, γδT cells are remarkably enriched in human barrier tissues and make up about 8–20% of resident pulmonary lymphocytes in the lung, functioning as a bridge between innate and adaptive immune responses in host defense, tumor surveillance, and autoimmune diseases (Silva-Santos et al. 2015; Cheng and Hu 2017). γδT cells can be functionally divided into two subtypes: interferon (IFN)-γ-producing γδT1 cells and IL-17-producing γδT17 cells. In mice, the tumor necrosis factor (TNF)-receptor CD27 can functionally define these two subsets, as CD27 is expressed on the surface of IFN-γ-producing γδT cells but is absent on IL-17-producing γδT cells (Raverdeau et al. 2019). Instead, IL-17+γδT cells preferentially express C–C Motif Chemokine Receptor 6 (CCR6) and the transcription factor PLZF (Ribot et al. 2009), while the differentiation into IL-17+γδT cells involves the expression of lymphotoxin-β receptor (LTBR), transforming growth factor(TGF)-β, IL-7, B lymphoid kinase (Blk), RORC, and the Notch-transcription factor Hes1 (Michel et al. 2012; Chien et al. 2013; Zuberbuehler et al. 2019; Parker and Ciofani 2020; Cheng and Hu 2017). However, γδT cells in humans are not differentiated based on the differential expression of CD27. How to distinguish the functional discrepancy between the two subsets is still under investigation (Ribot et al. 2009). Unlike γδT1 cells accumulating in the peripheral blood, human γδT cells are not programmed to secret IL-17 at a steady state, but cells mature and differentiate to produce IL-17 under a highly inflammatory milieu when encountering infection or some cancers (Raverdeau et al. 2019; Silva-Santos et al. 2015). Elevated levels of the cytokines IL-1β, IL-6, IL-23, and IL-7 in tumor microenvironment (TME) benefit the enrichment of IL-17-producing γδT cells rather than γδT1 cells (Raverdeau 2019).
In our previous study, we determined that levels of γδT17 cells were higher in elderly non-small cell lung cancer (NSCLC) patients (aged ≥ 75 years) than in their younger counterparts (aged ≤ 50 years) (Cheng et al. 2020). However, how γδT 17 cells become enriched in aged lung cancer patients and their prognostic significance remain unclear. In this study, using immunohistochemical staining, we investigated the frequency of γδT17 cells in human lung adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC) tissues from patients stratified by age, and subsequently examined the clinical significance of tumor-infiltrating γδT17 cells. We also analyzed differential gene expression between tumor and normal lung tissues, as well as among tumor tissues from patients of different ages, using the Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) datasets, to illustrate the key factors controlling γδT17 cell aggregation in the aged lung TME.
Materials and methods
Patients
Human NSCLC tissue arrays, including 168 LUAD (Catalog number: HLug-Ade180Sur-01, HLug-Ade180Sur-03, HLug-Ade180Sur-06) and 144 LUSC (Catalog number: HLug-Squ150Sur-02, OD-CT-RsLug01-009 TMA), were purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, People’s Republic of China). None of the patients received any anticancer therapy prior to surgery. The median age of the patients was 62.4 years (range 30–86 years). LUAD patients were followed up from July 2004 to August 2016, with a mean survival time of 40.2 months (range, 1–121 months); while the LUSC patients were followed up from July 2004 to August 2012, with a mean survival time of 40.95 months (range, 0–92 months). Overall survival (OS) was measured from the date of surgery until the date of death or last known follow-up.
Immunohistochemistry
Anti-human γδTCR antibody (clone 5A6.E9, Invitrogen, Carlsbad, CA, USA), anti-human IL-17A polyclonal antibody (Catalog # PA5-79,470, Invitrogen, Carlsbad, CA, USA), and the double Staining Kit (Mo/HRP + Rb/AP, DS-0002, ZSGB-BIOTECH Co., LTD, Beijing, China) were used for immunohistochemical staining as previously described (Cheng 2020). Briefly, γδTCR+ cells are shown in brown and IL-17A+ cells are shown in red. The double-positive cells of γδTCR+IL-17A+ are indicated by brown–red color and are identified as γδT17 cells, while the single-positive brown γδTCR+ cells are identified as non-IL-17A-producing γδT cells, and the single-positive red IL-17A+ cells are identified as IL-17A-producing αβT cells. The sections were photographed using an Olympus IX73 microscope (Olympus, Tokyo, Japan). Each round dot with a diameter of 1.5 mm (1.76mm2) in the section represents one tumor sample. The number of γδTCR and IL-17A double-positive cells (γδT17 cells), γδTCR single-positive cells (non-IL-17A-producing γδT cells), as well as IL-17A single-positive lymphocytes in every round dot were counted in a 40 × field, respectively.
RNA‑seq data analysis
A total of 483 LUAD samples and 347 normal control lung tissues, as well as 486 LUSC samples and 338 normal control lung tissues with detailed standardized RNA-seq data, including CD4, CD8, CD56, CCR6, γδTCR, RORC, LTBR, HES1, IL1, and IL23A expression, were selected from the updated TCGA and GTEx databases and were analyzed using Gene Expression Profiling Interactive Analysis (GEPIA 2). Differential gene expression data including CCR6, IL7, LTBR, HES1, and IL23A in different age groups were obtained from TCGA database and were analyzed using UALCAN Analysis (http://ualcan.path.uab.edu/).
Statistical analysis
All data were analyzed using GraphPad Prism 6.0 software for Windows (GraphPad Software, Inc, San Diego, CA) and are expressed as means ± SEM. Comparisons between groups were carried out by Student’s t test. The Chi-square (χ2) test was used to analyze the correlations between IL-17A-producing γδT17 cells and clinical parameters. Survival curves were constructed using the Kaplan–Meier method and the log-rank (Mantel-Cox) test. P values < 0.05 were considered statistically significant.
Results
Tumor-infiltrating γδT cells in LUAD and LUSC were mainly γδT17 cells
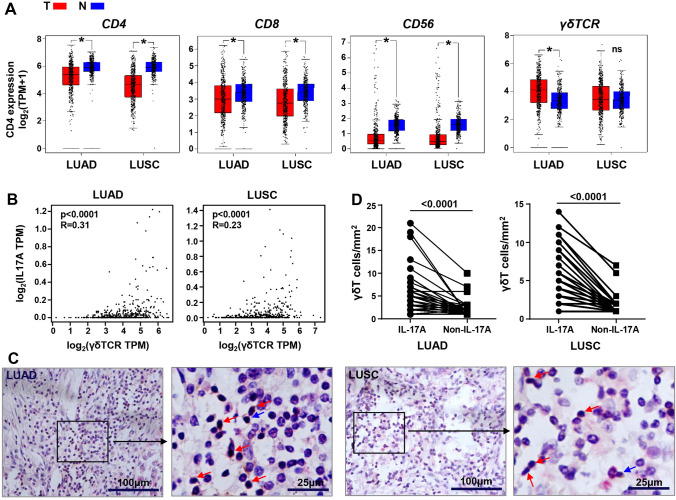
To identify the variations in the main lymphocyte subsets in the TME of LUAD and LUSC, RNA-seq data from TCGA and the GTEx databases were analyzed and compared the CD4, CD8, CD56, and γδTCR transcriptome in LUAD and LUSC tumor samples and normal healthy tissues. The expression of CD4, CD8, and CD56 were considerably lower both in LUAD and LUSC tumors than in normal tissues (p < 0.05). Conversely, γδTCR genes showed higher enrichment in tumors compared with normal lung tissues, especially in LUAD (p < 0.05), and presented a significant correlation with IL-17A expression (p < 0.0001) (Fig. 1A, B). To further verify whether the accumulation of γδT cells in the tumor are mainly IL-17A-producing cells, paraffin-embedded tissues from 168 LUAD patients and 144 LUSC patients were immunohistochemically stained with antibody against the γδTCR and IL-17A. As expected, we found a larger number of γδTCR and IL-17A double-positive cells (γδT17 cells), rather than single γδTCR-positive cells (non-IL-17A-producing γδT cells), infiltrated in the tumor tissues (Fig. 1C, D). Meanwhile, the frequencies of single IL-17A -positive lymphocytes, which may represent IL-17A-producing αβT cells, were lower than γδT17 cells in the tumors (data not shown). All of these indicated an implicated role for γδT17 cells in LUAD and LUSC.
Fig. 1.
IL-17A-producing γδT17 cells were enriched in the TME of LUAD and LUSC. A Analysis of CD4, CD8, CD56, and γδTCR transcripts (log2 TPM + 1) in LUAD and LUSC tumor tissues (T) and normal lung samples (N) through RNA-based next-generation sequencing (RNASeq) extracted from TCGA and GTEx datasets. B Analysis of the correlation between γδTCR and IL-17A transcripts (log2 TPM) in tumor tissues of LUAD and LUSC. C Infiltrated γδT 17 cells in the LUAD and LUSC tumor tissues through histochemical analysis. The double-positive cells of γδTCR+IL-17A+ are indicated by brown–red color and are indicated by red arrows, and the single-positive γδTCR+ cells (non-IL-17A-producing γδT cells) are shown in brown color and are indicated by blue arrows. D The numbers of γδT17 cells and non-IL-17A-producing γδT cells calculated in each sample. Student’s t test was used to compare the variations of the two groups. Data analyzed from TCGA and GTEx database (LUAD: Num (T) = 483, Num (N) = 347; LUSC: Num (T) = 486, Num (N) = 338). NS, no statistical differences; *p < 0.05; ****p < 0.0001
The correlation between tumor-infiltrating γδT17 cells and clinical pathological features in LUAD and LUSC
To investigate the association of γδT17 cells with clinical pathological profiles, such as age, sex, location, size, TNM classification (T: primary tumor; N: regional lymph node; M: distant metastasis), and the OS, 168 LUAD patients and 144 LUSC patients were divided into two groups based on the frequencies of γδT17 cells in the intratumor tissues (high and low infiltration groups). The χ2 test analysis revealed that LUAD patients, but not LUSC patients, with higher γδT17 infiltration were more likely to exhibit more moderate clinical pathological features with early T (p = 0.003) stages, as well as early clinical stages (p = 0.04), whereas higher levels of γδT17 cell infiltration for either patient groups did not correlate with sex, tumor location, size, N stages, M stages, or pathological stages (Table 1). Interestingly, we found that the frequency of γδT17 cells was significantly correlated with age in LUSC patients, but not LUAD patients, as older-aged LUSC patients (≥ 60 years) tended to have higher γδT17 cell infiltration in the tumor than younger patients (< 60 years) (p = 0.03). The percent of aged LUSC patients with higher γδT17 cells in the 63 cases was 74.6%, while the same frequency in the 56 cases of older-aged LUAD patients was 53.6% (Table 1).
Table 1.
Analysis of the correlations between γδT17 cells and clinical characteristic of LUAD and LUSC patients
| LUAD | LUSC | |||||
|---|---|---|---|---|---|---|
| High n = 56 | Low n = 112 | P-value | High n = 63 | Low n = 81 | P-value | |
|
Age (<60/≥60), % |
26/30, 46.4%/53.6% |
38/74 33.9%/66.1% |
0.12 |
16/47, 25.4%/74.6% |
35/46, 43.2%/56.8% |
0.03* |
|
Sex (M/F), % |
33/23, 58.9%/41.1% |
58/54, 51.8%/48.2% |
0.38 |
57/6, 90.5%/9.5% |
77/4, 95.1%/4.9% |
0.73 |
|
Location (L/R), % |
21/35, 37.5%/62.5% |
44/68, 39.3%/60.7% |
0.82 |
31/32, 49.2%/50.8% |
34/47, 42.0%/58.0% |
0.87 |
|
Size (<5/≥5cm), % |
44/12, 78.6%/21.4% |
81/31, 72.3%/27.7% |
0.38 |
31/32, 49.2%/50.8% |
42/39, 51.9%/48.1% |
0.10 |
|
cT (1+2/3+4), % |
50/6, 89.3%/10.7% |
76/36, 67.9%/32.1% |
0.003** |
50/13, 79.4%/20.6% |
65/16, 80.2%/19.8% |
1.00 |
|
cN (0+1/2+3), % |
40/16, 71.4%/28.6% |
75/37, 67.0%/33.0% |
0.56 |
49/14, 77.8%/22.2% |
61/20, 75.3%/24.7% |
0.84 |
|
cM (0/1), % |
55/1, 98.2%/1.8% |
110/2, 98.2%/1.8% |
1.00 |
63/0, 100.0%/0.0% |
80/1, 98.8%/1.2% |
0.32 |
|
cStage (1+2/3+4), % |
37/19, 66.1%/33.9% |
55/57, 49.1%/50.9% |
0.04* |
44/19, 69.8%/30.2% |
51/30, 63.0%/37.0% |
0.29 |
|
pStage (1+2/3+4), % |
35/21, 62.5%/37.5% |
75/37, 67.0%/33.0% |
0.57 |
46/17, 73.0%/27.0% |
58/23, 71.6%/28.4% |
0.46 |
|
3-year OS (<3/≥3), % |
18/38, 32.1%/67.9% |
60/52, 53.6%/46.4% |
0.009** |
12/51, 19.0%/81.0% |
26/55, 32.1%/67.9% |
0.08 |
|
5-year OS (<5/≥5), % |
35/21, 62.5%/37.5% |
95/17, 84.8%/15.2% |
0.001** |
42/21, 66.7%/33.3% |
67/14, 82.7%/17.3% |
0.03* |
Patients were divided into high (≥ 3.1 cells/mm2 in LUAD and ≥ 2.5 cells/mm2 in LUSC) and low (<3.1 cells/mm2 in LUAD and <2.5 cells/mm2 in LUSC) groups based on the levels of γδT 17 cells using cutoff values calculated by receiver operating characteristic curves. Parson’s χ2 test was used. *p<0.05; **p<0.01. M male, F female, L left, R right, cStage clinical stage, pStage pathological stage, OS overall survival
Tumor-infiltrating γδT17 cells are positively corelated with 5-year OS of older-aged patients with LUAD and LUSC
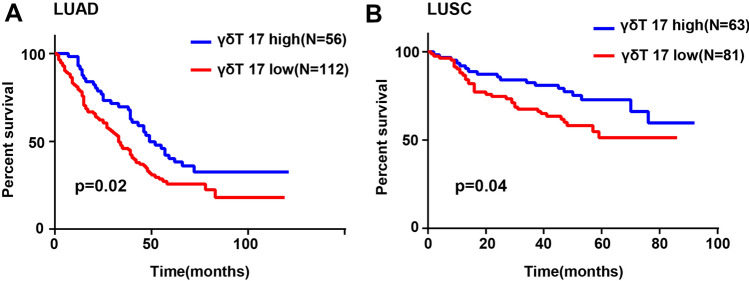
We also evaluated the prognostic significance of γδT17 cells in LUAD and LUSC. The data revealed that LUAD (p = 0.02) and LUSC (p = 0.04) patients with high γδT17 cell levels showed obviously improved OS compared to patients with low γδT17 cell levels (Fig. 2A, B). Moreover, further analysis demonstrated that the favorable outcome observed in LUAD (p = 0.001) and LUSC (p = 0.03) patients with high frequency of γδT17 cells was related to the 5-year OS rate, while in LUAD patients the high frequency of γδT17 cells was also related to the 3-year OS rate (p = 0.009). (Table 1). Since γδT17 cells are concentrated mainly in elderly patients, we next explored the 3-year and 5-year OS in older-aged (≥ 60 years) and younger-aged (< 60 years) patients with high or low γδT17 cell levels, respectively. As expected, younger patients with high or low levels of γδT17 cells did not differ significantly in terms of the 3-year and 5-year OS. However, a higher frequency of γδT17 cells indicated significantly higher 3- (0.02) and 5-year (p = 0.003) OS rates in elderly LUAD patients and higher 5-year OS rates in elderly LUSC (p = 0.02) patients compared to patients with low γδT17 cell levels, whereas no differences were observed between the two groups in the 3-year OS rates (Table 2).
Fig. 2.
Kaplan–Meier analyses of the prognostic significance of γδT 17 cells in LUAD and LUSC patients. Patients were divided into two groups based on the levels of γδT 17 cells using cut-off values calculated by receiver operating characteristic curves. Overall survival rates for A 168 LUAD and B 144 LUSC patients with high (≥ 3.1 cells/mm2 in LUAD and ≥ 2.5 cells/mm2 in LUSC) and low (< 3.1 cells/mm2 in LUAD and < 2.5 cells/mm2 in LUSC) levels of γδT 17 cells are shown
Table 2.
Analysis of the correlations between γδT17 cells and OS in older-aged LUAD and LUSC patients versus the younger-aged patients
| LUAD | LUSC | |||||
|---|---|---|---|---|---|---|
| High n = 26 | Low n = 38 | P-value | High n = 16 | Low n = 35 | P-value | |
| <60 | ||||||
| 3-year OS (<3/≥3), % | 9/17, 34.6%/65.4% | 19/19, 50.0%/50.0% | 0.22 | 1/15, 15.8%/84.2% | 7/28, 15.6%/84.4% | 0.21 |
| 5-year OS (<5/≥5), % | 18/8, 69.2%/30.8% | 33/5, 86.8%/13.2% | 0.07 | 9/7, 52.6%/47.4% | 26/9, 68.8%/31.2% | 0.20 |
| High n = 30 | Low n = 74 | P-value | High n = 47 | Low n = 46 | P-value | |
|---|---|---|---|---|---|---|
| ≥60 | ||||||
| 3-year OS (<3/≥3), % | 9/21, 30.0%/70.0% | 55.4%/44.6% | 0.02* | 11/36, 20.8%/79.2% | 19/27, 45.0%/55.0% | 0.06 |
| 5-year OS (<5/≥5), % | 17/13, 56.7%/43.3% | 62/12, 83.8%/16.2% | 0.003** | 33/14, 66.0%/34.0% | 41/5 87.5%/12.5% | 0.02* |
Patients were divided into high (≥ 3.1 cells/mm2 in LUAD and ≥ 2.5 cells/mm2 in LUSC) and low (< 3.1 cells/mm2 in LUAD and < 2.5 cells/mm2 in LUSC) groups based on the levels of γδT 17 cells using cutoff values calculated by receiver operating characteristic curves. Parson’s χ2 test was used. *p<0.05, **p<0.01. OS, overall survival
The tumor microenvironment of LUAD and LUSC was favorable for the enrichment of γδT17 cells
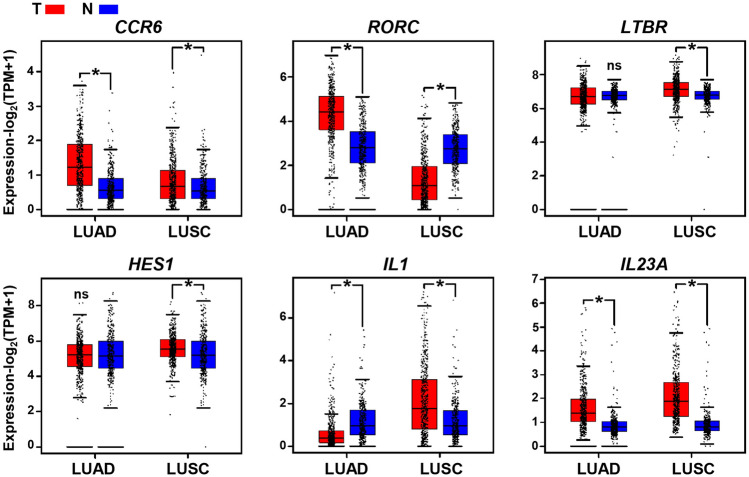
The results obtained above raised the question of why the γδT17 cells accumulated in the LUAD and LUSC tumor, while other lymphocytes such as CD4 + T, CD8 + T, and CD56 + NK or NKT cells were obviously repressed. Thus, we analyzed the gene expression features of tumors from TCGA and the GTEx databases. The data showed that transcription patterns associated with the development of γδT17 cells, such as elevated expression of RORC, were present in LUAD tumors. In addition, γδT17 cell differentiation involved upregulation in the expression of relevant genes including the chemokine receptor CCR6 as well as the cytokine IL23A in the TME of LUAD (Fig. 3). Similar to LUAD, transcription patterns in the LUSC tumor were characterized by high expression of genes CCR6, LTBR, HES1, IL1, and IL23A, which benefited the enrichment of γδT17 cells (Fig. 3).
Fig. 3.
The gene transcription patterns in the TME of LUAD and LUSC patients. Analysis of CCR6, RORC, LTBR, HES1, and IL23A transcripts (log2 TPM + 1) in LUAD and LUSC tumor tissues (T) and normal lung samples (N) through RNA-based next-generation sequencing (RNASeq) extracted from TCGA and GTEx datasets. LUAD: Num (T) = 483, Num (N) = 347; LUSC: Num (T) = 486, Num (N) = 338. NS, no statistical differences; *p < 0.05
Altered tumor microenvironment in elderly LUAD and LUSC patients led to γδT17 cell aggregation
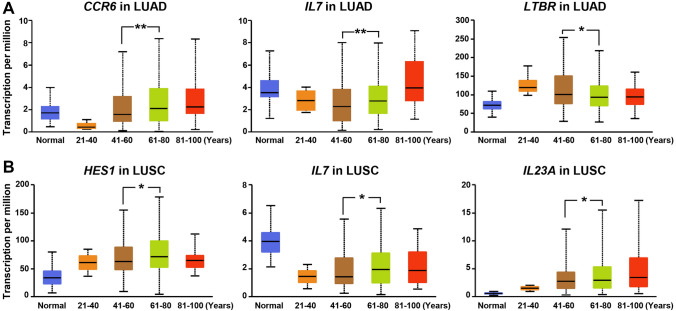
To investigate why γδT17 cells were more prone to accumulate in the lung of elderly patients than younger patients, we compared differences in γδT17 cell differentiation and the development of relevant gene patterns among different age groups ranging from 20 to 40 years, 41 to 60 years, 61 to 80 years, and 81 to 100 years. We found that lung cancer principally occurred in patients between the ages of 41 and 80, and thus, the 41–60 and 61–80 groups were selected as the main study subjects. In LUAD patients, the expression of CCR6 gradually increased with increasing age, and its gene levels were dramatically higher in the 61–80-year-old group than in the 41–60-year-old group (p = 0.007) (Fig. 4A). Although IL7 levels showed a decreased trend in all LUAD patients compared to normal individuals (data not shown), IL7 levels showed an obvious tendency of gradual increase in patients aged 41–100 years (41–60 vs 61–80 years, p = 0.008; 61–80 vs 81–100 years, p = 0.0008) (Fig. 4A). Nevertheless, similar differences were not found in HES1, RORC, and IL23A levels across different LUAD patient groups (Supplemental Fig. 1A), except for the decrease in LTBR expression in the 61–80-year-old group compared to the 41–60-year-old group (Fig. 4A). Although the TME in elderly LUSC patients was also favorable for the enrichment of γδT17 cells, it had different gene transcription features from LUAD. In particular, the gene levels of HES1 (p = 0.04), IL7 (p = 0.02), and IL23A (p = 0.04) were remarkably increased in the 61–80-year-old groups compared with patients aged 41–60 years (Fig. 4B), whereas there was no significant difference in the expression of CCR6, LTBR, and IL1B between the two groups (Supplemental Fig. 1B). These findings illustrated that the altered TME in elderly LUAD and LUSC patients leads to γδT17 lineage expansion, which subsequently protected against tumors probably through the production of IL-17A.
Fig. 4.
Variations in gene transcription in LUAD and LUSC patients stratified by ages. Analysis of CCR6, IL7, LTBR, HES1, and IL23A transcripts in tumor tissues of A LUAD or B LUSC patients in different age groups, as well as normal lung samples, through RNA-based next-generation sequencing (RNASeq) extracted from TCGA database. LUAD: Num (normal) = 59, Num (21–40 years) = 12, Num (41–60 years) = 90, Num (61–80 years) = 149, Num (81–100 years) = 32; LUSC: Num (normal) = 52, Num (21–40 years) = 2, Num (41–60 years) = 103, Num (61–80 years) = 361, Num (81–100 years) = 20. *p < 0.05
Discussion
Due to their developmental and functional plasticity, γδT cells engage in various human malignancies by playing contrasting roles including tumor-promoting effects or anti-tumor effects, which are dependent on the variable TME in different tumor types or in cell populations (Raverdeau et al. 2019; Gentles et al. 2015; Lee et al. 2020; Girardi et al. 2001). In this study, we found that IL-17-producing γδT17 cells, but not IFN-γ-producing γδT1 cells, were enriched in patients with LUAD and LUSC, especially in the elderly. Higher accumulation of γδT17 cells indicated favorable 3- and 5-year OS in LUAD patients aged ≥ 60 years and 5-year OS in LUSC patients aged ≥ 60 years. In particular, γδT17 cell infiltration in tumors was positively corelated with early T stages and clinical stages in LUAD, acting as an independent prognosis factor. Furthermore, we observed the gene transcription patterns in the LUAD and LUSC microenvironment favored γδT17 cell accumulation. With altered related-gene expression in the TME, aging intrinsically induced γδT cells to predominantly produce IL-17, which played a critical role in resistance to the development of LUAD and LUSC in the elderly.
Having both innate and adaptive properties, γδT cells comprise a small population of mostly tissue-resident lymphocytes and constitute an important first line of defense against infections, autoimmune diseases, and tumors, especially in the mucosal barrier and adipose tissues such as the skin, lung, liver, tongue, genital tract, and peritoneal cavity (McKenzie et al. 2018; Cheng et al. 2014). The γδT cells can undergo rapid migration and differentiation upon detection of stress, endogenous antigens, or chemokine and cytokine signaling, modulating the anti-infection or anti-tumor response through pro- or anti-inflammatory cytokines and their interactions with different types of innate and adaptive immune cells in the microenvironment (Lee et al. 2020). Of the two functional subsets of γδT cells, the role of γδT1 cells has been well elucidated in cancers principally due to the powerful anti-tumor effects of IFN-γ. However, the functions of IL-17-producing γδT17 cells in different tumors are still under debate (Silva-Santos et al. 2015), as IL-17 may act as a double-edged sword to facilitate tumor angiogenesis and enhance tumor immune evasion, as well as promote anti-tumor immunity (Murugaiyan and Saha 2009; Qian et al. 2017). Our previous study illustrated that age intrinsically altered lung-resident γδT cells which can control lung melanoma by producing IL-17A in the elderly (Cheng et al. 2020). Consistently, the frequencies of CD4+T cells, CD8+T cells, and CD56+ NK and NKT cells were decreased in the tumors of LUAD and LUSC patients, whereas the tumor-infiltrated γδT17 cells levels were significantly elevated (Fig. 1), especially in the elderly (Table 1).
Referring to the prognostic significance of IL-17 in the lung cancer, there have been mixed reports from different research groups. For instance, Chen et al. (2010) demonstrated that high expression of IL-17, mainly produced by CD4+T cells in NSCLC patients, acted as an independent prognostic factor for poor OS and disease-free survival. Conversely, high Th17 cell levels in the peripheral blood have also been reported to be positively associated with favorable 5-year OS in patients with late TNM stage, whereas no significant association was observed between peripheral γδT17 cells and OS (Song et al. 2019). Nevertheless, no studies have elucidated the predictive role of tumor-infiltrating γδT17 cells in lung cancer. Our present study observed that the number of IL-17A-producing αβT cells infiltrated in the tumor was much less than that of γδT17 cells (data not shown), and that high tumor infiltration of γδT17 cells indicated favorable 3- and 5-year OS in patients with LUAD and 5-year OS in patients with LUSC, especially in the elderly (Tables 1 and 2, Fig. 2). Furthermore, for LUAD patients, the γδT17 cell levels in the tumor were positively corelated with early T stages and clinical stages (Table 1). This may be a reason for the lower incidence and slower development of lung cancer in the elderly.
Notably, we and others have previously demonstrated that commensal microbiota controlled regional immunity and modulated tumoral immune surveillance through γδT17 cell-dependent mechanisms (Cheng et al. 2014; Li et al. 2017; Naik et al. 2012). For example, the homeostasis of liver-resident γδT17 cells was maintained by gut commensal microbes in a lipid antigen/CD1d-dependent manner (Li et al. 2017). In addition, IL-1R signaling was involved in microbiota-mediated IL-17A release by peritoneal cavity lung-resident γδT cells (Duan et al. 2010). Furthermore, commensal microbiota-sustained host immune response of γδT17 cells against B16/F10 melanoma and Lewis lung carcinoma required IL-6 and IL-23 expression (Cheng et al. 2014). Studies in murine lung adenocarcinoma induced by KRAS mutation and p53 loss have also indicated that local microbiota stimulated release of myeloid cells-derived IL-1β and IL-23, which induced proliferation and activation of lung-resident γδT cells that produced IL-17 and other effector molecules (Jin et al. 2019). Altogether, these findings suggest that tissue-specific factors possibly influence the clonal selection of γδT cells, raising the question of which factors control the activation and expansion of the human γδT17 cell subset. Our prior studies in murine melanoma also revealed that aged lung γδT17 cells were intrinsically altered independently of the commensal microbiota (Cheng et al. 2020), suggesting the involvement of other microenvironment factors.
In the present study, we observed genes associated with γδT17 cell development and polarization such as LTBR, HES1, RORC, CCR6, IL1, and IL23A, were elevated in the TME, although there was a slight difference in the gene types between LUAD and LUSC (Fig. 3). As mentioned above, IL-17+γδT cells preferentially express CCR6 (Ribot et al. 2009), while the differentiation into IL-17+γδT cells critically involves the expression of the Notch-transcription factor Hes1 (Shibata et al. 2011; Muro et al. 2019). The release of IL-17 by γδT cells can be predominantly stimulated solely by innate-derived cytokines such as IL-23 and IL-1β, but also by IL-7 and IL-18 (McKenzie et al. 2018). IL-23, alone or in combination with IL-1β, can induce innate IL-17 production from γδT cells (Papotto et al. 2017; Sutton et al. 2009). It has been reported that increased IL-7 production in the lymph nodes of aged mice provides a selective niche for the expansion of the γδT 17 lineage, for that TCRδ chain usage and clonal substructure are altered on aging (Chen et al. 2019). Interestingly, in our current study, the expressions of these genes related with γδT17 cell development and differentiation were higher in the lung TME of elderly patients than in the TME of younger patients. For instance, IL7 gene levels increased both in LUAD and LUSC patients with increasing age. Likewise, obvious elevations in CCR6 levels in LUAD, as well as higher expression of HES1 and IL23A in LUSC, were observed in the patients aged ≥ 60 years than in younger patients aged < 60 years (Fig. 4). The induction of human γδT cells require a highly inflammatory milieu instead of normal physiological conditions (Silva-Santos et al. 2015). Coincidentally, elderly populations exhibit a basal systemic inflammatory state characterized by high levels of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, TNF-α, and C-reactive protein compared to the young (Shaw et al. 2013). Thus, it is reasonable to speculate that age may intrinsically alter the immune environment of the lung and subsequently influence the development and differentiation of γδT cells involved in the progression of multiple lung diseases in the elderly population, and this may explain why γδT17 cells prefer to enrich in the elderly LUAD and LUSC patients. However, it remains to be clarified whether enriched γδT 17 cells are lung-resident γδT cells, as we previously reported in a murine lung cancer model, and how the observed factors affect the γδT 17 cell accumulation in the lung TME.
Conclusion
Our study demonstrated that changes in the immune microenvironment in aging lungs lead to an increase in γδT 17 cells, thereby controlling the progression of LUAD and LUSC, and may act as an independent predictor indicating favorable 3- and 5-year OS in the elderly. With the remarkable rise in the aging population, how to control age-related immune inflammation in multiple lung diseases becomes extremely important. Though the emergence of immune checkpoint inhibitors has opened a new chapter of cancer treatment, a decline in the function of the immune system, known as “immunosenescence” accompanies physiological aging, could impact their efficacy and safety in the elderly patients, and subsequently complicates the immunotherapy for the elderly (Elias et al. 2018). Therefore, there is a definite need for more studies specifically depict the landscape of immune changes with aging. Whether beneficial γδT17 cells can be used as anti-tumor immunotherapy in the elderly, and whether elevated γδT17 cells resulting from the alterations of the aged lung microenvironment are involved in other chronic obstructive pulmonary disease or pathogen infections, warrant further investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- Blk
B lymphoid kinase
- CCR6
C–C motif chemokine receptor 6
- GTEx
Genotype-tissue expression
- IFN
Interferon
- IL
Interleukin
- LTBR
Lymphotoxin-β receptor
- LUAD
Lung adenocarcinoma
- LUSC
Squamous cell carcinoma
- NK
Natural killer
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- TCGA
The cancer genome atlas
- TGF
Transforming growth factor
- TME
Tumor microenvironment
- TNF
Tumor necrosis factor
Author contributions
KC performed the study design, acquisition and analysis of data, and drafted the manuscript. XM participated in the data analysis. MC obtained the funding, conceived the study, and participated in the experimental design, data analysis, and the paper writing.
Funding
This work was supported by Natural Science Foundation of Anhui Province (2008085MH277), and National Natural Science Foundation of China (81471552).
Availability of data and material
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Code availability
Graphpad 6.0 software.
Declarations
Conflicts of interest
The authors have declared no conflict of interests.
Ethics approval
All the experiments referred to human specimens have been approved by the ethics committee of Shanghai Outdo Biotech Company.
Consent to participate
All the enrolled patients signed informed consent.
Consent for publication
All the authors agreed to the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bade BC, Dela Cruz CS (2020) Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 41:1–24. 10.1016/j.ccm.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Bourke W, Milstein D, Giura R, Donghi M, Luisetti M, Rubin AH, Smith LJ (1992) Lung cancer in young adults. Chest 102:1723–1729. 10.1378/chest.102.6.1723 [DOI] [PubMed] [Google Scholar]
- Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z (2010) Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 69:348–354. 10.1016/j.lungcan.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Chen YM, Lai CH, Rau KM, Huang CH, Chang HC, Chao TY, Tseng CC, Fang WF, Chen YC, Chung YH, Wang YH, Su MC, Huang KT, Liu SF, Chen HC, Chang YC, Chang YP, Wang CC, Lin MC (2016) Advanced non-Small cell lung cancer patients at the extremes of age in the era of epidermal growth factor receptor tyrosine kinase inhibitors. Lung Cancer 98:99–105. 10.1016/j.lungcan.2016.05.020 [DOI] [PubMed] [Google Scholar]
- Chen HC, Eling N, Martinez-Jimenez CP, O'Brien LM, Carbonaro V, Marioni JC, Odom DT, de la Roche M (2019) IL-7-dependent compositional changes within the gammadelta T cell pool in lymph nodes during ageing lead to an unbalanced anti-tumour response, EMBO Rep, 20: e47379. 10.15252/embr.201847379 [DOI] [PMC free article] [PubMed]
- Cheng M, Hu S (2017) Lung-resident gammadelta T cells and their roles in lung diseases. Immunology 151:375–384. 10.1111/imm.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, Shen G, Hu S (2014) Microbiota modulate tumoral immune surveillance in lung through a gammadeltaT17 immune cell-dependent mechanism. Cancer Res 74:4030–4041. 10.1158/0008-5472.CAN-13-2462 [DOI] [PubMed] [Google Scholar]
- Cheng M, Chen Y, Huang D, Chen W, Xu W, Chen Y, Shen G, Xu T, Shen G, Tian Z, Hu S (2020) Intrinsically altered lung-resident gammadeltaT cells control lung melanoma by producing interleukin-17A in the elderly. Aging Cell. 10.1111/acel.13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Zeng X, Prinz I (2013) The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends Immunol 34:151–154. 10.1016/j.it.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Chung H, Troy E, Kasper DL (2010) Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 7:140–150. 10.1016/j.chom.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE (2018) Aging, immune senescence, and immunotherapy: A comprehensive review. Semin Oncol 45:187–200. 10.1053/j.seminoncol.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21:938–945. 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC (2001) Regulation of cutaneous malignancy by gammadelta T cells. Science 294:605–609. 10.1126/science.1063916 [DOI] [PubMed] [Google Scholar]
- Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, Whary MT, Meyerson M, Germain R, Blainey PC, Fox JG, Jacks T (2019) Commensal microbiota promote lung cancer development via gammadelta T cells. Cell 176(998–1013):e16. 10.1016/j.cell.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Chung YS, Kim TJ (2020) Heterogeneity of human gammadelta T cells and their role in cancer immunity. Immune Netw 20:e5. 10.4110/in.2020.20.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Hao X, Chen Y, Bai L, Gao X, Lian Z, Wei H, Sun R, Tian Z (2017) The microbiota maintain homeostasis of liver-resident gammadeltaT-17 cells in a lipid antigen/CD1d-dependent manner. Nat Commun 7:13839. 10.1038/ncomms13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie DR, Comerford I, Silva-Santos B, McColl SR (2018) The emerging complexity of gammadeltaT17 cells. Front Immunol 9:796. 10.3389/fimmu.2018.00796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC (2012) Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proc Natl Acad Sci U S A 109:17549–17554. 10.1073/pnas.1204327109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro R, Takayanagi H, Nitta T (2019) T cell receptor signaling for gammadeltaT cell development. Inflamm Regen 39:6. 10.1186/s41232-019-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Saha B (2009) Protumor vs antitumor functions of IL-17. J Immunol 183:4169–4175. 10.4049/jimmunol.0901017 [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y (2012) Compartmentalized control of skin immunity by resident commensals. Science 337:1115–1119. 10.1126/science.1225152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J (2018) The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 19:10–19. 10.1038/s41590-017-0006-x [DOI] [PubMed] [Google Scholar]
- Papotto PH, Goncalves-Sousa N, Schmolka N, Iseppon A, Mensurado S, Stockinger B, Ribot JC, Silva-Santos B (2017) IL-23 drives differentiation of peripheral gammadelta17 T cells from adult bone marrow-derived precursors. EMBO Rep 18: 1957–1967. 10.15252/embr.201744200. [DOI] [PMC free article] [PubMed]
- Parker ME, Ciofani M (2020) Regulation of gammadelta T cell effector diversification in the thymus. Front Immunol 11:42. 10.3389/fimmu.2020.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y (2017) Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine 89:34–44. 10.1016/j.cyto.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Raverdeau M, Cunningham SP, Harmon C, Lynch L (2019) gammadelta T cells in cancer: a small population of lymphocytes with big implications. Clin Transl Immunol 8:e01080. 10.1002/cti2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA (2012) Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol 24:482–487. 10.1016/j.coi.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B (2009) CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 10:427–436. 10.1038/ni.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR, Montgomery RR (2013) Age-dependent dysregulation of innate immunity. Nat Rev Immunol 13:875–887. 10.1038/nri3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, Hara H, Yamasaki S, Kageyama R, Iwakura Y, Kawamoto H, Toh H, Yoshikai Y (2011) Notch-Hes1 pathway is required for the development of IL-17-producing gammadelta T cells. Blood 118:586–593. 10.1182/blood-2011-02-334995 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Silva-Santos B, Serre K, Norell H (2015) gammadelta T cells in cancer. Nat Rev Immunol 15:683–691. 10.1038/nri3904 [DOI] [PubMed] [Google Scholar]
- Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T (2012) Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 24:331–341. 10.1016/j.smim.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Song L, Ma S, Chen L, Miao L, Tao M, Liu H (2019) Long-term prognostic significance of interleukin-17-producing T cells in patients with non-small cell lung cancer. Cancer Sci 110:2100–2109. 10.1111/cas.14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331–341. 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Zuberbuehler MK, Parker ME, Wheaton JD, Espinosa JR, Salzler HR, Park E, Ciofani M (2019) The transcription factor c-Maf is essential for the commitment of IL-17-producing gammadelta T cells. Nat Immunol 20:73–85. 10.1038/s41590-018-0274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Graphpad 6.0 software.