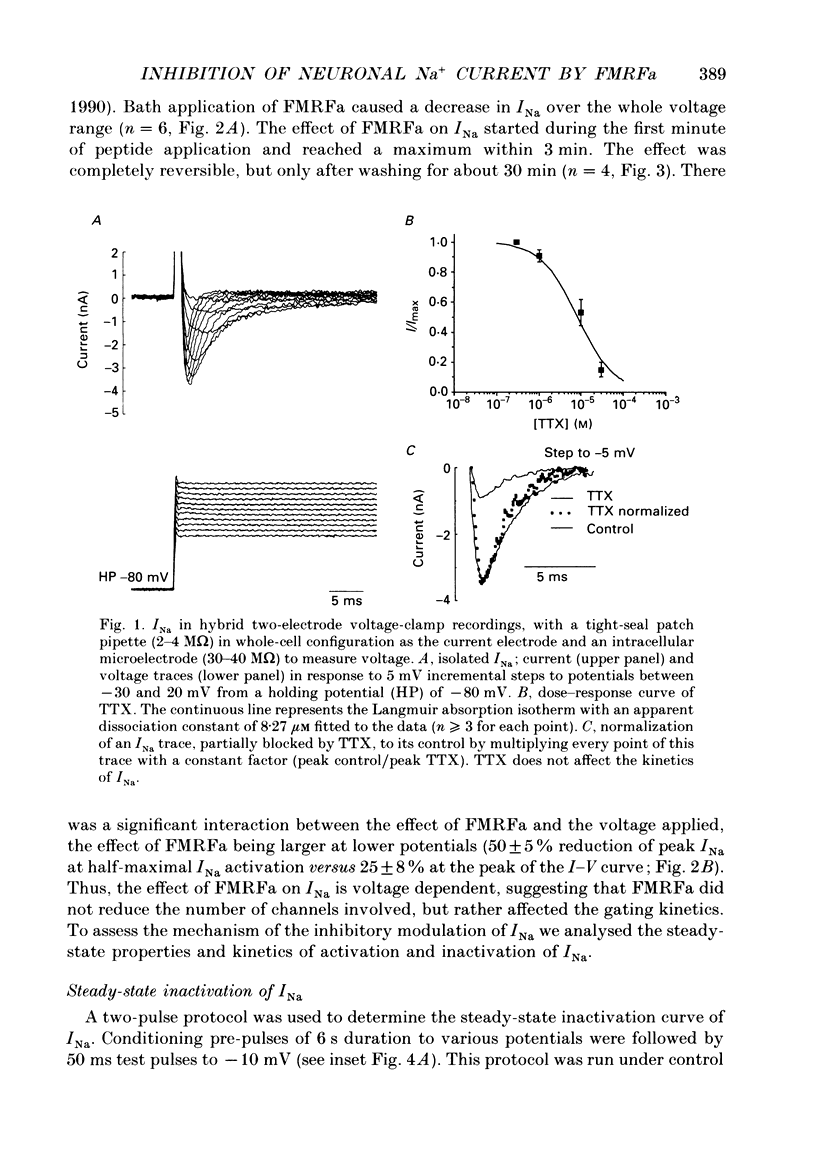
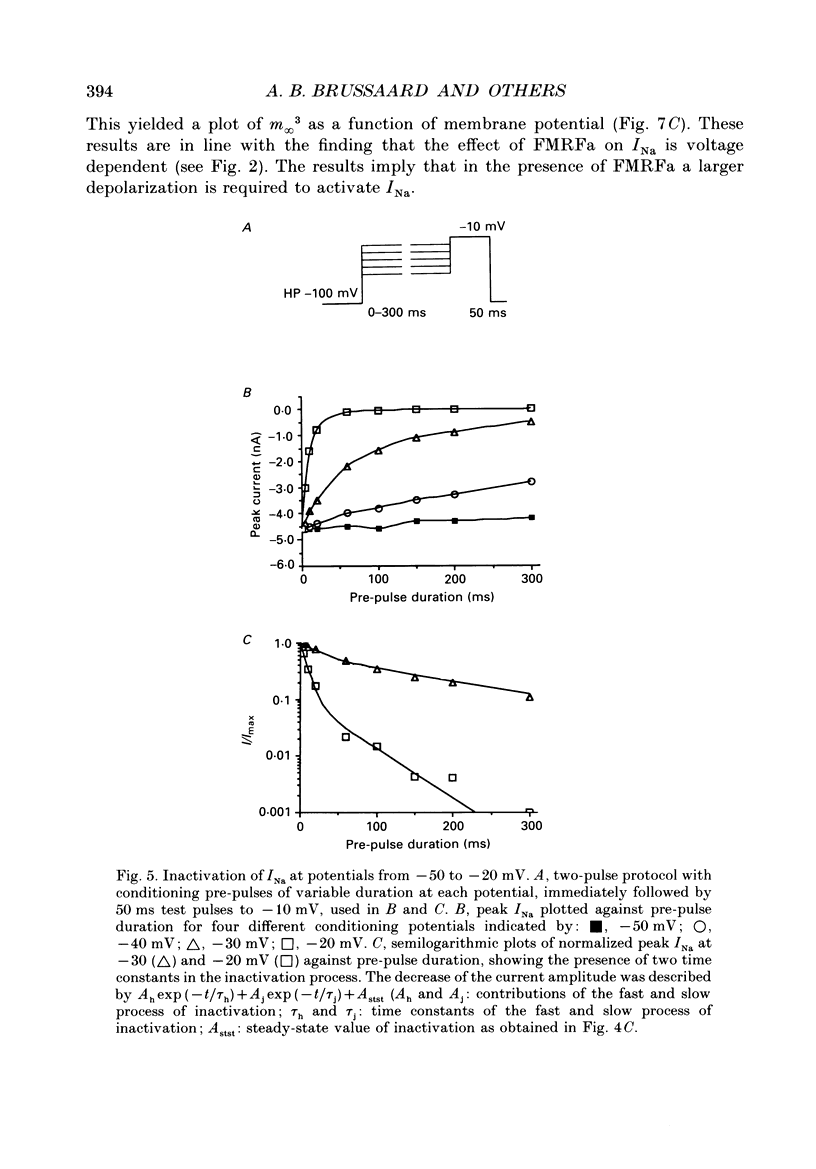
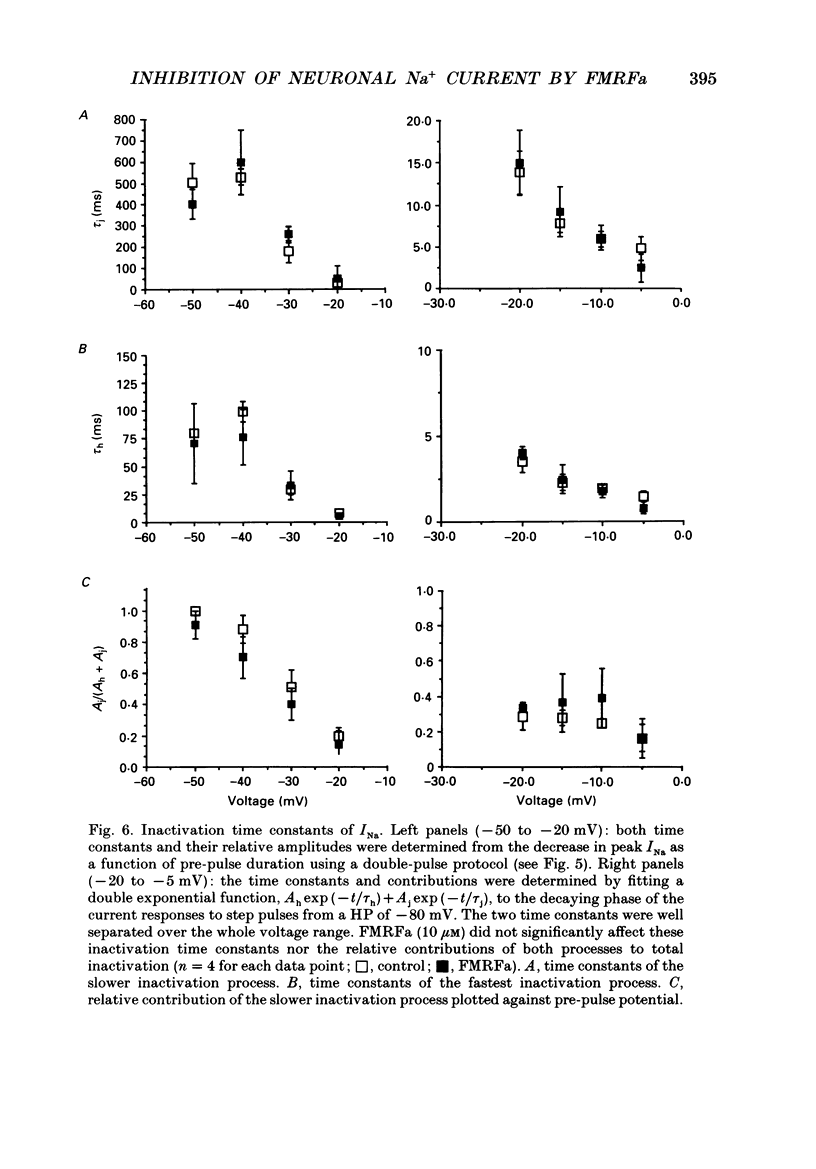
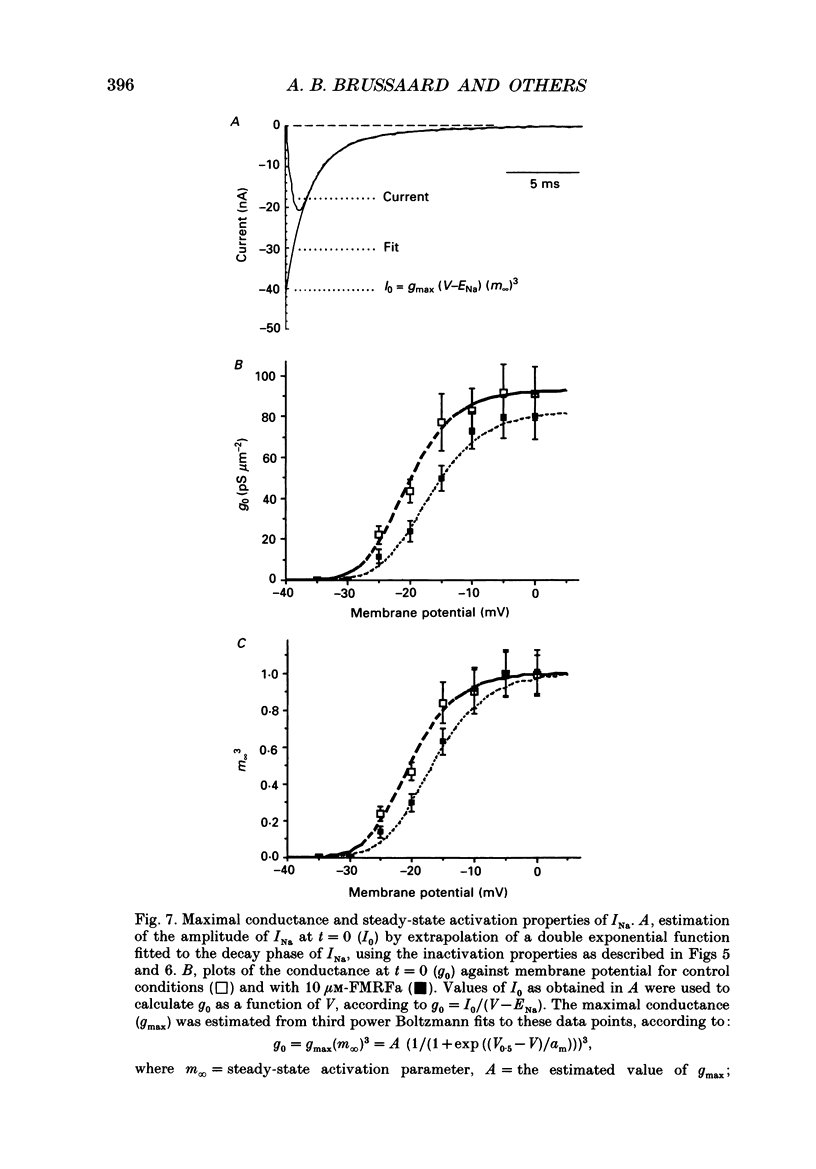
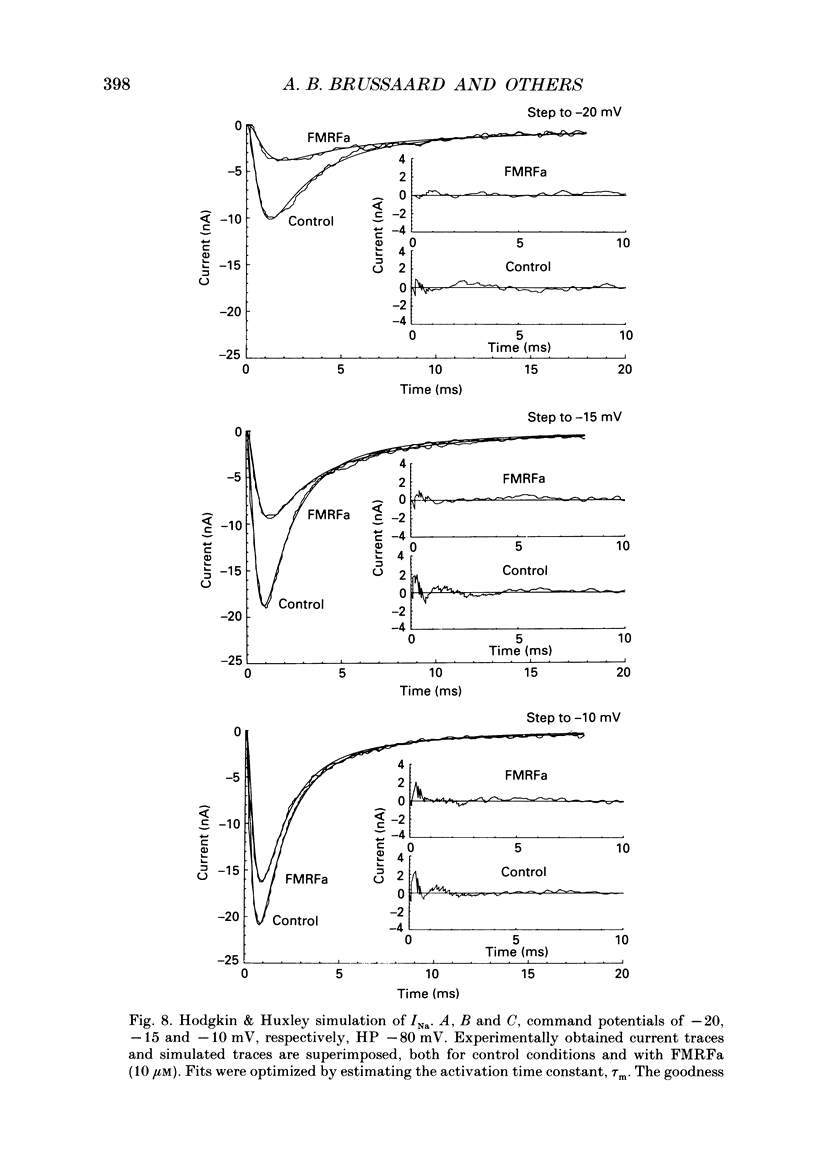
Abstract
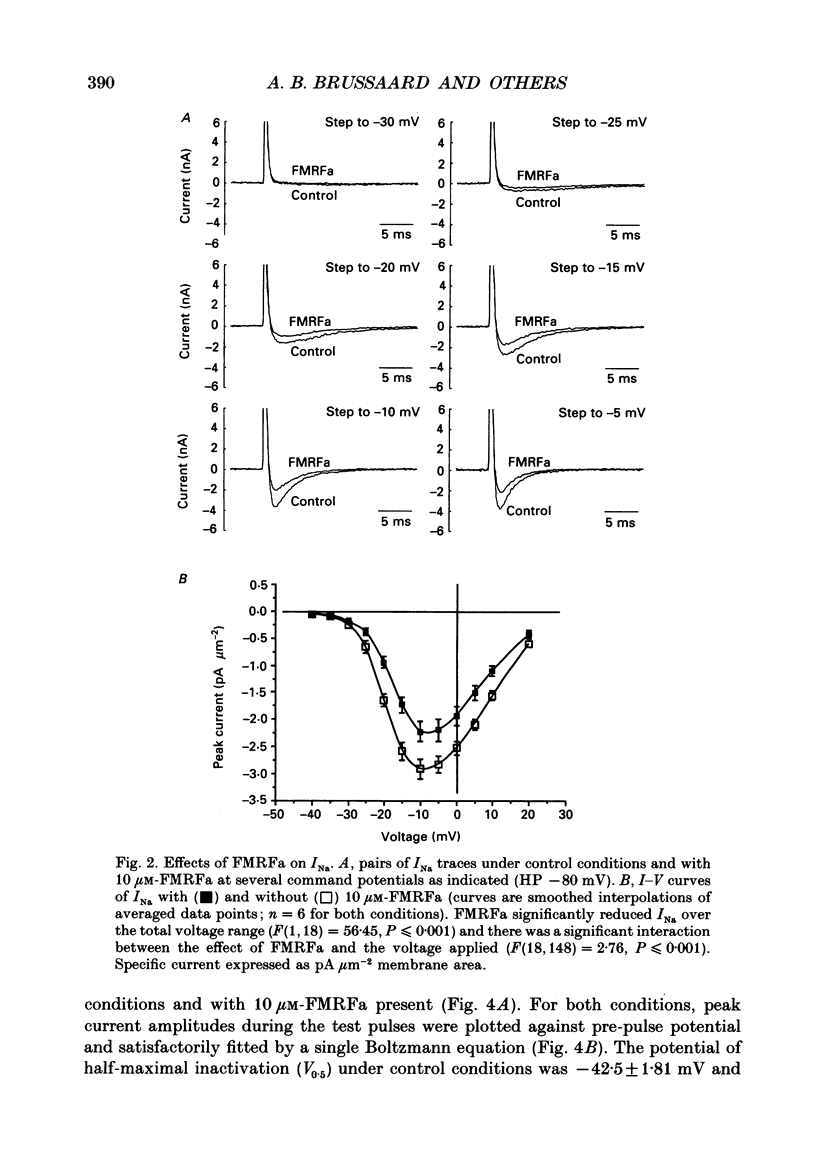
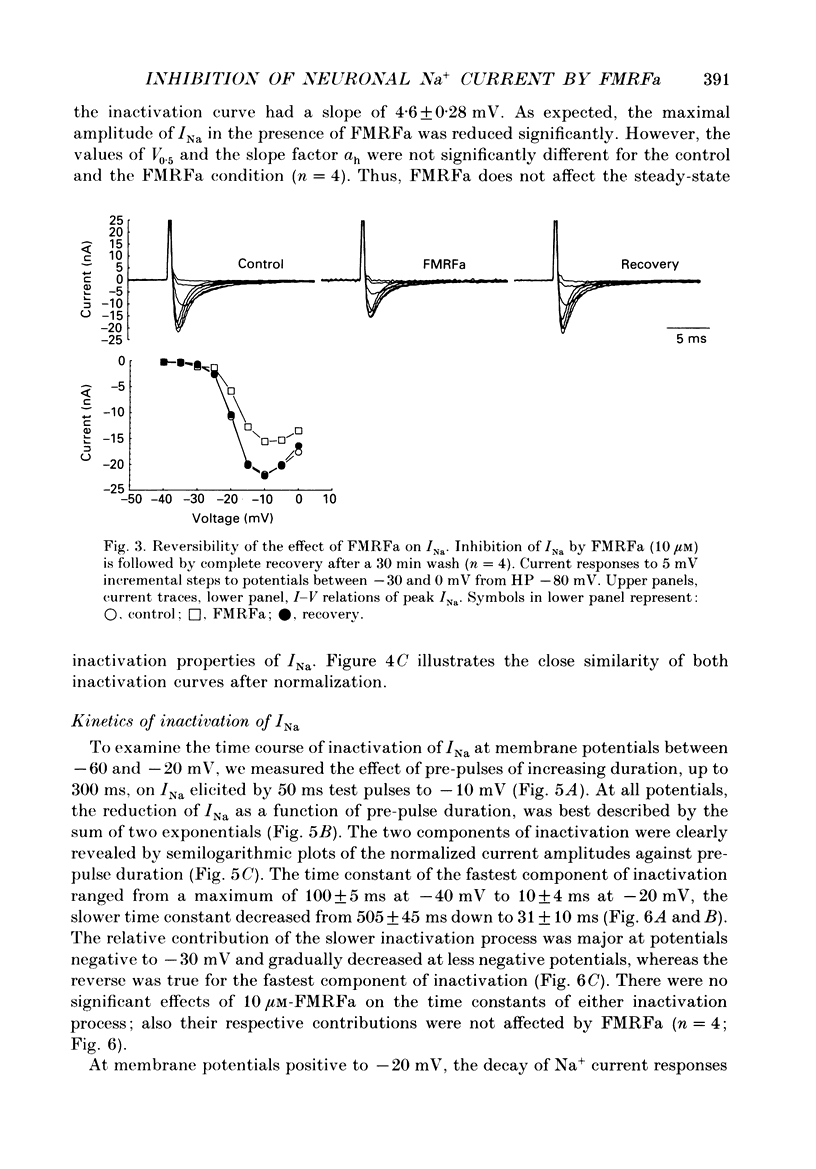
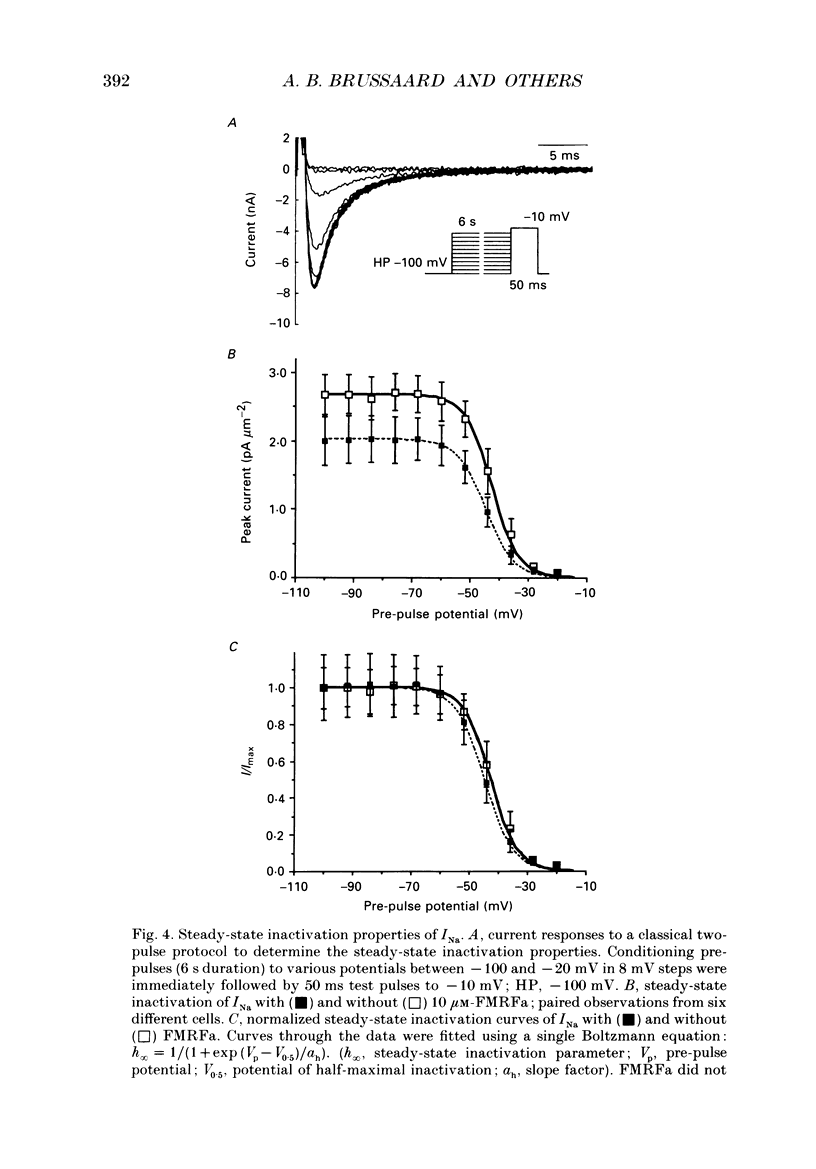
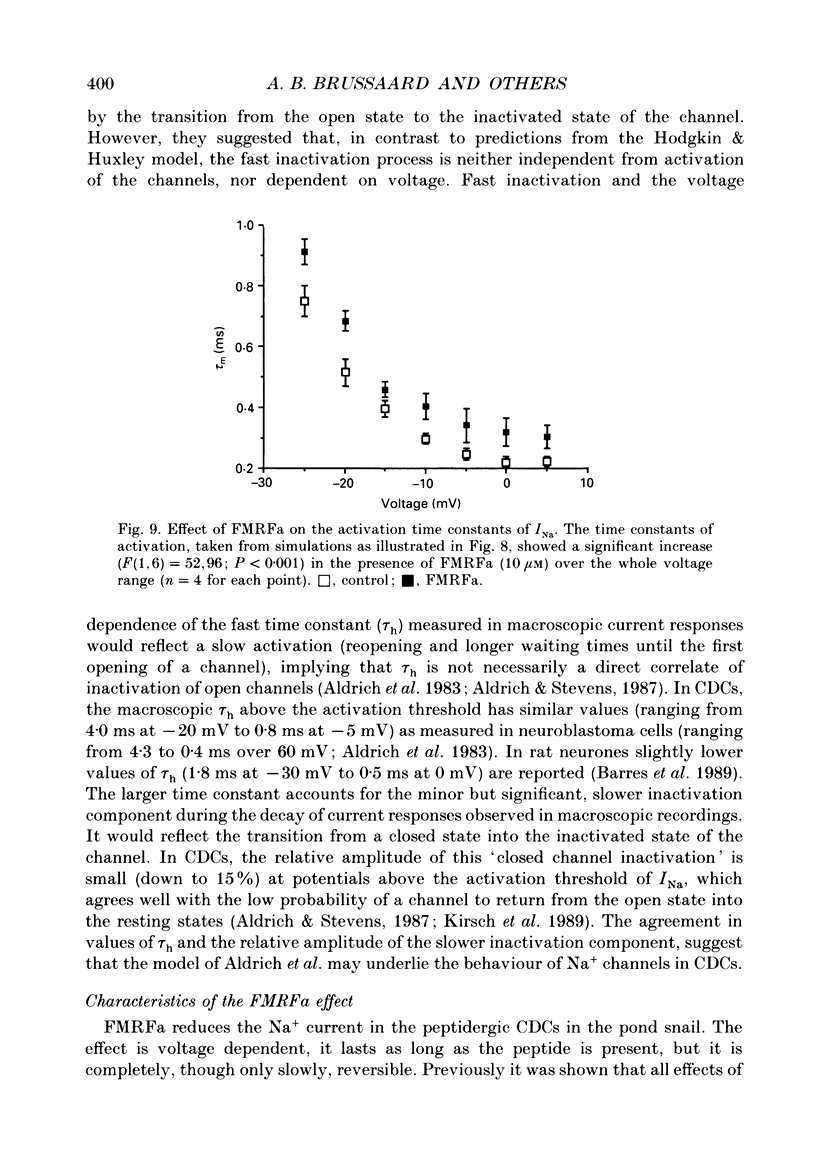
1. The putative neurotransmitter FMRFa (Phe-Met-Arg-Phe-amide) caused an inhibitory modulation of the voltage-gated sodium current (INa) in central neurones, the peptidergic caudo dorsal cells (CDCs) of the mollusc Lymnaea stagnalis. FMRFa reduced INa at all command potentials tested (ranging from -35 to +20 mV), but the amplitude of the effect of FMRFa was voltage dependent, inhibition being stronger at more negative potentials (50 +/- 5% reduction at half-maximal INa activation versus 25 +/- 8% at the peak of the I-V curve). 2. INa current traces were well fitted by a Hodgkin & Huxley based model, using m3 activation kinetics and two time constants for inactivation. 3. The steady-state inactivation curve of INa was characterized by half-maximal inactivation at -42.5 +/- 1.81 mV and a slope factor of 4.6 +/- 0.28 mV. The fastest time constant of inactivation ran from 100 +/- 5 to 0.8 +/- 0.32 ms and the slower time constant from 505 +/- 45 to 4.8 +/- 1.40 ms in the range -40 to -5 mV. 4. FMRFa had no significant effect on either component of inactivation, nor on the voltage dependence of steady-state inactivation, nor on the maximal conductance. 5. FMRFa affected the activation of INa. The activation time constant was increased, ranging from 0.75 +/- 0.050 to 0.22 +/- 0.017 ms under control and from 0.91 +/- 0.043 to 0.31 +/- 0.038 ms with FMRFa in the voltage range -25 to +5 mV. The steady-state activation curve was shifted to less negative potentials: half-maximal activation occurred at -26.5 +/- 1.2 mV under control and at 23.6 +/- 1.4 mV with FMRFa; the slope factor (4.6 +/- 1.4 mV in control experiments) was not affected. The combination of slower activation kinetics and a shift in the voltage dependence of activation in the Hodgkin & Huxley based model, adequately explained the reduction of INa by FMRFa. 6. The physiological consequence is that the spiking threshold is increased, causing an arrest of on-going firing activity and a decrease in excitability.
Full text
PDF






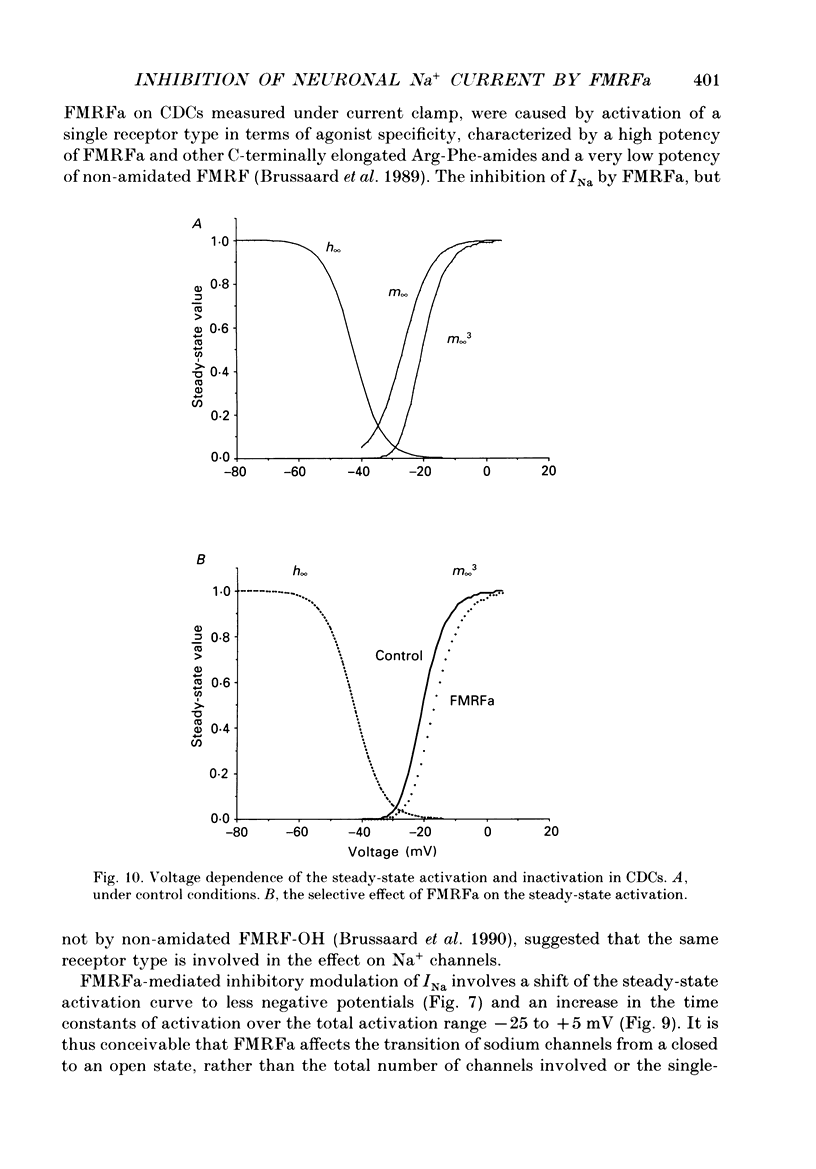



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Kandel E. R., Siegelbaum S. A. Neuronal inhibition by the peptide FMRFamide involves opening of S K+ channels. Nature. 1987 Jan 8;325(7000):153–156. doi: 10.1038/325153a0. [DOI] [PubMed] [Google Scholar]
- Brussaard A. B., Kits K. S., Ter Maat A., Van Minnen J., Moed P. J. Dual inhibitory action of FMRFamide on neurosecretory cells controlling egg laying behavior in the pond snail. Brain Res. 1988 Apr 26;447(1):35–51. doi: 10.1016/0006-8993(88)90963-8. [DOI] [PubMed] [Google Scholar]
- Brussaard A. B., Kits K. S., ter Maat A. One receptor type mediates two independent effects of FMRFa on neurosecretory cells of Lymnaea. Peptides. 1989 Mar-Apr;10(2):289–297. doi: 10.1016/0196-9781(89)90032-6. [DOI] [PubMed] [Google Scholar]
- Brussaard A. B., Ter Maat A., de Vlieger T. A., Kits K. S. Inhibitory modulation of neuronal voltage-dependent sodium current by Phe-Met-Arg-Phe-amide. Neurosci Lett. 1990 Apr 6;111(3):325–332. doi: 10.1016/0304-3940(90)90283-f. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M., Garty H., Sokolovsky M. G-protein mediates voltage regulation of agonist binding to muscarinic receptors: effects on receptor-Na+ channel interaction. Biochemistry. 1988 Jan 12;27(1):368–374. doi: 10.1021/bi00401a055. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M., Sokolovsky M., Dascal N. Modulation of the voltage-dependent sodium channel by agents affecting G-proteins: a study in Xenopus oocytes injected with brain RNA. Brain Res. 1989 Sep 4;496(1-2):197–203. doi: 10.1016/0006-8993(89)91066-4. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Phosphorylation of the alpha subunit of the sodium channel by protein kinase C. Cell Mol Neurobiol. 1984 Sep;4(3):291–297. doi: 10.1007/BF00733592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebberink R. H., van Loenhout H., Geraerts W. P., Joosse J. Purification and amino acid sequence of the ovulation neurohormone of Lymnaea stagnalis. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7767–7771. doi: 10.1073/pnas.82.22.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Skattebøl A., Possani L. D., Brown A. M. Modification of Na channel gating by an alpha scorpion toxin from Tityus serrulatus. J Gen Physiol. 1989 Jan;93(1):67–83. doi: 10.1085/jgp.93.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kits K. S. States of excitability in ovulation hormone producing neuroendocrine cells of Lymnaea stagnalis (gastropoda) and their relation to the egg-laying cycle. J Neurobiol. 1980 Jul;11(4):397–410. doi: 10.1002/neu.480110406. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., Lacerda A. E., Brown A. M. Angiotensin II modulates cardiac Na+ channels in neonatal rat. Circ Res. 1989 Dec;65(6):1804–1809. doi: 10.1161/01.res.65.6.1804. [DOI] [PubMed] [Google Scholar]
- Nilius B., Tytgat J., Albitz R. Modulation of cardiac Na channels by angiotensin II. Biochim Biophys Acta. 1989 Dec 14;1014(3):259–262. doi: 10.1016/0167-4889(89)90221-8. [DOI] [PubMed] [Google Scholar]
- Sah P., Gibb A. J., Gage P. W. The sodium current underlying action potentials in guinea pig hippocampal CA1 neurons. J Gen Physiol. 1988 Mar;91(3):373–398. doi: 10.1085/jgp.91.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R. Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbera L. A., Morad M. Atrionatriuretic peptide transforms cardiac sodium channels into calcium-conducting channels. Science. 1990 Feb 23;247(4945):969–973. doi: 10.1126/science.2154853. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S., Agnew W. S. Molecular diversity of voltage-sensitive Na channels. Annu Rev Physiol. 1989;51:401–418. doi: 10.1146/annurev.ph.51.030189.002153. [DOI] [PubMed] [Google Scholar]


