Abstract
Objective
Recent studies have indicated that CD47, interacting with SIRP-α, conveys “don’t eat me” signal in evasion of tumor cells and serves as a potential target for cancer immunotherapy. The purpose of this study was to investigate the clinical correlation of CD47 and uncover prognostic implications of CD47 and CD68 in non-small cell lung cancer (NSCLC).
Methods
The specimens from 384 patients with completely resected NSCLC were collected for immunohistochemical assays of CD47 and CD68. Cox multivariate proportion hazard analyses were conducted to confirm the independent prognostic value of CD47 and CD68. TCGA database and GSE37745 were used to identify the association between CD47 and immune cells.
Results
In 186 pairs of lung cancer and adjacent tissues, the RNA of CD47 was overexpressed in lung cancer tissues (P < 0.001). High expression of CD47 was associated with worse recurrence-free survival in RNA and protein level (P = 0.032 and P < 0.001, respectively). High expression of CD47 was significantly associated with large tumor size (P = 0.004), advanced pathologic TNM stage (P < 0.001), and histology (P = 0.003). Further analyses demonstrated that CD47 and CD68 predicted outcomes of patients independently. In addition, the expression of CD47 correlated with neutrophils, and did not correlated with B cells and CD4 + T cells in the TCGA database and GSE37745.
Conclusion
Combined use of CD47 and CD68 exhibited excellent performance in predicting survival of patients with NSCLC. CD47 was a potential therapeutic target for immune therapy of lung cancer.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03477-3) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, Prognostic analyses, Immune cell infiltration, Immunohistochemical assays
Introduction
Despite high morbidity and mortality of malignancies, immune-checkpoint inhibitors have significantly improved survival of patients, suggesting that activating immune system helps to kill tumor cells. As a major component of the body’s immune system, the innate immune system, associated with the clearance of cancer cells through phagocytosis, serves as the first defense against infections and malignancies (Iwasaki and Medzhitov 2010). Macrophages, which were characterized by CD68, play an important role in the innate immune system (Biswas and Mantovani 2010). Macrophages could be divided into two distinct types (M1 and M2). To our knowledge, M1 macrophages were correlated with less tumor invasiveness and good survival, while M2 macrophages were associated with tumor growth and poor survival (Mony and Schuchert 2018).
CD47, interacting with SIRP-α, conveys “don’t eat me” signal in the evasion of tumor cells from macrophages (Liu et al. 2019) and serves as a target for cancer immunotherapy (Logtenberg et al. 2019). CD47 is overexpressed in solid tumors and acts as an oncogene in previous studies (Chan et al. 2009; Edris et al. 2012; Zhao et al. 2016). Moreover, high expression of CD47 is associated with worse survival in several kinds of tumors (Chao et al. 2010; Nagahara et al. 2010; Suzuki et al. 2012). However, there are few studies focusing on the prognostic role of CD47 in lung cancer and its association with CD68, particularly in eastern-Asian patients.
In this study, we evaluated the expression of CD47 and CD68 in specimens from 384 patients with non-small cell lung cancer (NSCLC) by immunohistochemical assays. The results provided extra evidences for the anti-CD47 immune therapy of lung cancer.
Materials and methods
Tissue samples
The cohort to evaluate CD47 RNA, previously described in detail (Chen et al. 2019), consisted of 186 lung adenocarcinoma (LUAD) cases. The cohort to perform immunohistochemical (IHC) assay consisted of 384 NSCLC cases from Fudan University Shanghai Cancer Center diagnosed between 2009 and 2015. Patients with neoadjuvant treatment were excluded. Clinicopathologic parameters are showed in Table 1. The study was approved by the Institutional Review Board of FUSCC (IRB#090,977–1). Informed consents of patients were waived due to the retrospective study design.
Table 1.
Correlation between CD47 expression and clinicopathologic characteristics of patients with non-small cell lung cancer
| Variables | CD47 | P values | ||
|---|---|---|---|---|
| All cases (N = 384) | Low expression (N = 197) | High expression (N = 187) | ||
| Age (years) | 0.601 | |||
| Median (IQR) | 61 (55–67) | 61 (55–67) | 61 (55–68) | |
| Mean ± SD | 60.54 ± 9.88 | 60.28 ± 10.46 | 60.81 ± 9.24 | |
| Range | 26–83 | 26–83 | 36–80 | |
| Gender | 0.315 | |||
| Male | 220 (57.3) | 108 (54.8) | 112 (59.9) | |
| Female | 164 (42.7) | 89 (45.2) | 75 (40.1) | |
| Smoking history | 0.982 | |||
| Ever | 162 (42.2) | 83 (42.1) | 79 (42.2) | |
| Never | 222 (57.8) | 114 (57.9) | 108 (57.8) | |
| Pathological size, cm | 0.004 | |||
| Mean ± SD | 3.28 ± 1.98 | 3.00 ± 1.69 | 3.58 ± 2.21 | |
| p-TNM stage | < 0.001 | |||
| I | 203 (52.9) | 121 (61.4) | 82 (43.9) | |
| II | 69 (18.0) | 39 (19.8) | 30 (16.0) | |
| III | 112 (29.2) | 37 (18.8) | 75 (40.1) | |
| Histology | 0.003 | |||
| MIA | 9 (2.3) | 9 (4.6) | 0 (0) | |
| LPA | 25 (6.5) | 18 (9.1) | 7 (3.7) | |
| APA | 146 (38.0) | 65 (33.0) | 81 (43.3) | |
| PPA | 32 (8.3) | 16 (8.1) | 16 (8.6) | |
| IMA | 12 (3.1) | 10 (5.1) | 2 (1.1) | |
| MPA | 5 (1.3) | 2 (1.0) | 3 (1.6) | |
| SPA | 37 (9.6) | 16 (8.1) | 21 (11.2) | |
| SQCC | 118 (30.7) | 61 (31.0) | 57 (30.5) | |
| Mutational status | 0.160 | |||
| EGFR( +)/KRAS(-) | 215 (56.0) | 101 (51.3) | 114 (61.0) | |
| KRAS( +)/EGFR(-) | 23 (6.0) | 13 (6.6) | 10 (5.3) | |
| EGFR(−)/KRAS(−) | 146 (38.0) | 83 (42.1) | 63 (33.7) | |
The P values in bold indicated statistical significance (P < 0.05)
IQR interquartile range, SD standard deviation, LPA lepidic pattern-predominant adenocarcinoma, MIA minimally invasive adenocarcinoma APA acinar pattern-predominant adenocarcinoma, PPA papillary pattern-predominant adenocarcinoma, IMA invasive mucinous adenocarcinoma; MPA, micropapillary pattern-predominant adenocarcinoma SPA solid pattern-predominant adenocarcinoma, SQCC squamous cell carcinoma
Immunohistochemistry
Formalin-fixed paraffin-embedded primary specimens of NSCLC were obtained to construct 4-μm-thick sections. IHC assays for CD47 and CD68 were carried out using IHC kits (KIHC-5, Proteintech, USA) in accordance with the manufacture’s protocol under the following conditions: CD47 (20,305-1-AP, 1:200, Proteintech) and CD68 (D4B9C, #76,437, 1:400, Cell Signaling Technology). The total set of tissue specimens was processed and immunostained in one time to guarantee the objective comparison between samples.
Evaluation of immunohistochemical staining
The images of CD47 IHC staining were evaluated using the immunoreactivity score (IRS) (Kahlmeyer et al. 2019; Lindner et al. 2015). The intensity of staining was defined in four categories (no staining = 0, weak staining = 1, moderate staining = 2, and strong staining = 3) and multiplied by the proportion of positive cells (no = 0, < 10% = 1, 10%-50% = 2, 51%-80% = 3, and > 80% = 4). The images of CD68 IHC staining were assessed as the percentage of CD68-positive cells to all visible cells. The cut-offs for the expression of CD47 and CD68 were determined by their median values. The cutoff for IRS of CD47 was 3, and that for CD68 was 5%. Two independent persons, blinded to the outcomes of patients, reviewed pathologic images. For cases with different results, a consensus was reached through discussion.
Statistical analysis
Data were analyzed using IBM SPSS software (version 25.0). Recurrence-free survival (RFS) was defined as the time from date of diagnosis to date of recurrence attributed to lung cancer. Overall survival (OS) was defined as the time from date of diagnosis to data of death due to any causes. Paired Student t tests were conducted to compare CD47 expression in 186 pairs of NSCLC cases. Kaplan–Meier analyses, log-rank tests, and Cox proportion hazard regression were applied to perform survival analyses. Multivariable Cox analyses were used to identify independent factors related to survival. Spearman’s tests were carried out to evaluate the correlation between CD47 and CD68 or the infiltrations of immune cells. The analyses of immune cells infiltration were conducted by TIMER (https://cistrome.shinyapps.io/timer/) in the TCGA database and GSE37745 (Botling et al. 2013; Li et al. 2017). All tests were two-tailed, and statistical significance was set at P < 0.05.
Results
High expression of CD47 is found in lung cancer tissues and associated with worse survival
To investigate CD47 expression in tumor tissues, we compared the RNA expression of CD47 in 186 pairs of lung cancers and adjacent tissues from our previous study (Chen et al. 2019). The results demonstrated that CD47 was overexpressed in lung cancer tissues (P < 0.001, Fig. 1a). Among 95 patients with invasive adenocarcinoma, high expression of CD47 RNA was associated with worse RFS (P = 0.032, Fig. 1b).
Fig. 1.
The expression of CD47 in primary lung cancer. a The relative mRNA expression level of CD47 in 186 pairs of primary lung cancer and its adjacent tissues. b Kaplan–Meier Curve of recurrence-free survival according to mRNA expression of CD47 in patients with lung adenocarcinoma
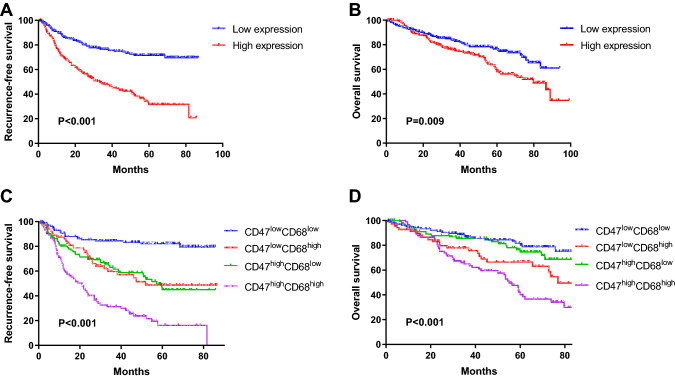
Next, we evaluate the protein level of CD47 and CD68 in completely resected tissues using immunochemical assays. A total of 384 NSCLC patients, undergoing surgical resection in Fudan University Shanghai Cancer Center, were enrolled in the study. The represent IHC images of CD47 and CD68 are shown in Fig. 2. CD47 protein was mainly localized in the membrane of cancer cells, whereas CD68 in the membrane and cytoplasm of macrophages. Further analyses demonstrated that the high expression of CD47 was associated with worse RFS and OS (RFS, P < 0.001, Fig. 3a; OS, P = 0.009, Fig. 3b).
Fig. 2.
Representative images of immunohistochemical staining for high and low expressions of CD47 and CD68 in lung cancer specimens. The cutoffs for the expression of CD47 and CD68 were determined by their median values
Fig. 3.
CD47 and CD68 were adverse predictor in non-small cell lung cancer (NSCLC). a–b Kaplan–Meier Curve of recurrence-free survival (a) and overall survival (b) according to protein expression of CD47 in patients with NSCLC. c–d Kaplan–Meier Curve of recurrence-free survival (c) and overall survival (d) according to protein expression of CD47 and CD68 in patients with NSCLC. The cut-offs for the expression of CD47 and CD68 were determined by their median values
Patients characteristics according to CD47 expression
Next, we investigated baseline of patients stratified by CD47 expression. The results are showed in Table 1. Among 384 enrolled patients, 187 (187/384, 48.7%) showed high expression of CD47, and 197 (197/384, 51.3%) showed low expression. High expression of CD47 was significantly associated with large tumor size (P = 0.004), advanced pathologic TNM stage (P < 0.001), and histology (P = 0.003). Additionally, there were no differences in age (P = 0.601), gender (P = 0.315), smoking history (P = 0.982), or mutational status (P = 0.160) between the CD47high and CD47low groups.
CD47 and CD68 predict survival of patients independently
Previous studies reported that CD47, the immune-suppressive marker expressed in the membrane of tumor cell, has a key role in tumor-mediated immune escape by interacting with tumor-associated macrophages (TAMs) characterized by CD68 (Feng et al. 2019), suggesting that CD47 expression might affect the amount of TAMs. Therefore, we explored the relationship between CD47 and CD68 by Spearman correlation analyses in the TCGA database and our cohort. The results demonstrated that CD68 was positively correlated with CD68 in both the TCGA database (r = 0.18, P = 2.3E-8, Supplementary Fig. 1a) and our cohort (r = 0.2642, P < 0.001, Supplementary Fig. 1b). Additionally, we investigated the combined use of CD47 and CD68 as prognostic factors. The survival analyses revealed that the RFS and OS in the CD47low CD68low group were the best, those in the CD47high CD68high group were the worst, and those in the CD47low CD68high and CD47high CD68low groups were intermediate (RFS, P < 0.001, Fig. 3c; OS, P < 0.001, Fig. 3d). Furthermore, Cox proportion analyses demonstrated that the expressions of CD47 and CD68 independently predicted RFS [CD47, hazard ration (HR) 2.090, 95% confident interval (CI) 1.475–2.962, P < 0.001; CD68, HR 2.550, 95% CI 1.847–3.521, P < 0.001) for operable NSCLC (Table 2).
Table 2.
Univariate and multivariable Cox regression analyses of factors associated with recurrence-free survival (N = 384)
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.012 (0.996, 1.029) | 0.128 | ||
| Sex | 0.122 | |||
| Female | Reference | |||
| Male | 1.282 (0.935, 1.758) | |||
| Smoking status | 0.088 | 0.337 | ||
| Never | Reference | Reference | ||
| Ever | 1.319 (0.959, 1.814) | 1.179 (0.842, 1.651) | ||
| Histology | 0.358 | |||
| Adenocarcinoma | Reference | |||
| Squamous cell carcinoma | 0.836 (0.571, 1.225) | |||
| p-TNM stage | < 0.001 | < 0.001 | ||
| I | Reference | Reference | ||
| II | 2.585 (1.635, 4.086) | < 0.001 | 2.663 (1.649, 4.301) | < 0.001 |
| III | 5.804 (4.041, 8.337) | < 0.001 | 4.703 (3.241, 6.825) | < 0.001 |
| CD47 | < 0.001 | < 0.001 | ||
| Low expression | Reference | Reference | ||
| High expression | 2.980 (2.124, 4.181) | 2.090 (1.475, 2.962) | ||
| CD68 | < 0.001 | < 0.001 | ||
| Low expression | Reference | Reference | ||
| High expression | 2.763 (2.012, 3.793) | 2.550 (1.847, 3.521) | ||
The P values in bold indicated statistical significance (P < 0.05)
Then, we conducted subgroup analyses to investigate whether mutational status could influence the prognostic effect of CD47. Among patients with EGFR mutation, CD47 (HR 2.625, 95% CI 1.691–4.076, P < 0.001) and CD68 (HR 2.222, 95% CI 1.492–3.309, P < 0.001) were associated with RFS independently (Table 3). Among patients with KRAS mutation, there is no prognostic effect of CD47 or CD68. Among patients without EGFR and KRAS mutation, CD47 (HR 3.139, 95% CI 1.680–5.866, P < 0.001) and CD68 (HR 3.638, 95% CI 1.962–6.746, P < 0.001) were also prognostic factors (Table 3).
Table 3.
Cox regression analysis of CD47 and CD68 on recurrence-free survival in patients stratified by mutational status
| Variables | Cox regression | |
|---|---|---|
| HR (95% CI) | P | |
| EGFR(+)/KRAS(-) | ||
| CD47 | < 0.001 | |
| Low expression | Reference | |
| High expression | 2.625 (1.691, 4.076) | |
| CD68 | < 0.001 | |
| Low expression | Reference | |
| High expression | 2.222 (1.492, 3.309) | |
| KRAS( +)/EGFR(−) | ||
| CD47 | 0.115 | |
| Low expression | Reference | |
| High expression | 3.243 (0.752, 13.989) | |
| CD68 | 0.370 | |
| Low expression | Reference | |
| High expression | 1.840 (0.485, 6.979) | |
| EGFR(-)/KRAS(-) | ||
| CD47 | < 0.001 | |
| Low expression | Reference | |
| High expression | 3.139 (1.680, 5.866) | |
| CD68 | < 0.001 | |
| Low expression | Reference | |
| High expression | 3.638 (1.962, 6.746) | |
The P values in bold indicated statistical significance (P < 0.05)
Correlation with between CD47 and immune cells
Since CD47 played a significant role in immune evasion of tumor cells (Liu et al. 2019; Logtenberg et al. 2019), we further used the TIMER (Li et al. 2017) to investigate the relationship between CD47 and immune infiltration in LUAD and lung squamous carcinoma (LUSC) from the TCGA database, and GSE37745 was used as a validation dataset (Botling et al. 2013). The results showed that the expression of CD47 in LUAD and LUSC was significantly correlated with the infiltration level of neutrophils in both datasets (Fig. 4 and Supplementary Fig. 2). Moreover, B cells and CD4 + T cells were not associated with CD47 in the two databases, supporting the conclusion that CD47 was a key factor in innate immunity rather than adaptive immunity.
Fig. 4.
The correlation between CD47 and infiltration of immune cells from innate (a) and adaptive (b) immune system in the TCGA database
Discussion
Immune-checkpoint inhibitors (ICIs) have been a major breakthrough in past decades. However, only ICIs (PD-1, PD-L1, or CTLA-4) targeting the lymphoid lineage, modulating the adaptive T-cell response, are currently used in clinical practice. CD47 is an emerging checkpoint for myeloid cells and the innate immune system, and serves as a potential target for cancer immune therapy. In several malignancies, the results of anti-CD47 therapy were promising (Chao et al. 2010, 2011; Lee et al. 2014). Nevertheless, in lung cancer, therapies of monoclonal antibodies targeting CD47 are still at early stage of developments. In this study, we reported that the high expression of CD47 was upregulated in lung cancer tissues. To our knowledge, for the first time, the study revealed the combine use of CD47 and CD68 could predict survival in eastern-Asian patients with NSCLC and the expression of CD47 correlated with the infiltration of cells from the innate immune system.
CD47 has been considered as an adverse biomarker for survival in several cancers (Zhao et al. 2018). Animal experiments revealed that CD47 blockade could strengthen the effect of anti-angiogenic therapy on NSCLC by enhancing macrophage infiltration and tumor cell destruction (Zhang et al. 2019). Furthermore, anti-CD47 siRNA could even suppress the growth of lung cancer cells in vitro (Wu et al. 2018). However, there are few papers studying the exact role of CD47 in the prognosis of lung cancer. Barrera et al. found that CD47 overexpression in white blood cells was associated with poor survival in patients with NSCLC (Barrera et al. 2017). Liu et al. showed that the mRNA level of CD47 could predict the survival of NSCLC in eastern Asians (Liu et al. 2017). Nevertheless, Arrieta et al. investigated the expression of CD47 in Mexicans and demonstrated that the prognostic role of CD47 was only shown in patients with EGFR mutations rather than the whole population. In our study, we performed immunohistochemical staining in primary NSCLC tissues from 384 Chinese patients and found CD47 predicted RFS and OS in protein level. The above studies indicated that the prognostic value of CD47 might be more apparent in eastern Asians. Moreover, the high expression of CD47 was associated with large pathological size and advanced TNM stage, implicating that CD47 played an important role in development and progression of lung cancer. These results suggested that anti-CD47 therapy may be more effective in advanced NSCLC.
In our study, we found CD47 did not have prognostic value in KRAS( +) patients, while it did in KRAS(−) patients. Ma et al. demonstrated patients with KRAS mutation had worse survival compared with those without (Ma et al. 2020). Perhaps, KRAS( +) patients had unfavorable outcomes, which covered the prognostic effect of CD47.
TAMs were a key part of microenvironment of cancer which affected tumor progression and metastasis (Karnevi et al. 2014; Mantovani et al. 2017). In general, high density of TAMs (characterized by CD68) indicated poor survival in lung cancer (Zhang et al. 2012). In this study, we found the prognostic value of CD68 was independent of CD47. The high expression of CD68 and CD47 simultaneously indicated the worst survival of patients with NSCLC. Furthermore, CD68 positively correlated CD47 in the TCGA database, implicating the close connection of CD47 and macrophages.
The immune cells played a crucial role in tumor-immune network and evasion of tumor cell (Bindea et al. 2013; Mlecnik et al. 2010). In fact, if we better understand the immune infiltration status, we could choose the patients who were most likely to benefit. In this study, we found that CD47 was closely correlated with neutrophils and not correlated with B cells and CD4 + T cells in LUAD and LUSC, supporting the conclusion that CD47 was a key factor in innate immunity rather than adaptive immunity.
It need be addressed that there are some limitations in the study. First, the study is based on the specimens from one institution, which may cause selection bias. Future validations from other centers are needed. Second, in this study, we analyzed the infiltration of immune cells only by bioinformatic methods based on the data from TCGA database rather than IHC assays. However, previous studies have demonstrated that data from RNA-seq could reliably predict immune cell infiltration (Aran et al. 2017; Gentles et al. 2015; Newman et al. 2019). Therefore, we believe that it is acceptable to use RNA-seq to evaluate the infiltration of immune cells.
In conclusion, our study showed that the high expression of CD47 was associated with poor survival in NSCLC. The combination of CD47 and CD68 could predict outcomes of NSCLC patients. The expression of CD47 correlated with neutrophils and did not correlate with B cells and CD8 + T cells, and CD47 was a therapeutic target for immune therapy of lung cancer.
Electronic supplementary material
Supplementary Figure 1. The association between CD47 and CD68 in the TCGA database (A) and our cohort (B). Spearman’s tests were carried out to investigate the correlation between CD47 and CD68.
Supplementary Figure 2. The correlation between CD47 and infiltration of immune cells from innate (A) and adaptive (B) immune system in GSE37745.
Below is the link to the electronic supplementary material.
Abbreviations
- CI
Confidential interval
- HR
Hazard ratio
- ICIs
Immune-checkpoint inhibitors
- IHC
Immunohistochemistry
- IRS
Immunoreactivity score
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous carcinoma
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- RFS
Recurrence-free survival
- TAMs
Tumor-associated macrophages
Funding
This work was supported by the National Natural Science Foundation of China (81930073 and 81772466), Shanghai Municipal Science and Technology Major Project (Grant No. 2017SHZDZX01, VBH1323001/026), Shanghai Municipal Key Clinical Specialty Project (SHSLCZDZK02104), and Pilot Project of Fudan University (IDF159034).
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fangqiu Fu, Yang Zhang, and Zhendong Gao have contributed equally to this work
References
- Aran D, Hu Z, Butte AJ (2017) xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 18:220. 10.1186/s13059-017-1349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera L et al (2017) CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer 117:385–397. 10.1038/bjc.2017.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G et al (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39:782–795. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896. 10.1038/ni.1937 [DOI] [PubMed] [Google Scholar]
- Botling J et al (2013) Biomarker discovery in non-small cell lung cancer: integrating gene expression profiling, meta-analysis, and tissue microarray validation. Clin Cancer Res 19:194–204. 10.1158/1078-0432.Ccr-12-1139 [DOI] [PubMed] [Google Scholar]
- Chan KS et al (2009) Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA 106:14016–14021. 10.1073/pnas.0906549106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP et al (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142:699–713. 10.1016/j.cell.2010.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL (2011) Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 118:4890–4901. 10.1182/blood-2011-02-338020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H et al (2019) Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun 10:5472. 10.1038/s41467-019-13460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edris B et al (2012) Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A 109:6656–6661. 10.1073/pnas.1121629109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL (2019) Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 19:568–586. 10.1038/s41568-019-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles AJ et al (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21:938–945. 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327:291–295. 10.1126/science.1183021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlmeyer A et al. (2019) Expression of PD-1 and CTLA-4 Are Negative Prognostic Markers in Renal Cell Carcinoma. J Clin Med 8 doi:10.3390/jcm8050743 [DOI] [PMC free article] [PubMed]
- Karnevi E, Andersson R, Rosendahl AH (2014) Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol 92:543–552. 10.1038/icb.2014.22 [DOI] [PubMed] [Google Scholar]
- Lee TK et al (2014) Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology 60:179–191. 10.1002/hep.27070 [DOI] [PubMed] [Google Scholar]
- Li T et al (2017) TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res 77:e108–e110. 10.1158/0008-5472.CAN-17-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner JL et al (2015) Expression of secreted protein acidic and rich in cysteine (SPARC) in breast cancer and response to neoadjuvant chemotherapy. Ann Oncol 26:95–100. 10.1093/annonc/mdu487 [DOI] [PubMed] [Google Scholar]
- Liu L et al (2017) Anti-CD47 antibody as a targeted therapeutic agent for human lung cancer and cancer stem cells. Front Immunol 8:404. 10.3389/fimmu.2017.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, O’Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL (2019) Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated “don’t-eat-me” signal. Nat Immunol 20:265–275. 10.1038/s41590-018-0292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg MEW et al (2019) Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPalpha axis and a target for cancer immunotherapy. Nat Med 25:612–619. 10.1038/s41591-019-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhang Y, Deng C, Fu F, Deng L, Li Y, Chen H (2020) The prognostic value of Kirsten rat sarcoma viral oncogene homolog mutations in resected lung adenocarcinoma differs according to clinical features. J Thorac Cardiovasc Surg. 10.1016/j.jtcvs.2020.05.097 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B et al (2010) Data integration and exploration for the identification of molecular mechanisms in tumor-immune cells interaction. BMC Genomics 11(Suppl 1):S7. 10.1186/1471-2164-11-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony JT, Schuchert MJ (2018) Prognostic implications of heterogeneity in intra-tumoral immune composition for recurrence in early stage lung cancer. Front Immunol 9:2298. 10.3389/fimmu.2018.02298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara M et al (2010) Correlated expression of CD47 and SIRPA in bone marrow and in peripheral blood predicts recurrence in breast cancer patients. Clin Cancer Res 16:4625–4635. 10.1158/1078-0432.CCR-10-0349 [DOI] [PubMed] [Google Scholar]
- Newman AM et al (2019) Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 37:773–782. 10.1038/s41587-019-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S et al (2012) CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep 28:465–472. 10.3892/or.2012.1831 [DOI] [PubMed] [Google Scholar]
- Wu J et al (2018) A glutamine-rich carrier efficiently delivers anti-CD47 sirna driven by a “glutamine trap” to inhibit lung cancer cell. Growth Mol Pharm 15:3032–3045. 10.1021/acs.molpharmaceut.8b00076 [DOI] [PubMed] [Google Scholar]
- Zhang QW et al (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE 7:e50946. 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X et al (2019) Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J Immunother Cancer 7:346. 10.1186/s40425-019-0812-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H-J, Pan F, Shi Y-C, Luo X, Ren R-R, Peng L-H, Yang Y-S (2018) Prognostic significance of CD47 in human malignancies: a systematic review and meta-analysis. Trans Cancer Res 7:609–621. 10.21037/tcr.2018.05.31 [Google Scholar]
- Zhao H et al (2016) CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep 6:29719. 10.1038/srep29719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.