Abstract
Introduction
The aim of this study was to evaluate prognostic factors in patients with non-metastatic Merkel cell carcinoma (MCC), with a particular focus on immunological markers such as TILs subtyping (CD3, CD8, CD68, FoxP3, PD-L1 and PD-1) and MCPyV.
Methods
Patients treated for a non-metastatic MCC with oncologic surgical resection followed or not by adjuvant radiotherapy between 01/2007 and 12/2018 were analyzed. Local and regional control (LC, RC), distant metastasis-free survival (DMFS) and overall survival (OS) were evaluated. Clinical variables analyzed included age, gender, performance status, comorbidity, tumor size, location and presentation type, extension, oncologic resection and adjuvant radiotherapy. Pathological variables analyzed included type of tumor-infiltrating lymphocytes, CD3, CD8, CD68, PD-L1 expression on immune cells and tumors cells, PD-1, FoxP3 and MCPyV, assessed with immunohistochemistry (IHC).
Results
77 patients were included. After a median follow-up of 18 months (range 0.2–144), the 1-year LC, RC, DMFS and OS were 83%, 60%, 82% and 75%, respectively. In multivariate analysis, a percentage of PD-L1 expression by immune cells ≥ 1% was significantly correlated with improvement of RC (p = 0.012), DMFS (p = 0.003) and OS (p = 0.006). Adjuvant radiotherapy significantly improved DMFS (p = 0.021) and OS (0.041) rates. There was a correlation between the presence of MCPyV + and the expression of PD-L1 on IC (p = 0.05) and TC (p = 0.03).
Conclusion
PD-L1 expression by immune and tumor cells in non-metastatic MCC seems to significantly improve outcome in patients who did not received PD-1/PD-L1 inhibitors. Prospective studies are needed to confirm our hypothesis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03676-6.
Keywords: Merkel cell carcinoma, PD-L1 expression, Prognostic, Immunological markers
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer, which was first described by Tocker (1972). The incidence of MCC varies considerably in the world’s regions and has significantly increased during the last decade, due to population aging, increased sun exposure and/or improvement of diagnostic immunohistochemical tools as well as improved registration(Allen et al. 2005; Fitzgerald et al. 2015; Agelli and Clegg 2003; Lemos and Nghiem 2007; Lanoy et al. 2010).The two main etiologic factors known to be involved in MCC pathogenesis are Merkel cell polyomavirus (MCYpV) infection, detected in approximatively 80% cases, and accumulation of UV-induced mutations in the MCPyV negative MCC(Agelli and Clegg 2003; Lanoy et al. 2010; Sihto et al. 2009; Kassem et al. 2009). Many other risk factors have been described such as immunosuppression (organ transplant, medication, human immunodeficiency virus [HIV]), male sex, and young age (Sihto et al. 2012; Andea et al. 2008; Sihto and Joensuu 2012; Bichakjian et al. 2007).
Standard treatment for non-metastatic MCC is multimodal: an excision with 1–2 cm margin and sentinel lymph node biopsy is recommended, depending on the primary tumor location and size, followed by therapeutic lymph node dissection in case of positive sentinel node. Adjuvant radiotherapy of the tumor bed and lymph nodes is indicated after surgery to reduce the risk of recurrence (Veness et al. 2005a, b; Lewis et al. 2006; Maubec et al. 2014; Bhatia et al. 2014; Jouary et al. 2012; Jabbour et al. 2007). Several disease characteristics including performance status, comorbidities, and stage at diagnosis can influence the choice of treatment.
Historically, chemotherapy consisting of platinum salts, etoposide or topotecan was used for metastatic MCCs but the progression-free survival remained poor (1.9–4.6 months) (Colunga et al. 2018; Villani et al. 2019; Miller et al. 2013). Nowadays, this modality is only used for palliative treatment or when immunotherapy is contraindicated. Indeed, over the past decade, immune checkpoint inhibitors (ICIs) such as programmed cell Death-Ligand 1 (PDL-1), programmed cell Ligand 1(PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors have emerged in the treatment of stage IIIB/IV MCC and have been shown to provide substantial benefit in patients with advanced disease, compared to conventional chemotherapy (Cassler et al. 2016; D'Angelo et al. , 2018; Nghiem et al. 2019). Since 2017, both the PD-L1 inhibitor avelumab and the PD-1 inhibitor pembrolizumab are recommended in the treatment of metastatic MCCs.
Several clinical and pathological factors have been investigated as potential prognosis factors in MCC with the aim to personalize treatment. Beyond clinical factors such as lymph nodes involvement or MCYpV infection (Rodig et al. 2012; Becker et al. 2009), prognosis may also be influenced by the presence and composition of tumor-infiltrating lymphocytes (TILs), highlighting the regulation of this cancer by the immune system (Sihto et al. 2012; Andea et al. 2008; Sihto and Joensuu 2012; Bichakjian et al. 2007). This is of particular interest in the context of radiation. Indeed, by increasing antigens presentation and subsequent CD8 recruitment, local radiation therapy could enhance the immune response and the effectiveness of immunotherapies in MCC (Paulson et al. 2014).
The aim of this study was to evaluate the influence of clinical and pathological prognostic factors on outcome in patients with non-metastatic MCC, with a particular focus on immunological markers such as TILs subtyping (CD3, CD8, CD68, FoxP3), PD-L1, PD-1 and MCYpV.
Materials and methods
Patient’s characteristics
Patients treated for a non-metastatic MCC between 01/2007 and 12/2018 at our two institutions were retrospectively included in this study. Inclusion criteria were: patients older than 18 years, histologically confirmed MCC and treatment with oncologic surgical resection followed or not by adjuvant radiotherapy.
Staging was defined by nodal ultrasound (US), thoraco-abdomino-pelvic computed tomography (CT) with contrast enhancement and/or 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). Therapeutic management was suggested at multidisciplinary team meetings including dermatologists, oncologists, radiation oncologists, pathologists, and radiologists. All tumors were re-staged according to the AJCC Cancer Staging Manual, 8th edition for the purpose of this study.
Clinical parameters including gender, age, comorbidities known to influence prognosis (immunosupression, hematologic disorders), performance status, anatomic location, type of presentation of primary tumor, size, nodal status, quality of surgical resection as well as radiotherapy volumes, dose and toxicities have been collected.
This study was approved by our institutional review board and all samples were included in a registered tumor tissue collection (CHRU Brest, CPP No. DC-2008-214).
Surgical procedure
Surgical treatment included wide excision of the primary lesion with 1–2 cm margins when possible. Mohs micrographic surgery was an option and was done at the surgeon’s discretion. Sentinel node biopsy (SNB) was usually performed irrespective of primary tumor’s size, followed by regional lymph node dissection in case of occult SNB involvement.
Radiotherapy treatment planning
Radiotherapy was planned according to clinical and radiological parameters including tumor site, size, proximity to organs at risk and lymph node involvement. Irradiation was CT planned. Custom-made bolus to reduce the skin sparing effect and lead shields were frequently used. Local radiotherapy to the primary disease consisted of wide-field RT with minimum 1–2 cm margins around the surgical bed and was applied with megavoltage photon beams (6-MV photons of a linear accelerator) or with 6–12-MeV electrons. Usually a dose of 50 Gy (specified at the 95% isodose line) was delivered in 25 daily fractions but protocol could be modified at the discretion of the treating radiation oncologist depending on performance status and geographical remoteness from radiation center. Adjoining regional lymph nodes were irradiated either with in-continuity electron fields or with photons to a dose of 50 Gy at the depth appropriate to the target volume. A boost up to 60–66 Gy EQD2 could be administered in case of positive margins or macroscopic node. Planning target volume margins were 5–10 mm depending on immobilization and disease location. Treatment plans were generated for a CLINAC (Oncor impression 3D Plus, with 160 leaves) before 2013, then for a TrueBeam STX linac (Varian Medical Systems, Palo Alto, CA with 120 leaves) either using 3D conformational or intensity modulated radiotherapy technique.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on serial 3 μm thick tissue sections from one formalin-fixed paraffin-embedded (FFPE) tumor sample per patient, when these were available. The CD3 (polyclonal antibody, 1:100; Dako, Glostrup, Denmark), CD8 (clone C8/144B, 1:25; Dako), FOXP3 (clone FJK-16 s, 1:100; Thermo Fisher Scientific, Waltham, MA, USA), CD68 (clone PG-M1, 1:100, Dako), MCPyV large T-antigen (clone CM2B4, Santa Cruz Biotechnology, Inc. Dallas, TX, USA), PD-1 (clone NAT105, previously diluted; Roche Diagnostics), and PD-L1 (clone 22C3, 1:50; Dako) markers were analyzed.
Tonsillar samples were used as a positive control for PD-L1 testing. Tissue sections were laid on Superfrost Plus slides. The Ventana Benchmark Ultra automated slide preparation system (Roche Diagnostics) and ultraView Universal DAB Detection Kit (Roche Diagnostics for CD3, CD8, CD68, FOXP3, PD-1 and MCPyV) or OptiView DAB IHC Detection Kit (Roche Diagnostics for PD-L1 IHC) were used. The IHC staining procedure consisted of treatment with Cell Conditioner 1, followed by incubation with appropriately diluted antibody at 37 °C. Antibody incubation (and signal amplification for PD-L1 IHC) was followed by counterstaining with hematoxylin, washing, and mounting.
Slides scanning and reading
IHC slides were scanned using a 3DHistech Panoramic Midi scanner (3DHistech, Budapest, Hungary). The CaseViewer software (3DHistech) was used for manual analyses of digitalized slides without performing any automated quantification. Slides were analyzed by two examiners to reach a consensual result for interpretation.
Analyzes were performed using whole tumors slides for CD3, PD-L1 and MCPyV IHC. TIL (defined as lymphocytes present at the interface of the tumor and stroma) were quantified and classified into 3 groups: not identified (no lymphocyte or lymphocytes present but not infiltrating the tumor), non-brisk (lymphocytes infiltrating the tumor only focally or not along the entire base of the vertical growth) and brisk (lymphocytes diffusely infiltrating the entire base of the dermal tumor or the entire invasive component of the tumor).
PD-L1 IHC expression was assessed in terms of percentage of tumor cells (TC) and immune cells (IC) with membranous straining, as follows: < 1% stained cells, 1–9% stained cells, 10–49% stained cells and ≥ 50% stained cells.
MCPyV IHC was considered as positive in case of any focus of moderate to strong stained cells in tumor cells.
Second line analyses were performed using selected areas of tumor slides of serial tissue sections (corresponding to one field of view × 40, 0.98 mm diameter, 3.02mm2 in our software). CD3, CD8, CD68, FOXP3 and PD-1 were classified according to the following, depending of their amount of expression: absence (< 1%), low (1–9%), moderate (10–49%) or strong (≥ 50%).
Statistical analyses
Standard descriptive statistics were used to describe the general data behavior. Local (LC) and regional control (RC) were defined as the time from surgery to the time of local and regional nodal relapse, respectively. Distant metastasis-free survival (DMFS) was calculated from the date of surgery to the time of any distant metastasis. Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up.
To investigate the prognostic role of individual variables, the log-rank test or univariate Cox regression were used, for categorical and numerical data, respectively. Due to the important number of tested variables, statistical tests for univariate analysis were considered significant at p < 0.01. The receiver operating characteristic (ROC) curve was used to determine cut-off values of significant parameters according to the Youden index. Multivariate Cox model was used as a method to estimate the independent association of a variable set with OS, LC, RC and DMFS. Prognostic factors analyzed were age (> or ≤ 79), gender, performance status (≤ 1 or > 1), comorbidities (immunosupression and hematologic disorder), location (cervico-encephalic versus others), presentation (nodular versus others) and size (> 2 cm or ≤ 2 cm) of primary tumor, surgical margins status (R0 or R1), administration of radiotherapy (yes/no), radiotherapy dose including administration of a boost on the tumor bed, and finally immunohistochemical markers factors (type of TILs, CD3, CD8, CD68, PD-L1 tumor and immune cells, PD-1, FOXP3, MCPyV). Multivariate analysis were considered significant at p < 0.05.
Correlation between immunohistochemical markers and the presence of MCPyV was tested using the Spearman’s rho correlation coefficient.
All statistical analyses were performed using MedCalc Statistical Software version 15.8 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015).
Results
Patients and tumors characteristics
A total of 77 patients treated with surgery with or without adjuvant radiotherapy for a non-metastatic MCC between 01/2007 and 12/2018 were included in this retrospective study.
Median age at diagnosis was 83 years (49–101). The majority of patients were female (56%) with a median PS of 2 (0–3). Thirty-six percent (n = 28) of patients had immunosupression but only 4% (n = 3) had associated hematological malignancies. The predominant location of the primary lesion was cervico-encephalic (52%, n = 40) with a size less than 2 cm in 53% of patients (n = 41). One third of patients (n = 24) presented with a lymph node involvement at diagnosis (Table 1). Median follow-up was 25.7 (0.7–219.9) months.
Table 1.
Patients characteristics
| N = 77 | % | ||
|---|---|---|---|
| Gender | |||
| Female | 43 | 56 | |
| Male | 34 | 44 | |
| Age (median, range) | 83 (49–101) | ||
| Performance status (median, range) | 2 (0–3) | ||
| Size | |||
| < 2 cm | 41 | 53 | |
| > 2 cm | 36 | 47 | |
| Comorbidity | |||
| Skin carcinoma | 20 | 25 | |
| Immunosupression | 28 | 36 | |
| Hemopathy | 3 | 4 | |
| Location | |||
| Cervico-encephalic | 40 | 52 | |
| Trunk | 9 | 12 | |
| Upper extremity | 9 | 12 | |
| Lower extremitity | 19 | 24 | |
| Presentation | |||
| Nodular | 39 | 51 | |
| Ulcerar | 20 | 26 | |
| Cyst | 11 | 14 | |
| Nodal | 7 | 9 | |
| Extension | |||
| Local | 53 | 69 | |
| Locoregional | 24 | 31 | |
| Surgical Margins > 2 cm | |||
| Yes | 20 | 26 | |
| No | 57 | 74 | |
| Sentinel node biopsy | |||
| Yes | 10 | 13 | |
| No | 67 | 87 | |
| Nodal dissection | |||
| Yes | 22 | 29 | |
| No | 55 | 71 | |
| Radiotherapy | 61/77 | 79 | |
| < 50 Gy | 11 | 18 | |
| ≥ 50 Gy | 50 | 82 | |
| Boost on tumor bed | 34 | 56 | |
| Nodal | 43 | 70 |
Treatment characteristics
After diagnostic procedure that included ultrasound (n = 55) and/or FDG-PET (n = 28), all patients underwent surgery of the primary tumor. Overall, 32% (n = 25) needed a revision surgery due to insufficient initial margins (< 2 cm). SNB was performed in 13% (n = 10) and lymph node dissection in 29% (n = 22) of patients. Only one patient underwent dissection following positive lymph node biopsy, in the remaining 21, straight dissection was performed due to clinical or radiological lymph nodes involvement). Adjuvant radiotherapy was performed in 61 patients (79%) and was not administered due to poor performance status in the 16 other patients. Median radiotherapy dose was 50 Gy (range 37.5–64, EQD2). A boost of 10 Gy (EQD2) on the tumor bed was performed in 56% and a nodal radiotherapy in 70% of patients. Mean overall treatment time was 43.0 ± 11.3 days.
Only one patient received concomitant chemotherapy consisting of carboplatin (6 cycles, 40 mg/m2) and another patient received adjuvant chemotherapy (cisplatin and etoposide for 4 cycles). No patients were treated using anti-PD-1/PD-L1 ICI at the time of the study.
Pathology
FFPE primary tumor samples were available in 58/77 patients (75%) and immunohistochemistry was performed in all these cases. The majority of patients (53%) had non-brisk disposal for tumor-infiltrating lymphocytes, 28% had brisk disposal and 19% had lymphocytes present but without infiltrating tumor at all. Tumors were MCPyV positive in 29% (17/58). Overall, 44/58 (76%) tumors contained at least 1% of FoxP3 IHC stained tumor cells and 30/58 (52%) tumors contained at least 1% of PD-1 IHC stained tumor cells. Regarding PD-L1 expression, 69% of immune cells contained at least 1% of PD-L1 IHC stained versus only 19% for tumor cells, if the whole slide was considered. IHC stained at least 1% tumors cells for CD3, CD8 and CD68 in 88% (n = 51), 50% (n = 29), and 57% (n = 33) patients, respectively (Table 2).
Table 2.
Immunohistochemistry (IHC) markers
| N = 58 | % | |
|---|---|---|
| Type of TILs | ||
| No TILs | 11 | 19 |
| No brisk | 31 | 53 |
| Brisk | 16 | 28 |
| CD3 | ||
| Absence | 7 | 12 |
| Low | 22 | 38 |
| Moderate | 23 | 40 |
| Strong | 6 | 10 |
| CD8 | ||
| Absence | 0 | 0 |
| Low | 29 | 50 |
| Moderate | 20 | 34 |
| Strong | 9 | 16 |
| CD68 | ||
| Absence | 0 | 0 |
| Low | 25 | 43 |
| Moderate | 23 | 40 |
| Strong | 10 | 17 |
| PD-L1 IC | ||
| < 1% | 18 | 31 |
| 1–9% | 19 | 33 |
| 10–49% | 20 | 34 |
| ≥ 50% | 1 | 2 |
| PD-L1 TC | ||
| < 1% | 47 | 81 |
| 1–9% | 9 | 16 |
| 10–49% | 2 | 3 |
| ≥ 50% | 0 | 0 |
| PD-1 | ||
| < 1% | 28 | 49 |
| 1–9% | 10 | 17 |
| 10–29% | 17 | 29 |
| ≥ 30% | 3 | 5 |
| MCPyV | ||
| Positive | 17 | 29 |
| Negative | 41 | 67 |
| FOXP3 | ||
| Absence | 14 | 24 |
| Low | 21 | 37 |
| Moderate | 13 | 22 |
| Strong | 10 | 17 |
TILs tumor-infiltrating lymphocytes, CD3-8–6 cluster of differentiation 3–8-68, PD-L1 programmed cell death-ligand 1, IC immune cells, TC tumor cells, PD-1 programmed cell death 1, MCPyV Merkell cell polyomavirus, FOXP3 forkhead box P3
Outcome analysis
Local and regional control
Local failures occurred in 20/77 (25.9%) patients. LC at 6-months and 1-year were 86% and 83%, respectively. On univariate analysis, clinical factors recorded as influencing positively LC were age ≤ 79 years, absence of lymphovascular emboli, adjuvant radiotherapy and administration of an additional radiation boost on the tumor bed. Increased expressions of CD3 and PD-L1 + IC were pathological factors that influenced positively LC. On multivariate analysis, only the absence of lymphovascular emboli remained statistically associated with LC (p < 0.001) (supplementary table 1), yielding an estimated 1-year LC rates of 59% versus 92% in patients with and without lymphovascular invasion (p < 0.001).
Regional lymph nodes failures occurred in 41/77 (53.2%) yielding a 6-months and 1-year RC rates of 70% and 60%, respectively. On univariate analysis, factors influencing RC positively were age ≤ 79 years, tumor size ≤ 2 cm, surgical margins > 2 cm, absence of angiolympatic vascular emboli or perinervous sheatings, stage I disease, adjuvant radiotherapy and lymph node radiotherapy. A brisk disposal of tumor-infiltrating lymphocytes, increased expression of CD3, PD-L1 + IC and presence of MCPyV were pathological factors that positively influenced RC. In multivariate analysis, factors that remained statistically associated with better RC were: age ≤ 79 years (p = 0.006), absence of angiolymphatic vascular emboli (p = 0.027) and expression of PD-L1 + IC ≥ 1% (p = 0.012) (supplementary table 2). The estimated 1-year LR rates were 62% and 33% in patients with and without PD-L1 + IHC IC ≥ 1%, respectively (p = 0.01).
Distant metastasis-free survival
Distant metastasis occurred in 16/77 patients (20.8%) during the follow-up. DMFS at 6-months and 1-year were 88% and 82%, respectively. On univariate analysis, factors influencing positively DMFS rate were: stage I disease, absence of angiolympatic vascular emboli and perinervous sheatings, adjuvant radiotherapy, and expression of PDL-1 + IHC IC (≥ 1%). In multivariate analysis, stage I (p = 0.009), adjuvant radiotherapy (p = 0.021) and rate of PD-L1 IHC stained immune cells (p = 0.003) remained significantly correlated with DMFS (supplementary table 3). The estimated 1-year DMFS rate was 91% versus 65% in patients with PD-L1 + IHC IC ≥ 1% versus others (p < 0.001).
Overall survival
At last follow-up, 23 of 77 patients were alive (29.9%). The overall survival rate at 1 and 2-year were 75% and 55%, respectively. On univariate analysis, age ≤ 79 years, PS ≤ 1 adjuvant radiotherapy with a dose > 50 Gy, absence of angiolymphatic vascular emboli, expression of PD-L1 + IHC IC and TC ≥ 1% and presence of MCPyV were significantly correlated with improved survival. On multivariate analysis, age (p = 0.037), PS (p = 0.045), absence of angiolymphatic vascular emboli (p = 0.006), adjuvant radiotherapy (p = 0.041) and expression of PD-L1 IHC stained IC ≥ 1% (p = 0.006) remained statistically associated with improvement of OS (Table 3). Regarding adjuvant radiotherapy, the estimated 1-year OS rate with or without adjuvant RT was 85% and 59%, respectively (p < 0.001) (Fig. 1). The estimated 1-year OS rate was 82% versus 40% in patients with ≥ 1% versus < 1% PD-L1 IHC stained immune cells (p = 0.0.01) (Fig. 2).
Table 3.
Univariate and mutivariate analysis for overall survival (OS)
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (> 79 vs. ≤ 79) | 3.65 | 2.03–6.55 | < 0.001 | 1.09 | 1.005–1.18 | 0.037 |
| Gender (male vs. female) | 0.79 | 0.44–1.42 | 0.44 | |||
| PS (> 1 vs. ≤ 1) | 2.63 | 1.37–5.014 | 0.002 | 2.36 | 1.01–5.53 | 0.045 |
| Immunosupression (yes vs. no) | 1.25 | 0.68–2.28 | 0.48 | |||
| Location (cervico-encephalic vs. others) | 1.54 | 0.85–2.76 | 0.15 | |||
| Presentation (nodular vs. others) | 1.16 | 0.65–2.09 | 0.61 | |||
| Stage (> I vs. ≤ I) | 1.34 | 0.74–2.44 | 0.35 | |||
| Angiolymphatic vascular emboli (yes vs. no) | 2.25 | 1.05–4.79 | 0.007 | 4.84 | 1.58–14.80 | 0.006 |
| Perinervous sheathings (yes vs. no) | 1.53 | 0.73–3.21 | 0.19 | |||
| Lymph node involvement (yes vs. no) | 1.08 | 0.57–2.08 | 0.80 | |||
| SBLN (yes vs. no) | 0.38 | 0.17–0.86 | 0.09 | |||
| Lymph node dissection (yes vs. no) | 0.62 | 0.31–1.25 | 0.24 | |||
| Radiotherapy (yes vs. no) | 0.33 | 0.15–0.73 | < 0.001 | 0.45 | 0.37–0.92 | 0.041 |
| Dose (> 50 Gy vs. ≤ 50 Gy) | 0.28 | 0.08–0.89 | < 0.001 | 0.95 | 0.51–1.63 | 0.25 |
| Lymph node radiotherapy (yes vs. no) | 0.47 | 0.21–1.12 | 0.063 | |||
| Type of TILs (B vs. NB) | 0.39 | 0.18–0.83 | 0.043 | |||
| CD3 (≥ 1% vs < 1%) | 0.35 | 0.12–0.53 | 0.026 | |||
| CD8 (≥ 1% vs < 1%) | 0.47 | 0.23–0.97 | 0.045 | |||
| CD68 (≥ 1% vs. < 1%) | 0.86 | 0.32–2.35 | 0.79 | |||
| PD-L1 IC (≥ 1% vs. < 1%) | 0.34 | 0.09–0.69 | 0.010 | 0.43 | 0.20–0.79 | 0.006 |
| PD-L1 TC (≥ 1% vs. < 1%) | 0.39 | 0.18–0.83 | 0.009 | 0.82 | 0.31–2.15 | 0.69 |
| PD-L1 Tt (≥ 1% vs < 1%) | 0.76 | 0.34–1.69 | 0.47 | |||
| PD1 (≥ 1% vs. < 1%) | 1.06 | 0.51–2.19 | 0.87 | |||
| FoxP3 (≥ 1% vs. < 1%) | 0.94 | 0.43–2.09 | 0.89 | |||
| MCPyV (positive vs. negative) | 0.34 | 0.16–0.71 | 0.004 | 0.69 | 0.26–1.80 | 0.45 |
Significant results are given in bold
PS performance status, TB tumor bed, SBLN sentinel lymph node biopsy, TILs tumor-infiltrating lymphocytes, CD3-8–68 cluster of differentiation 3–8-68, PD-L1 programmed cell death-ligand 1, IC immune cells, TC tumor cells, TT total, PD-1 programmed cellle death 1, MCPy Merkel cell polyomavirus, FOXP3 forkhead box P3
Fig. 1.

Probability of overall survival depending on treatment with adjuvant radiotherapy
Fig. 2.
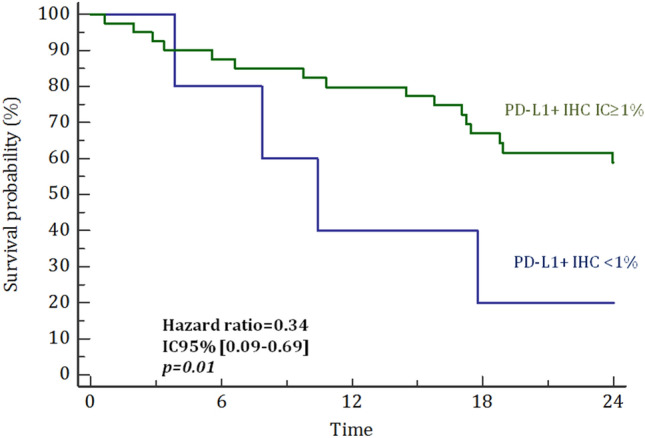
Probability of overall survival depending on percentage of PD-L1 IHC stained immune cells (IC)
Outcomes in the subgroup of irradiated patients depending on immune markers
Among patients who were treated with radiotherapy, LC, DMFS and OS were significantly improved when PDL-1 was expressed by immune cells (≥ 1%) (p < 0.001, 0.006, and 0.01, respectively) (supplementary table 4). Others immune markers did not significantly affect outcomes.
Correlation between immune markers
There was a correlation between MCPyV + tumors and PD-L1 + IC (p = 0.05) as well as with PD-L1 + TC (p = 0.03). Approximately 50% of MCPyV + tumors expressed PD-L1 by TC and 88% expressed PD-L1 by IC. We also found a correlation between PD-L1 + (TC and IC) and other lymphocyte subclasses: CD3 (p < 0.0001), CD8 (p = 0.001), CD68 (p = 0.01), indicating an immune-active environment at the interface of these different cell populations. No other immune marker was correlated with MCPyV.
Discussion
Many treatment-related prognostic factors of MCCs have already been identified in the literature. Among them, sentinel lymph node biopsy (Maubec et al. 2014), adjuvant radiotherapy with a boost on tumor bed (Veness et al. 2005a; Lewis et al. 2006; Bhatia et al. 2014; Jabbour et al. 2007), and lymph node radiotherapy (Jouary et al. 2012; Veness et al. 2005b) have been shown to improve outcome: Indeed, in one of the largest meta-analysis published so far that included 1665 patients, median OS was 63 months in patients treated with surgery followed by adjuvant radiotherapy versus 45 months in patients treated with surgery alone (p = 0.0002) (Petrelli et al. 2019a). In the present study, we also showed that DMFS and OS were improved with the use of adjuvant radiotherapy (p = 0.021 and 0.041, respectively).
Both the PD-L1 inhibitor avelumab and the PD-1 inhibitor pembrolizumab are recommended in the treatment of metastatic MCC since they have been approved by the FDA in 2017 and 2019 respectively, regardless of PD-L1/PD-1 status. The use of avelumab is based on the multicenter randomized phase II JAVELIN Merkel 200 trial, which included patients with metastatic MCC who had failed to conventional chemotherapy. The most recently reported results have demonstrated a 33% objective response rate at 2 years. Durable responses were noted with stable progression-free survival of 29% over the first 2 years. When one considers treatment-naïve patients only, the objective response rate increased to 62.1% (D'Angelo et al. , 2018). Nivolumab and Pembrolizumab, PD-1 inhibitors, have also demonstrated objective response rates ranging from 45 to 65%, with durable response up to 9 months (Nghiem et al. 2019; Choi et al. 2020; Topalian et al. 2020).
Additional markers are needed to improve the management of this disease. In contrast to other solid carcinoma such as malignant melanoma, the expression of PD-L1 has been shown to be associated with improved survival parameters in MCC (Villani et al. 2019; Lipson et al. 2013; Wang et al. 2016; Paulson et al. 2017). Our results confirmed this hypothesis with an improvement in RC (p = 0.012), DMFS (p = 0.003) and OS (p = 0.006) rates when PD-L1 was expressed by immune cells.
FFPE primary tumor samples were available for 58 patients in our cohort. Among these, 43/58 patients were treated with radiotherapy and had a significantly better OS than patients who did not receive radiation (p = 0.041). Our findings also suggest that radiation therapy may be more effective in patients with higher rate of PD-L1 + IC. In fact, in vivo studies have shown that stimulation of the immune system by irradiation improves antigen presentation and recruitment of CD8 + T cells (Ko and Formenti 2019). This phenomenon leads to the enhancement of autoimmune response and could also improve survival parameters by the occurrence of an abscopal effect (Xu et al. 2018; Iyer et al. 2015). In MCC, this phenomenon has been reported in two patients who progressed after immunotherapy and received single-fraction palliative radiotherapy (8 Gy). Not only local control at irradiated regions was observed, but also a durable abscopal response at unirradiated sites of metastatic disease, with a timing of response of 2 and 4 months. One can hypothesize that there is a synergic effect between radiotherapy and immunotherapy, where irradiation acts as an in situ vaccine (Vanpouille-Box et al. 2018). Based on this rational, a study is on-going to evaluate the interaction between immunotherapy and stereotactic body radiation therapy in metastatic MCC (NCT03071406).
MCPyV is known to be clonally integrated in approximately 80% of MCC (Agelli and Clegg 2003; Lanoy et al. 2010; Sihto et al. 2009; Kassem et al. 2009). Surprisingly, in our cohort, only 29% of MCCs were MCPyV + . This finding can suggest another etiology for MCC, in particular neo-epitopes in relation with UV-induced mutational burden. Another explanation could be the fact that MCPyV was analyzed with IHC and not polymerase chain reaction (PCR). The high percentage of patients MCPyV- could explain the modest median OS in our cohort (18 months), with a median OS of 31 months (3–209) versus 9 months (0.1–111) in MCPyV + and MCPyV- MCCs, respectively. MCPyV- MCCs are known to have a poorer prognosis than MCPyV + MCCs (Ricci et al. 2020). In a large cohort of 282 patients, Moshiri et al. showed that virus-negative MCC patients had significantly increased risk of disease progression (HR = 1.77) and death (HR = 1.85) from MCC compared with virus-positive MCC patients (2017).
Several studies have already investigated a possible correlation between MCPyV expression and immune response in MCC, but results remain discordant. Sihto et al. showed that MCPyV + MCC had higher rate of CD3, CD8, and FoxP3 + lymphocytes subclasses compared to MCPyV–MCC (2009). In contrast, Feldmeyer et al. found that MCPyV + and MCPyV– MCCs only differ by their PD-L1 expression rates in inflammatory and tumor cells (2016). Ricci et al. found no correlation between MCPyV and immune markers when these were singly evaluated, but a strong association when immune markers were globally evaluated with a specific immunoscore including different rate of CD3, CD8, FoxP3, and PD-L1, suggesting a tumor-specific immune response with the involvement and the cooperation of numerous lymphocyte subclasses in MCPyV + MCC (2020). In contrast, in our study, we only found a correlation between MCPyV + MCC and expression of PD-L1 by immune and tumor cells. No other immune markers were associated with MCPyV, although we should mention that we evaluated them only singly and not globally. In the literature, both patients with virus-positive and virus-negative MCC seem to benefit from ICIs, whatever of PD-L1 expression (Nghiem et al. 2019; Choi et al. 2020; Samimi 2019).
Ricci et al. have also showed that CD8 + lymphocytes are the only singly evaluated lymphocyte subclass that strongly influenced OS and disease specific-survival (2020). This study supports the results found by Paulson et al. who showed that increase in CD8 + lymphocytes is associated with improved MCC-specific survival, thus adding prognostic information to conventional staging (2014). Here, we did not find any correlation with CD8 rate.
Our study has several limits. First, inherent biases are present due to its retrospective nature. Our cohort is mainly constituted of elderly patients with a median age of 83, compared to 66.5–77 in the meta-analysis of Petrelli et al. which included 17,179 patients (2019b). As such, follow-up was short due to early death. Additionally, older age is usually associated with performance status worsening and this also impacted radiotherapy administration which has been omitted in 21% of them. Additionnally, the radiation dose was suboptimal in 11 of patients who received a dose of 50 Gy EQD2 or less. This is a possible explanation for less good outcome compared to literature. Regional relapses (53%) were also surprisingly frequent in our population. This may be explained by the low rate of patients who benefit from a lymph node biopsy (which should be standard) or dissection (32/77, 41.6%). Here again, older age and impaired performance status may be the reason for suboptimal treatment.
Moreover, no correlation was feasible with any response to anti PD-1/PD-L1 treatment as no patient was treated using these drugs at the time of study. We also acknowledge that our cohort is heterogeneous, and results should be interpreted with caution given the number of variables included. Our results differ from other studies in the selection criteria for IHC, different median value and cut-offs for immune markers. Indeed, the use of different methodologies to identify «PD-L1 + tumors», «PD-L1- tumors» and «presence of immune cells» has generated heterogeneous data in the literature, and as such, results are difficult to compare. We previously showed that a high heterogeneity exists in PD-L1 expression within the same tumor sample: 19% of MCC tumors were «PD-L1 + tumors» when using a cut-off value of ≥ 1% of PD-L1 stained tumor cells versus 55% if only a small foci of PD-L1 + tumor cells was considered. This hypothesis may explain why «PD-L1- tumors» MCC respond to ICIs (Benigni et al. 2020). Further work will look for better biomarkers to predict the response/non response to immune checkpoint inhibitors prior to treatment initiation in MCC.
In conclusion, PD-L1 expression by immune and tumor cells in non-metastatic Merkel cell carcinoma seems to be associated with better RC, DMFS and OS in patients who did not received PD-1/PD-L1 inhibitors. Prospective studies are needed to confirm our hypothesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No financial disclosure to declare.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agelli M, Clegg LX (2003) Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 49(5):832–841 [DOI] [PubMed] [Google Scholar]
- Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG (2005) Merkel cell carcinoma: prognosis and treatment of patients from a single institution. JCO 23(10):2300–2309 [DOI] [PubMed] [Google Scholar]
- Andea AA, Coit DG, Amin B, Busam KJ (2008) Merkel cell carcinoma: histologic features and prognosis. Cancer 113(9):2549–2558 [DOI] [PubMed] [Google Scholar]
- Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D (2009) MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol 129(1):248–250 [DOI] [PubMed] [Google Scholar]
- Benigni P, Guenole M, Bonsang B, Marcorelles P, Schick U, Uguen A (2020) Foci of programmed cell death-ligand 1 (PD-L1)-positive tumor areas with tumor-infiltrating leukocytes (TILs) evocative of a PD-1/PD-L1-related adaptive immune resistance are frequent in merkel cell carcinoma. App Immunohistochem Mol Morphol 28(1):17–22 [DOI] [PubMed] [Google Scholar]
- Bhatia S, Iyer JG, Storer B et al (2014) Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: survival analysis of 6,908 cases from the National Cancer Data Base. JCO 32(15_suppl):9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichakjian CK, Lowe L, Lao CD et al (2007) Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer 110(1):1–12 [DOI] [PubMed] [Google Scholar]
- Cassler NM, Merrill D, Bichakjian CK, Brownell I (2016) Merkel cell carcinoma therapeutic update. Curr Treat Options Oncol 17(7):36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi FD, Kraus CN, Elsensohn AN et al (2020) Programmed cell death 1 protein and programmed death-ligand 1 inhibitors in the treatment of nonmelanoma skin cancer: a systematic review. J Am Acad Dermatol 82(2):440–459 [DOI] [PubMed] [Google Scholar]
- Colunga A, Pulliam T, Nghiem P (2018) Merkel cell carcinoma in the age of immunotherapy: facts and hopes. Clin Cancer Res 24(9):2035–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo SP, Russell J, Lebbe C et al (2018) Efficacy and safety of first-line avelumab treatment in patients with stage iv metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol 4(9):e180077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer L, Hudgens CW, Ray-Lyons G et al (2016) Density, distribution, and composition of immune infiltrates correlate with survival in merkel cell carcinoma. Clin Cancer Res 22(22):5553–5563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE (2015) Dramatic increase in the incidence and mortality from merkel cell carcinoma in the united states. Am Surg 81(8):802–806 [DOI] [PubMed] [Google Scholar]
- Iyer JG, Parvathaneni U, Gooley T et al (2015) Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med 4(8):1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour J, Cumming R, Scolyer RA, Hruby G, Thompson JF, Lee S (2007) Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease–results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol 14(6):1943–1952 [DOI] [PubMed] [Google Scholar]
- Jouary T, Leyral C, Dreno B et al (2012) Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol 23(4):1074–1080 [DOI] [PubMed] [Google Scholar]
- Kassem A, Technau K, Kurz AK et al (2009) Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer 125(2):356–361 [DOI] [PubMed] [Google Scholar]
- Ko EC, Formenti SC (2019) Radiation therapy to enhance tumor immunotherapy: a novel application for an established modality. Int J Radiat Biol 95(7):936–939 [DOI] [PubMed] [Google Scholar]
- Lanoy E, Costagliola D, Engels EA (2010) Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer 126(7):1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos B, Nghiem P (2007) Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol 127(9):2100–2103 [DOI] [PubMed] [Google Scholar]
- Lewis KG, Weinstock MA, Weaver AL, Otley CC (2006) Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol 142(6):693–700 [DOI] [PubMed] [Google Scholar]
- Lipson EJ, Vincent JG, Loyo M et al (2013) PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 1(1):54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubec E, Servy A, Boitier F et al (2014) Sentinel node biopsy in the initial evaluation of 87 patients with Merkel cell carcinoma. JCO 32(15_suppl):9015 [Google Scholar]
- Miller NJ, Bhatia S, Parvathaneni U, Iyer JG, Nghiem P (2013) Emerging and mechanism-based therapies for recurrent or metastatic Merkel cell carcinoma. Curr Treat Options Oncol 14(2):249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri AS, Doumani R, Yelistratova L et al (2017) Polyomavirus-negative Merkel cell carcinoma: a more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol 137(4):819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Bhatia S, Lipson EJ et al (2019) Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. JCO 37(9):693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson KG, Iyer JG, Simonson WT et al (2014) CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: a population-based study. Am J Clin Pathol 142(4):452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson KG, Lewis CW, Redman MW et al (2017) Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer 123(8):1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F, Ghidini A, Torchio M et al (2019a) Adjuvant radiotherapy for Merkel cell carcinoma: a systematic review and meta-analysis. Radiother Oncol 134:211–219 [DOI] [PubMed] [Google Scholar]
- Petrelli F, De Stefani A, Trevisan F et al (2019b) Combination of radiotherapy and immunotherapy for brain metastases: a systematic review and meta-analysis. Crit Rev Oncol Hematol 144:102830 [DOI] [PubMed] [Google Scholar]
- Ricci C, Righi A, Ambrosi F et al (2020) Prognostic impact of MCPyV and TIL subtyping in merkel cell carcinoma: evidence from a large european cohort of 95 patients. Endocr Pathol 31(1):21–32 [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Cheng J, Wardzala J et al (2012) Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest 122(12):4645–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi M (2019) Immune checkpoint inhibitors and beyond: an overview of immune-based therapies in merkel cell carcinoma. Am J Clin Dermatol 20(3):391–407 [DOI] [PubMed] [Google Scholar]
- Sihto H, Joensuu H (2012) Tumor-infiltrating lymphocytes and outcome in Merkel cell carcinoma, a virus-associated cancer. Oncoimmunology 1(8):1420–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H (2009) Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst 101(13):938–945 [DOI] [PubMed] [Google Scholar]
- Sihto H, Bohling T, Kavola H et al (2012) Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res 18(10):2872–2881 [DOI] [PubMed] [Google Scholar]
- Toker C (1972) Trabecular carcinoma of the skin. Arch Dermatol 105(1):107–110 [PubMed] [Google Scholar]
- Topalian SL, Bhatia S, Amin A, et al. Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020: JCO2000201. [DOI] [PMC free article] [PubMed]
- Vanpouille-Box C, Formenti SC, Demaria S (2018) Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res 24(2):259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veness MJ, Perera L, McCourt J et al (2005a) Merkel cell carcinoma: improved outcome with adjuvant radiotherapy. ANZ J Surg 75(5):275–281 [DOI] [PubMed] [Google Scholar]
- Veness MJ, Morgan GJ, Gebski V (2005b) Adjuvant locoregional radiotherapy as best practice in patients with Merkel cell carcinoma of the head and neck. Head Neck 27(3):208–216 [DOI] [PubMed] [Google Scholar]
- Villani A, Fabbrocini G, Costa C, Carmela Annunziata M, Scalvenzi M (2019) Merkel cell carcinoma: therapeutic update and emerging therapies. Dermatol Ther 9(2):209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Teng F, Kong L, Yu J (2016) PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9:5023–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MJ, Wu S, Daud AI, Yu SS, Yom SS (2018) In-field and abscopal response after short-course radiation therapy in patients with metastatic Merkel cell carcinoma progressing on PD-1 checkpoint blockade: a case series. J Immunother Cancer 6(1):43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


