Abstract
Purpose
Previous research on the association between proton pump inhibitor (PPI) use and the risk of progression to high-grade dysplasia (HGD)/esophageal adenocarcinoma (EAC) in Barrett’s Esophagus (BE) patients has generated inconsistent findings. This meta-analysis was performed to clarify the association.
Methods
We performed a comprehensive search strategy to select relevant studies up to September 2020. Heterogeneity was assessed using the I-squared statistic. Odds ratios (OR) and 95% confidence intervals (CI) were calculated through either fixed-effects or random-effects model. Duration-response was also performed to assess the gain effects of different PPI intake duration. Sensitivity analysis, subgroup analyses, and tests for publication bias or other small-study effects were conducted.
Results
Twelve studies with 155,769 subjects were included. The PPI use was associated with the reduced risk of BE progression to HGD/EAC (OR = 0.47, 95% CI = 0.32–0.71). In the duration–response analysis, the estimated OR for decreased risk of HGD/EAC with PPI intake duration of 12 months was 0.81 (95% CI = 0.71–0.91). Sensitivity analysis suggested the results of this meta-analysis were stable. No publication bias was detected.
Conclusions
PPI use is associated with a decreased risk of HGD/EAC in patients with BE. For further investigation, that more well-designed studies are still needed to elucidate the protective effect of PPI usage on BE patients to prevent HGD/EAC.
Keywords: Proton pump inhibitor, High-grade dysplasia, Esophageal adenocarcinoma, Barrett’s esophagus, Meta-analysis
Background
More than 570,000 people are diagnosed with esophageal cancer (EC) each year globally (Bray et al. 2018). In 2018, more than 500,000 deaths were attributed to esophageal cancer, which ranks the 7th in terms of incidence and the 6th on the list of cancer-related mortality worldwide (Bray et al. 2018). Esophageal adenocarcinoma (EAC) incidence is significantly related to the increase in the incidence of Barrett’s esophagus (BE) (Badgery et al. 2020a, b). BE is a metaplastic change in the lower part of the esophagus caused by gastroesophageal reflux disease (GERD) (Badgery et al. 2020a, b). In GERD, the stratified squamous epithelium is replaced by the abnormal columnar epithelium with gastric and intestinal phenotypes (Badgery et al. 2020a, b). In BE, the progression occurs from low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and EAC.
Despite improvements in overall outcomes with current surveillance and treatment strategies, the poor prognosis of EAC is still worse than that of most other cancer types. The 5-year survival rate of EAC is only 20% among patients of western populations (Badgery et al. 2020a, b; Coleman et al. 2018). Therefore, more attention is needed for preventive intervention. Based on previous studies, nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs) and statins have substantial impacts on the risk or incidence of esophageal adenocarcinoma in people with Barret’s esophagus (Schneider et al. 2015; Singh et al. 2014; Thomas et al. 2018). Among the above medications, the strong acid suppression property allows PPI to be theoretically effective, as it could reverse or delay the pathophysiological process of BE in GERD patients. However, emerging evidence shows that PPI use may be associated with adverse reactions, including dementia, fracture, cardiovascular disease, fatty liver disease, and hypomagnesemia (Bhatt et al. 2010; Wei et al. 2020; Zhang et al. 2020; Pyo et al. 2020; Srinutta et al. 2019). Besides, the controversy of whether PPIs may affect preventing BE from progressing to cancer always exists (Kastelein et al. 2013; Tan et al. 2018; Nguyen et al. 2010; Hvid-Jensen et al.2014). The outcome of Kastelein’s (Kastelein et al. 2013) and Tan’s (Tan et al. 2018) studies indicated that PPIs intake was associated with a lower risk of HGD/EAC while Nguyen (Nguyen et al. 2010) and Hvid-Jensen (Hvid-Jensen et al. 2014) did not conclude with such correlations. Therefore, considering its adverse effects, such as dementia (Zhang et al. 2020), fracture (Wei et al. 2020), cardiovascular diseases (Bhatt et al. 2010), fatty liver disease (Pyo et al. 2020), and hypomagnesemia (Srinutta et al. 2019), as well as the controversial conclusions from previous meta-analyses (Singh et al. 2014; Hu et al. 2017), it is necessary to reassess PPI's effects on preventing HGD/EAC among BE patients, by an updated meta-analysis.
Methods
This meta-analysis has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42020206511. It was also performed in the light of the Preferred Reporting Project declared by the Systematic Review and Meta-Analysis (PRISMA), which is a recommended guideline for the performance of meta-analyses, often used to refer to the detailed step-by-step procedures (Moher et al. 2010).
Search strategy
We performed a comprehensive search strategy to select relevant studies. PubMed, Embase, Cochrane Library, Web of Science, CBM (China Biomedical Database) CNKI (China National Knowledge Infrastructure), VIP (Chinese) database, and Wanfang Data were searched up to September 2020. We used the following terms: (“PPI” OR “PPIs” OR “proton pump inhibitors” OR “proton pump inhibitor” OR “Omeprazole” OR “lansoprazole” OR “dexlansoprazole” OR “esomeprazole” OR “pantoprazole” OR “rabeprazole” OR “acid suppress” OR “acid suppression”) AND (“cancer” OR “carcinoma” OR “neoplasms” OR “tumor” OR “adenocarcinoma” OR “metaplasia” OR “HGD” OR “High-Grade Dysplasia” OR “High-Grade Dysplasia”) AND (“oesophageal” OR “esophageal” OR “esophagus” OR “oesophagus”) AND (“Barrett’s esophagus” OR “Barrett esophagus” OR “Barrett’s oesophagus” OR “Barrett oesophagus”). In the Chinese database, Chinese phrases with the same meanings were used, respectively.
Inclusion criteria
Articles can be regarded as eligible if they met the following criteria. (1) The research type was a cohort or case–control design. (2) The subjects of the study were patients with Barrett’s esophagus from any region, country or race. (3) The outcome index was the incidence of EAC or HGD. (4) The experimental group was PPI use, and the control group was non-PPI use. (5) The language of the article is English or Chinese. (6) It provided relative risks (RRs) or odds ratios (ORs) or hazard ratios (HRs) with its 95% confidence intervals (CIs) or provided sufficient original data to calculate the effect size.
Exclusion criteria
Exclusion criteria were as follows: (1) The type of research was not clearly stated and cannot be judged; (2) unable to extract valid ending data from the article; (3) duplicate or identical articles for the same data; (4) outcome index was the incidence of low-grade dysplasia or any grade dysplasia; (5) reviews, animal studies, in vitro studies or meta-analyses.
Data extraction
Two reviewers (Y. Chen and C. Sun) independently carried out literature screening and data extraction according to the inclusion and exclusion criteria. In case of disagreement, they discussed and resolved or consulted a third researcher (Y. Wu). Data extraction content includes included the following: author, year, research type, country, duration of PPI use, study period, the total number of patients with baseline dysplasia status, the incidence of HGD/EAC, adjusted confounders, Newcastle–Ottawa Scale (NOS) scores, etc.
Assessment of study quality
Two authors worked independently using the NOS to evaluate the quality of the included studies. If there was a disagreement, a consensus was achieved through discussion or seeking for a third person's opinion. The NOS scale is particularly applicable to the scoring of observational studies. Case-controls were evaluated in terms of selection, comparability, and exposure, whereas cohort studies were evaluated in terms of selection, comparability, and outcome. Totally, it includes eight items and has a maximum score of nine stars. Furthermore, seven and above stars were considered as high-quality documents, while less than seven stars were considered as low-quality documents (Stang 2010).
Statistical analysis
The association between PPI use and HGD/EAC outcomes was analyzed through pooling the adjusted ORs with 95% CIs of included studies by inverse variance method. Since the absolute risk of HGD/EAC is relatively low, the RRs and HRs were expected to yield similar estimates as ORs and thus were all pooled as ORs. When multiple effect values were provided in a single study, the one that controlled the most confounders was selected. The heterogeneity across studies was assessed with the Q test and I2 statistics (Higgins and Thompson 2002). When substantial heterogeneity was detected (I2 > 50%), the DerSimonian and Laird random-effects model was applied to estimate the pooled OR and 95% CIs (DerSimonian and Laird 1986). Otherwise, the fixed-effects model was applied. Sensitivity analyses were performed by changing the random-effects or fixed-effects models, and by omitting one study at a time (Haidich 2010). Subgroup analysis was performed according to study location, follow-up period, study design, sample size and adjusted cofounders. Duration–response relation between duration of PPI use and risk of HGD/EAC was performed using the method of restricted cubic splines with four knots of the distribution in the glst module of Stata 14.0 (Orsini et al. 2012). Publication bias was detected by using visual inspection of the funnel plot, Begg’s test and Egger’s test (Begg and Mazumdar 1994; Egger et al. 1997). All statistical analyses were performed using RevMan software (version 5.3; Cochrane library) and STATA 14.0 statistical software (Stata Corp., College Station, TX), and a P value less than 0.05 was considered statistically significant.
Results
Study selection and study characteristics
A total of 1276 records were finally confirmed by electronic search and 994 potentially relevant studies were selected after removing duplicate literature, screening the titles and abstracts. Among 45 potentially relevant studies, a total of 12 studies met the inclusion criteria. The detailed process of literature screening is shown in Fig. 1.
Fig. 1.
PRISMA flow chart
In this meta-analysis, we identified 12 published articles with 155,769 subjects, published between 2006 and 2020, including six cohort studies (Srinutta et al. 2019; Thota et al. 2017; Krishnamoorthi et al. 2016; Gaddam et al. 2014; Jung et al. 2011; Nguyen et al. 2009). and six case–control studies (Tan et al. 2018; Nguyen et al. 2010; Hvid-Jensen et al. 2014; de Jonge et al. 2006; Masclee et al. 2015; Loomans-Kropp et al. 2014). Most of the studies were adjusted for the effects of common confounders, including age, sex, race, smoking, alcohol use, obesity, BE length, baseline dysplasia status, time of BE diagnosis, esophagitis or reflux symptoms, and medications (NSAIDs/aspirin/statins), etc. (Studies were variable in terms of adjustment for BE length, NSAID use, ASA use and statin use.) The NOS scores of ten included studies were ≥ 6 scores, except for two abstracts without full texts which did not provide sufficient information for quality assessment. (Table 1). We also extracted the baseline characteristics of the patients. (Table 2).
Table 1.
Characteristics of individual studies included in the meta-analysis
| Study | Country | Time period | Total no. of patients with BE with baseline dysplasia status | Incident EACand/or HGD | Variables adjusted for | Study design | OR (95% CI) | NOS scores | ||
|---|---|---|---|---|---|---|---|---|---|---|
| De jonge (2006) | Netherlands |
2003–2005 NA |
335 | EAC-91 | Adjusteda | Case–control | 0.09 (0.05–0.20) | 6 | ||
| Nguyen et al. (2009) | USA |
1982–2004 mean7.6y |
344NDBE-100% |
HGD-20 EAC-13 |
Adjustedb | Cohort | 0.39 (0.19–0.80) | 8 | ||
| Nguyen et al. (2010) | USA |
2000–2002 NA |
812 | EAC-116 | Adjustedc | Case–control | 1.50 (0.61–3.66) | 6 | ||
| Jung et al. (2011) | USA |
1974–2006 mean5.9y |
355NDBE-83% LGD-17% |
HGD-12 EAC-7 |
Adjustedd | Cohort | 0.23 (0.03–1.72) | 9 | ||
| Kastelein et al. (2013) | Netherlands |
2003–2009 mean5.2y |
540NDBE-86% LGD-14% |
HGD-28 EAC-12 |
Adjustede | Cohort | 0.21 (0.07–0.66) | 8 | ||
| Gaddam et al. (2014) | USA | NA | 3635 | 506 | Adjustedf | Cohort | 0.41 (0.22–0.75) | N/A | ||
| Hvid-Jensen et al. (2014) | Denmark |
1995–2009 mean10.2y |
1437NDBE-90% LGD-10% |
HGD-80 EAC-60 |
Adjustedg | Case–control | 1.90 (0.70–4.90) | 6 | ||
| Masclee et al. (2015) |
UK Netherlands |
1996–2011 1996–2012 |
1466 | 57 | Adjustedh | Case–control | 0.90 (0.30–2.30) | 7 | ||
| Krishnamoorthi et al. (2016) | UK |
1991–2010 mean4.8y |
12,373 | NA | Adjustedi | Cohort | 0.69 (0.52–0.92) | 9 | ||
| Thota et al. (2017) | USA |
2012–2015 mean3.6y |
843NDBE-82.2% LGD-17.8% |
HGD-214 EAC-149 |
Adjustedj | Cohort | 0.49 (0.27–0.89) | 9 | ||
| Tan et al. (2018) | USA | 2004–2009 | 1098 | 300 | Adjustedk | Case–control | 0.59 (0.35–0.99) | 6 | ||
| Loomans-Kropp et al. (2020) | USA | 1992–2013 | 132,286 | 12,026 | Adjustedl | Case–control | 0.30 (0.20–0.44) | N/A | ||
BE Barrett’s esophagus, HGD Barrett’s esophagus with high-grade dysplasia, LGD Barrett’s esophagus with low-grade dysplasia, NA not applicable, NDBE non-dysplastic Barrett’s esophagus, NSAID non-steroidal anti-inflammatory drug, PPI proton pump inhibitor, EAC esophageal adenocarcinoma, N/A Not applicable
aAdjusted for age, gender, educational level, smoking status, alcohol use, reflux symptoms
bAdjusted for gender, age at BE diagnosis, BE length
cAdjusted for race, number of outpatient encounters, noncancer disease comorbidity index, Veterans’ Affairs priority level, filled prescriptions for NSAIDs/aspirin and statins
dAdjusted for age, gender
eAdjusted for age, gender, BE length, histology, baseline PPI use, use of other medications
fAdjusted for presence of male gender, age, Caucasian race, BE length, smoking, H2RA use, aspirin or NSAID use
gAdjusted for presence of LGD, gender, use of statins NSAIDs low-dose aspirin and high-dose aspirin anti-diabetics
hAdjusted for duration of follow-up since BE diagnosis
iAdjusted for age, gender, smoking, BMI, hiatal hernia, Diabetes mellitus-type 2 PPI, NSAIDs, Statin, Metformin, Insulin, and Other anti-diabetic medications
jAdjusted for age, gender, BE length, and hiatal hernia size
kAdjusted for age at Barrett’s diagnosis, smoking status, BMI, number of EGDs after Barrett’s diagnosis statin use, H2RA use, aspirin use, and NSAID use
lAdjusted for age, history of gastroesophageal reflux disease, inflammatory bowel disease, and diabetes with complications
Table 2.
Baseline characteristics of patients in the included studies
| Study | Age at BO diagnosis | Sex (% men) | Race (% Caucasian) | Obesity (% with BMI > 30 kg/m2) | Smoking (% smokers) | Length of BE (% with LSBE) | Reflux symptoms or endoscopic esophagitis | Other medication use | |
|---|---|---|---|---|---|---|---|---|---|
| NSAIDs/aspirin | Statins | ||||||||
| De jonge (2006) | 62 (11) | 74 | 100 | 27 | 20 | NR | 72.5%, NR | 134 (40%) | NR |
| Nguyen et al. (2009) | 61 (12) | 94 | 90 | NR | NR | 29.1 | NR | 169 (49.1%) | 87 (25.3%) |
| Nguyen et al. (2010) | 65 (10) | 97 | 74 | NR | NR | NR | NR | 468 (57.6%) | 377 (46.4%) |
| Jung et al. (2011)a | 63 (14) | 69 | NR | 29b | 13 | 59 | 77, 31% | NR | NR |
| Kastelein et al. (2013) | 61 (53–68) | 71 | NR | 19 | 19 | 100 | 29, 9% | 110 (20.4%) | 102 (18.9%) |
| Gaddam et al. (2014)*,a | 60.9 (12.3) | 87.8 | 93 | NR | NR | NR | NR | NR | NR |
| Hvid-Jensen et al. (2014) | 62.6 (52.4–72.9) | 66.5 | NR | NR | NR | NR | NR |
966 (67.2%) 439 (30.5%) |
250 (17.4%) |
| Masclee et al. (2015) | 64.8 (13.8) | 63 | NR | 18.4 | 11.4 | NR | NR, 4% |
128 (22.8%) 183 (26.3%) |
248 (35.6%) |
| 61.2 (13.4) | 62 | NR | 11.4 | 49.5 | NR | NR, 30% |
104 (13.5%) 48 (6.2%) |
126 (16.4%) | |
| Krishnamoorthi et al. (2016) | 63 (13.8) | 64 | NR | NR | 52.1 | NR | NR | NR | NR |
| Thota et al. (2017)a | 59 (12.6) | 72 | 96 | 28b | NR | NR | NR |
676 (74.1%) 48 (5.3%) |
469 (51.4%) |
| Tan et al. (2018) | 64.8 (9.2) | 100 | NR | 38.6 | 15.10% | NR | 70.8%, NR |
473 (43.1%) 289 (26.3%) |
705 (64.2%) |
| Loomans-Kropp et al. (2020)* | 66–69 | NR | NR | NR | NR | NR | NR | NR | NR |
BMI body mass index, BE Barrett’s esophagus, LSBE long-segment Barrett’s esophagus, NR not reported, NSAID non-steroidal anti-inflammatory drug, EAC esophageal adenocarcinoma, PPI proton pump inhibitors
*For only abstract
aFor entire cohort of patients with BE
bMean BMI
Overall meta-analysis
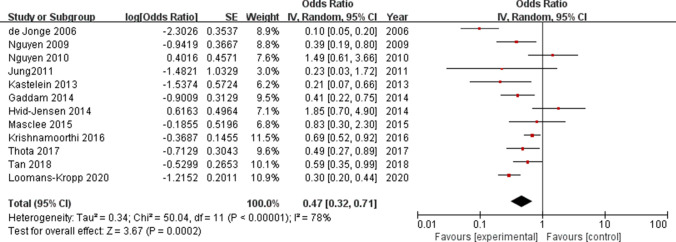
Heterogeneity was found among the studies (I2 = 78%, P < 0.001), so the random effect model was carried out. The result showed that PPI use was associated with reduced risk of progression of BE to HGD/EAC (OR = 0.47, 95% CI = 0.32–0.71, P < 0.001; Fig. 2).
Fig. 2.
Forrest plot: Association between proton pump inhibitor (PPI) use and the risk of progression to high-grade dysplasia (HGD)/esophageal adenocarcinoma
Subgroup analyses
In the subgroup analysis by location, a statistically significant association was observed in North America (OR = 0.47, 95% CI = 0.33–0.68, P < 0.001), whereas no significant association was detected in Europe (OR = 0.47, 95% CI = 0.18–1.22, P = 0.120). In the case of study designs, the pooled OR for HGD/EAC in the cohort studies was 0.55, (95% CI = 0.44–0.69, P < 0.001), and the pooled OR for esophageal cancer in the case–control studies was 0.55 (95% CI = 0.25–1.20, P = 0.130). For follow‐up duration, two studies that followed the study with participants of ≤ 5 years of duration indicated a significant impact of PPI use on reducing the risk of developing HGD/EAC (OR = 0.65, 95% CI = 0.50–0.84, P = 0.001); however, in the four studies with follow‐up periods of > 5 years, the association between the PPI use and incidence of HGD/EAC was not statistically significant (OR = 0.48; 95% CI = 0.17–1.31, P = 0.150). In terms of sample size, the outcomes were significant in both the groups with sample size > 1000 and ≤ 1000 group (OR = 0.57, 95% CI = 0.39–0.82, P = 0.003; OR = 0.32, 95% CI = 0.11–0.88, P = 0.030, respectively). On stratified analysis by adjusted confounders, the subgroup of studies that was multivariable-adjusted, age-gender adjusted, and unadjusted, all showed the statistical significance (OR = 0.46, 95% Cl = 0.30–0.71, P < 0.001; OR = 0.09, 95% Cl = 0.05–0.17, P < 0.001; OR = 0.57, 95% Cl = 0.41–0.81, P = 0.002; respectively). For NOS scores, the results were different when confined to different subgroups (high quality: OR = 0.59, 95% CI = 0.47–0.74, P < 0.001; moderate quality: OR = 0.62, 95% CI = 0.18–2.14, P = 0.440). All these results are represented in Table 3.
Table 3.
Results of subgroups analyses
| Groups | Categories | Number of studies | Effect estimate | P | Heterogeneity with groups (I2) | Pheterogeneity |
|---|---|---|---|---|---|---|
| Location | North America | 7 | 0.47 (0.33, 0.68) | < 0.001 | 53% | 0.040 |
| Europe | 5 | 0.47 (0.18, 1.22) | 0.120 | 89% | < 0.001 | |
| Follow-up period | ≤ 5 years | 2 | 0.65 (0.50, 0.84) | 0.001 | 4% | 0.310 |
| > 5 years | 4 | 0.48 (0.17, 1.31) | 0.150 | 71% | 0.020 | |
| Study design | Case control | 6 | 0.55 (0.25, 1.20) | 0.130 | 87% | < 0.001 |
| Cohort | 6 | 0.55 (0.44, 0.69) | < 0.001 | 36% | 0.160 | |
| Sample size | ≤ 1000 | 5 | 0.32 (0.11, 0.88) | 0.030 | 83% | < 0.001 |
| > 1000 | 7 | 0.57 (0.39, 0.82) | 0.003 | 69% | 0.003 | |
| Adjusted confounders | Multivariable-adjusted | 10 | 0.46 (0.30, 0.71) | < 0.001 | 81% | < 0.001 |
| Age-gender adjusted | 2 | 0.09 (0.05, 0.17) | < 0.001 | 0% | 0.340 | |
| Unadjusted | 7 | 0.57 (0.41, 0.81) | 0.002 | 59% | 0.020 | |
| Quality assessment | High | 6 | 0.59 (0.47–0.74) | < 0.001 | 31% | 0.200 |
| Moderate | 4 | 0.62 (0.18–2.14) | 0.440 | 91% | < 0.001 |
A total of five studies were included in this duration–response analysis, indicating a linear duration–response relationship. An inverse relationship between duration of PPI use and HGD/EAC risk was found. The estimated OR for the risk of HGD/EAC was 0.81 (OR = 0.81, 95% CI = 0.71–0.91) with 12 months of PPI intake. In two subsequent years, the ORs dropped to 0.65 and 0.52, respectively.
Sensitivity analyses
To test the stability of association, sensitivity analyses were conducted by excluding studies one by one. The fluctuation of the pooled ORs was found to be between 0.45 and 0.64 with an upper limit of 95% CI constantly remained less than 1, and P-value constantly remained less than 0.05, suggesting the stability of this meta-analysis. By changing the random-effect model to the fixed-effect model, the overall result was not altered significantly (OR = 0.49, 95% CI = 0.41–0.58), which further confirmed the stability.
Publication bias
No significant evidence of funnel plot asymmetry was observed (Fig. 3). Besides, Begg’s test (Z = 0.48; P = 0.631) and Egger’s test (t = – 0.30; P = 0.768) showed that publication bias might not exist.
Fig. 3.
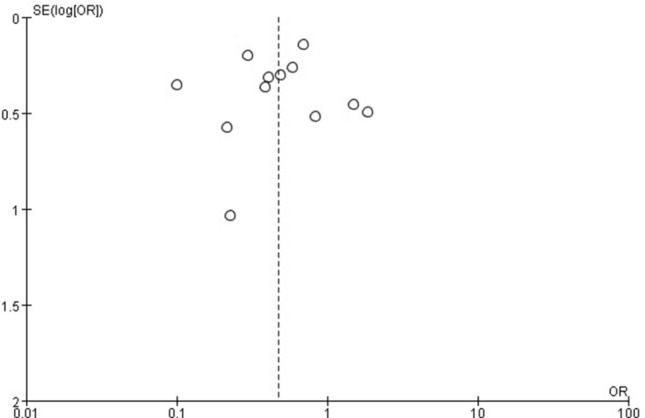
Funnel plot
Discussion
This meta-analysis of five cohort studies and five case––control studies found that the use of PPIs reduces BE progression to HGD/EAC. This finding was inconsistent with the previous meta-analysis of PPI use and the progression to HGD/EAC (Hu et al. 2017), where different inclusion criteria were adopted. Furthermore, three new articles (Tan et al. 2018; Thota et al. 2017; Krishnamoorthi et al. 2016) were also included in our meta-analysis compared with the previous one (Hu et al. 2017). In addition, two original studies (Hillman et al. 2004; Altawil et al. 2011) from the previous meta-analysis (Hu et al. 2017) were excluded as they did not meet our inclusion criteria. In the study conducted by Hillman et al., the study group had delayed PPI use in comparison to blank control (non-PPI) (Hillman et al. 2004). However, both previous meta-analysis (Hu et al. 2017) and this meta-analysis aimed at PPI use for the progression instead of delayed PPI use. Although the outcome in the inclusion criteria of the previous meta-analysis (Hu et al. 2017) was the incidence of HGD/EAC and not any degree of dysplasia, it included the study conducted by Altawil et al., whose outcome index was some degree of dysplasia or EAC (Altawil et al. 2011). According to Vienna classification of gastrointestinal epithelial neoplasia, high-grade adenoma/dysplasia and non-invasive carcinoma (carcinoma in situ) are both classified as category 4 which requires endoscopic or surgical local resection (Schlemper et al. 2000; Dixon 2002), similar to the treatment strategy for early-stage EAC (Shah and Gerdes 2015). Therefore, we included the studies that reported HGD/EAC, and Altawil et al. (Altawil et al. 2011) was excluded because it reported some degree of dysplasia or EAC instead of HGD/EAC.
In the subgroup analysis, the follow-up period of 5 years and less was associated with reduced progression from BE to HGD/EAC by 35%, but the follow-up period of over 5 years did not show the same association. This is consistent with Hu et al.’s findings (Hu et al. 2017). In the case of the location, a statistically significant reduction of 43% was observed in North America, whereas no significant association was detected in Europe. In addition, original studies from other regions, such as Asia and Australia, were very limited, indicating the need for more original studies to explore this role of PPI in populations from those regions. In terms of the study designs, a positive association was observed in cohort studies while case–control studies showed no such association. Due to the essential difference between case–control studies and cohort studies, selection bias and recall bias are inevitable in case–control studies, and the order of exposure and disease are difficult to be judged. In addition, the cohort studies included were of higher quality compared to case–control studies included. Therefore, the results of cohort studies are more reliable than case–control studies. Furthermore, in the stratified analysis by sample size, the outcomes were significant in both groups, and in groups with more than 1,000 people, low heterogeneity was observed. In the subgroup analysis stratified by adjusted confounders, the subgroup of studies that was multivariable-adjusted or age-gender adjusted or unadjusted all demonstrated protective effects of PPI use. The adjusted multivariable included the concurrent use of statins and aspirin, providing some evidence that the effect of PPIs appeared to be independent of aspirin/statin use, similar to the conclusion in the study by Singh et al. (2014).
According to the preclinical findings, the beneficial effect of PPIs and the risk of progression from BE to HGD/EAC is credible. PPIs, which include omeprazole, lansoprazole, pantoprazole, and others are a category of inhibitors treating acid-related diseases. The advantages of PPIs include strong acid suppression, high specificity, and long duration of acid-suppressive effect. The last step of gastric acid secretion is the proton pump in the parietal cells drives the exchange of H+ and K+ in the tubules. It is well known that PPIs inhibit H+ and K+-ATPase, which in turn blocks the last channel of gastric acid secretion (Ahmed and Clarke 2020). As a result of chronic GERD, BE represents the end stage of the natural course of GERD (Lekakos et al. 2011). One-fifth of the population in the United States has been estimated to suffer from gastroesophageal reflux (Locke et al. 1997) and that about 10% of patients with reflux are diagnosed with BE (Winters et al. 1987). BE, defined as salmon-colored mucosa extending ≥ 1 cm proximal to the gastroesophageal (GE) junction, can progress to HGD and ultimately EAC, and BE is the only known precursor for EAC (Kambhampati et al. 2020; Spechler 2013). With the replacement of normal squamous epithelium by metaplastic intestinal-type columnar epithelium (Spechler 2013), the further damage of acid exposure at the metaplastic epithelium is weakened by reducing gastroduodenal reflux (Fitzgerald et al. 2001). Therefore, PPI can promote the healing of esophageal mucosa by reducing exposure to acid (Fitzgerald 2005). In addition to anti-secretory effects, PPI can also directly act on neutrophils and monocytes with anti-inflammatory effects (Kedika et al. 2009). Therefore, it lightens the irritation to the esophagus and prevents epithelial cells from transforming into cancer cells. This is consistent with our overall result of decreased incidence of HGD/EAC in BE patients who take PPIs, as well as the result of duration–response analysis, indicating that the risk of HGD/EAC decreases with different duration of PPIs use (A reduction of 19, 35, and 48% for 12, 24, and 36 months, respectively.). Generally, there is a critical dose for each drug. In this study, the two common drug doses were elaborated. Pantoprazole is effective against ulcers and H. pylori at 40 mg/d. It not only effectively prevents inflammatory lesions during hospitalization at 20 mg/d, but is also available at lower doses freely (Cremer et al. 1995; Kahrilas et al. 2008). For those with severe GERD symptoms or erosive.
Esophagitis, oral omeprazole 20–40 mg once daily is required (Waghray et al. 2019). As to bile reflux, previous study revealed that control of biliary reflux may inhibit the progression from metaplasia to adenocarcinoma (Pera et al. 1993); but it was controversial whether PPIs had the effect on the elimination of biliary reflux. Dellon et al. (2010) concluded that bile reflux can intensify acid reflux-induced erosive esophagitis and cause PPI-refractory reflux symptoms without acid exposure but other researchers have considered that proton pump inhibitors can significantly reduce the duration of bile reflux in GERD patients even when bile reflux is not reduced to the normal range (Champion et al. 1994; Marshall et al. 1998). Some studies also found that long-term use of PPI might give rise to increased gastrin production, potentially promoting cell proliferation and up-regulating COX-2 expression, thus resulting in esophageal cancer (Abdalla et al. 2004; Haigh et al. 2003). In addition, previous studies have shown that long-term use of PPI can cause dementia, fracture, cardiovascular diseases, fatty liver disease, and hypomagnesemia (Bhatt et al. 2010; Wei et al. 2020; Zhang et al. 2020; Pyo et al. 2020; Srinutta et al. 2019). Therefore, prolonged use of PPIs to prevent HGD/EAC for patients with BE requires extra caution to weigh the benefits versus potential side effects.
This meta-analysis has several inherent limitations. First, there was heterogeneity among included studies, but it was reduced among subgroups in relation to study location, follow-up period, study design, sample size, adjusted cofounders, and quality assessment. Second, information to explore the relationship between the different types of PPI and the progression to HGD/EAC was insufficient in stratified analysis. Third, one of the studies included did not have pathology confirmation of adenocarcinoma, though the authors presumed the EC as EAC due to the fact that most EC cases developed in BE were EACs (Krishnamoorthi et al. 2016). Nevertheless, omitting this study did not significantly alter the overall results in the sensitivity analysis. Last but not least, no subgroup analysis comparing different doses of PPIs were perform due to the lack of sufficient data. Notably, a recent trial conducted by Jankowski et al. did not find differences of HGD and EAC risk, but a high-dose PPI is superior to low-dose PPI in terms of all-cause mortality (Jankowski et al. 2018).
Despite the limitations, the advantages of this meta-analysis should be highlighted. First, this meta-analysis included a total number of 19,828 people. The large sample size provided stronger evidence for the meta-analysis. Second, compared with the previous meta-analysis (Hu et al. 2017), the inclusion criteria of this meta-analysis are more stringent, ensuring the quality of research. Third, this meta-analysis stratified study location, follow-up period, study design, sample size and adjusted cofounders. Fourth, a duration-response analysis was performed. Finally, to calculate the pooled OR indicators, multivariable-adjusted ORs were used in most studies, making the research results more accurate.
In conclusion, PPI use is associated with a significantly decreased risk of progression to HGD/EAC in BE patients, especially in patients from North America. This protective effect of PPIs appeared to be independent of aspirin/NSAID/statin use, smoking status, alcohol use, age, and other confounding factors. Given the high incidence of HGD/EAC in BE patients and the high mortality of EAC, chemo-preventive agents could be the modality of choice. More well-designed studies from different populations, including randomized control trials and prospective cohort studies with large sample sizes, high-quality, and long follow-up periods, are needed to elucidate the protective effect of PPIs usage on BE patients to prevent HGD/EAC.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- EAC
Esophageal adenocarcinoma
- EC
Esophageal cancer
- GE
Gastroesophageal
- GERD
Gastroesophageal reflux disease
- HGD
High-grade dysplasia
- HR
Hazard ratio
- LGD
Low-grade dysplasia
- LSBE
Long-segment Barrett’s esophagus
- NA
Not applicable
- NDBE
Non-dysplastic Barrett’s esophagus;
- NOS
Newcastle–Ottawa Scale
- NR
Not reported
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- OR
Odd ratio
- PPI
Proton pump inhibitor
- PRISMA
Preferred reporting project declared by the systematic review and meta-analysis
- PROSPERO
Prospective register of systematic reviews
- RR
Relative risk
Author contributions
YC performed literature search, collected the data, statistical analysis and wrote the manuscript. CS designed the study, performed literature search, collected the data, performed statistical analysis and wrote the manuscript. YW performed statistical analysis, revised the manuscript and provided critical opinion. XC analyzed and interpreted the data. SK, ZK, and GL provided critical opinion and revised the manuscript. ZG and HY revised the manuscript LH participated in the literature search. QZ provided critical opinion, participated in literature search, and revised the manuscript. All authors approved the final manuscript.
Funding
No sources of funding were used to conduct this study.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdalla SI, Lao-Sirieix P, Novelli MR, Lovat LB, Sanderson IR, Fitzgerald RC (2004) Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res 10:4784–4792 [DOI] [PubMed] [Google Scholar]
- Ahmed A, Clarke JO (2020) Proton pump inhibitors (PPI). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557385/ [PubMed]
- Altawil JIB, Jinjuvadia R, Antaki F (2011) Can progression to dysplasia in Barrett’s esophagus be prevented by proton pump inhibitors? Am J Gastroenterol 106:S31 [Google Scholar]
- Badgery H, Huang Q, Georgy SR, Wang DH, Read M (2020a) Recent insights into the biology of Barrett’s esophagus. Ann N Y Acad Sci. 10.1111/nyas.14432 [DOI] [PubMed] [Google Scholar]
- Badgery H, Read M, Winter NN, Taylor ACF, Hii MW (2020b) The role of esophagectomy in the management of Barrett’s esophagus with high-grade dysplasia. Ann N Y Acad Sci. 10.1111/nyas.14439 [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101 [PubMed] [Google Scholar]
- Bhatt DL, Cryer BL, Contant CF et al (2010) Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 363:1909–1917 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424 [DOI] [PubMed] [Google Scholar]
- Champion G, Richter JE, Vaezi MF, Singh S, Alexander R (1994) Duodenogastroesophageal reflux: relationship to pH and importance in Barrett’s esophagus. Gastroenterology 107:747–754 [DOI] [PubMed] [Google Scholar]
- Coleman HG, Xie SH, Lagergren J (2018) The epidemiology of esophageal adenocarcinoma. Gastroenterology 154:390–405 [DOI] [PubMed] [Google Scholar]
- Cremer M, Lambert R, Lamers CB, Delle Fave G, Maier C (1995) European Pantoprazole Study Group. A double-blind study of pantoprazole and ranitidine in treatment of acute duodenal ulcer: a multicenter trial. Dig Dis Sci 40:1360–1364 [DOI] [PubMed] [Google Scholar]
- de Jonge PJ, Steyerberg EW, Kuipers EJ et al (2006) Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol 101:1421–1429 [DOI] [PubMed] [Google Scholar]
- Dellon ES, Shaheen NJ (2010) Persistent reflux symptoms in the proton pump inhibitor era: the changing face of gastroesophageal reflux disease. Gastroenterology 139:7-13.e3 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188 [DOI] [PubMed] [Google Scholar]
- Dixon MF (2002) Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 51:130–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RC (2005) Barrett’s oesophagus and oesophageal adenocarcinoma: how does acid interfere with cell proliferation and differentiation? Gut Suppl 1:i21–i26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RC, Lascar R, Triadafilopoulos G (2001) Review article: Barrett’s oesophagus, dysplasia and pharmacologic acid suppression. Aliment Pharmacol Ther 15:269–276 [DOI] [PubMed] [Google Scholar]
- Gaddam S, Thota PN, Vennalaganti P et al (2014) Proton pump inhibitor use but not statin use is associated with decreased risk for high-grade dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus: results from a large Multicenter Cohort Study. Gastroenterology 146:s-123 [Google Scholar]
- Haidich AB (2010) Meta-analysis in medical research. Hippokratia Suppl 1:29–37 [PMC free article] [PubMed] [Google Scholar]
- Haigh CR, Attwood SE, Thompson DG et al (2003) Gastrin induces proliferation in Barrett’s metaplasia through activation of the CCK2 receptor. Gastroenterology 124:615–625 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC (2004) Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med J Aust 180:387–391 [DOI] [PubMed] [Google Scholar]
- Hu Q, Sun TT, Hong J, Fang JY, Xiong H, Meltzer SJ (2017) Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS ONE 12:e0169691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM (2014) Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett’s oesophagus: a nationwide study of 9883 patients. Aliment Pharmacol Ther 39:984–991 [DOI] [PubMed] [Google Scholar]
- Jankowski JAZ, de Caestecker J, Love SB et al (2018) Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet 392:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KW, Talley NJ, Romero Y et al (2011) Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol 106:1447–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrilas PJ, Shaheen NJ, Vaezi MF et al (2008) American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 135:1383–1391 [DOI] [PubMed] [Google Scholar]
- Kambhampati S, Tieu AH, Luber B, Wang H, Meltzer SJ (2020) Risk factors for progression of Barrett’s esophagus to high grade dysplasia and esophageal adenocarcinoma. Sci Rep 10:4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelein F, Spaander MC, Steyerberg EW et al (2013) Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 11:382–388 [DOI] [PubMed] [Google Scholar]
- Kedika RR, Souza RF, Spechler SJ (2009) Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 54:2312–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthi R, Borah B, Heien H, Das A, Chak A, Iyer PG (2016) Rates and predictors of progression to esophageal carcinoma in a large population-based Barrett’s esophagus cohort. Gastrointest Endosc 84:40-46.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekakos L, Karidis NP, Dimitroulis D, Tsigris C, Kouraklis G, Nikiteas N (2011) Barrett’s esophagus with high-grade dysplasia: focus on current treatment options. World J Gastroenterol 17:4174–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd (1997) Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted county, Minnesota. Gastroenterology 112:1448–1456 [DOI] [PubMed] [Google Scholar]
- Loomans-Kropp HA, Chaloux M, Richmond E et al (2020) Proton pumpinhibitor and statin use decrease risk of esophageal adenocarcinoma among individuals with Barrett’s esophagus: a seer-medicare analysis. Gastroenterology 158:S87–S88 [Google Scholar]
- Marshall RE, Anggiansah A, Manifold DK, Owen WA, Owen WJ (1998) Effect of omeprazole 20 mg twice daily on duodenogastric and gastro-oesophageal bile reflux in Barrett’s oesophagus. Gut 43:603–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclee GM, Coloma PM, Spaander MC, Kuipers EJ, Sturkenboom MC (2015) NSAIDs, statins, low-dose aspirin and PPIs, and the risk of oesophageal adenocarcinoma among patients with Barrett’s oesophagus: a population-based case-control study. BMJ Open 5:e006640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG (2010) PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341 [PMC free article] [PubMed] [Google Scholar]
- Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE (2009) Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 7:1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DM, Richardson P, El-Serag HB (2010) Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Gastroenterology 138:2260–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M, Trastek VF, Carpenter HA et al (1993) Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. Ann Thorac Surg 55:1386–1392 [DOI] [PubMed] [Google Scholar]
- Pyo JH, Kim TJ, Lee H, Choi SC, Cho SJ, Choi Yh et al (2020) Proton pump inhibitors use and the risk of fatty liver disease: a nationwide cohort study. J Gastroenterol Hepatol. 10.1111/jgh.15236 [DOI] [PubMed] [Google Scholar]
- Schlemper RJ, Riddell RH, Kato Y et al (2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Zhao WK, Corley DA (2015) Aspirin and nonsteroidal anti-inflammatory drug use and the risk of Barrett’s esophagus. Dig Dis Sci 60:436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PM, Gerdes H (2015) Endoscopic options for early stage esophageal cancer. J Gastrointest Oncol 6:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB (2014) Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut 63:1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spechler SJ (2013) Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 310:627–636 [DOI] [PubMed] [Google Scholar]
- Srinutta T, Chewcharat A, Takkavatakarn K et al (2019) Proton pump inhibitors and hypomagnesemia: a meta-analysis of observational studies. Medicine 98:e17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605 [DOI] [PubMed] [Google Scholar]
- Tan MC, El-Serag HB, Yu X, Thrift AP (2018) Acid suppression medications reduce risk of oesophageal adenocarcinoma in Barrett’s oesophagus: a nested case-control study in US male veterans. Aliment Pharmacol Ther 48:469–477 [DOI] [PubMed] [Google Scholar]
- Thomas T, Loke Y, Beales ILP (2018) Systematic review and meta-analysis: use of statins is associated with a reduced incidence of oesophageal adenocarcinoma. J Gastrointest Cancer 49:442–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thota PN, Hajifathalian K, Benjamin T, Runkana A, Lopez R, Sanaka MR (2017) Lack of incremental effect of histamine receptor antagonists over proton pump inhibitors on the risk of neoplastic progression in patients with Barrett’s esophagus: a cohort study. J Dig Dis 18:143–150 [DOI] [PubMed] [Google Scholar]
- Waghray A, Waghray N, Perzynski AT, Votruba M, Wolfe MM (2019) Optimal omeprazole dosing and symptom control: a randomized controlled trial (OSCAR trial). Dig Dis Sci 64:158–166 [DOI] [PubMed] [Google Scholar]
- Wei J, Chan AT, Zeng C, Bai X, Lu N, Lei G, Zhang Y (2020) Association between proton pump inhibitors use and risk of hip fracture: a general population-based cohort study. Bone 139:115502 [DOI] [PubMed] [Google Scholar]
- Winters C Jr, Spurling TJ, Chobanian SJ et al (1987) Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 92:118–124 [PubMed] [Google Scholar]
- Zhang Y, Liang M, Sun C, Song EJ, Cheng C, Shi T, Min M, Sun Y (2020) Proton pump inhibitors use and dementia risk: a meta-analysis of cohort studies. Eur J Clin Pharmacol 76:139–147 [DOI] [PubMed] [Google Scholar]