Abstract
Cryptococcus neoformans is a pathogenic fungus with a defined sexual cycle involving haploid MATα and MATa cells. Interestingly, MATα strains are more common, are more virulent than congenic MATa strains, and undergo haploid fruiting in response to nitrogen limitation or MATa cells. Three genes encoding the MFα pheromone were identified in the MATα mating-type locus and shown to be transcriptionally induced by limiting nutrients and coculture with MATa cells. The MFα1, MFα2, and MFα3 genes were mutated, individually and in combination. MATα strains lacking MFα pheromone failed to induce morphological changes in MATa cells. Pheromoneless MATα mutants were fusion and mating impaired but not sterile and mated at ∼1% the wild-type level. The pheromoneless MATα mutants were also partially defective in haploid fruiting, and overexpression of MFα pheromone enhanced haploid fruiting. Overexpression of MFa pheromone also enhanced haploid fruiting of MATα cells and stimulated conjugation tube formation in MATa cells. A conserved G-protein activated mitogen-activated protein kinase signaling pathway was found to be required for both induction and response to mating pheromones. The MFα pheromone was not essential for virulence of C. neoformans but does contribute to the overall virulence composite. These studies define paracrine and autocrine pheromone response pathways that signal mating and differentiation of this pathogenic fungus.
Cryptococcus neoformans is an opportunistic human pathogenic fungus first identified more than 100 years ago (10, 55). The incidence of disease caused by C. neoformans has increased dramatically in the past two decades due to an increasing population of immunocompromised individuals. C. neoformans infection results from inhalation of spores or dessiccated yeast cells, which then spread via the bloodstream to infect the central nervous system and cause life-threatening meningoencephalitis. Studies of the pathogenesis of C. neoformans have defined two important virulence factors: a large polysaccharide capsule that impairs phagocytosis and promotes intracellular survival, and melanin, a pigmented polymer that ensheaths the fungal cells and serves as an antioxidant to protect from oxidative and nitrosative challenge by macrophages (12, 22, 40, 41, 54).
C. neoformans is a heterothallic basidiomycete with a defined sexual cycle involving haploid cells of two mating types: MATα and MATa (2, 37). Mating occurs when two strains of opposite mating type are cocultured under certain nutritional conditions, such as nitrogen limitation, and is thought to be regulated by pheromones (18, 45). After cell fusion, a heterokaryotic filamentous mycelium develops. The tips of the filaments differentiate to form a rounded structure, the basidium, where nuclear fusion, meiosis, and sporulation occur. Interestingly, strains of the MATα mating type differentiate by a process termed haploid or monokaryotic fruiting, which involves filamentation, basidia formation, and sporulation as haploids (64). This process occurs to a limited extent in response to nitrogen limitation alone and is dramatically stimulated when MATa cells are in close proximity (62).
The MATα mating-type locus is also linked to prevalence and virulence of C. neoformans. MATα strains are more common than MATa strains in the environment (38), virtually all clinical isolates are of the MATα mating type, and MATα strains are more virulent than congenic MATa strains in a murine tail vein injection model (39).
An ∼40-kb region of the genome containing part of the MATα locus was previously identified by a difference cloning method (45). One gene encoding a presumptive mating pheromone, MFα1, was identified and found to stimulate conjugation tube formation when transformed into MATa cells. The C. neoformans MFα1 pheromone is similar to Saccharomyces cerevisiae a-factor and basidiomycetous fungal pheromones, which all have a characteristic CAAX prenylation motif at the carboxy terminus (7, 8, 14, 24, 44, 46, 48). Recent studies have revealed that farnesylation and carboxymethylation directed by the CAAX motif are necessary for the activity of expressed or synthetic MFα1 pheromone (18).
Mating pheromones are known to be essential for the initial recognition and cell-cell fusion steps during mating in both budding and fission yeasts (17, 57, 65). In contrast, in the homobasidiomycetes Coprinus cinereus and Schizophyllum commune, cell fusion occurs promiscuously and is not regulated by pheromone. In these organisms, mating pheromones control nuclear migration and fusion of the hook or clamp cells that link the filament cells and function to ensure proper nuclear migration (11, 33, 34, 36, 59). In the hemibasidiomycetous plant fungal pathogen Ustilago maydis, mating pheromones play roles in both early and late stages of mating, controlling conjugation tube formation and cell fusion and also the stability of the heterokaryotic filaments (5, 35, 56).
Here we took a molecular genetics approach to analyze roles of the pheromones in mating, differentiation, and virulence of C. neoformans. Our studies reveal that the mating pheromones play a paracrine signaling role, activating morphological changes in mating partner cells that promote cell fusion and conjugation, similar to the role of pheromones in U. maydis. Our studies also reveal that the MFα mating pheromone plays an autocrine signaling role, stimulating filamentation and sporulation of haploid MATα cells in response to nutrient limitation. Studies of cell growth and mating in the protozoan ciliate Euplotes raikovi have revealed an analogous role for secreted pheromones in regulating mitogenesis via autocrine signaling and mating via paracrine signaling between cells of opposite mating type (6, 49, 61). Taken together, these findings suggest that an ancient autocrine role for pheromones in self-signaling was coopted to play a paracrine signaling role in mating partner recognition in both uni- and multicellular eukaryotes. Alternatively, the unique ability of MATα cells to differentiate via haploid fruiting may have in part evolved via mutations that enable MATα cells to sense and respond to their own mating pheromone.
MATERIALS AND METHODS
Strains.
All studies were conducted with congenic serotype D strains derived from JEC20 (MATa) and JEC21 (MATa) and their auxotrophic derivatives (30, 39, 45). All strains used are listed in Table 1.
TABLE 1.
C. neoformans strains
| Strain | Genotypea | Source (reference) |
|---|---|---|
| JEC20 | MATa | Kwon-Chung et al. (39) |
| JEC21 | MATα | Kwon-Chung et al. (39) |
| JEC30 | MATalys1 | J. Edman |
| JEC34 | MATaura5 | Moore and Edman (45) |
| JEC43 | MATα ura5 | Moore and Edman (45) |
| JEC50 | MATα ade2 | Moore and Edman (45) |
| JEC53 | MATaura5 lys1 | J. Edman |
| JEC155 | MATα ura5 ade2 | J. Edman |
| JEC169 | MATaura5 ade2 lys1 | J. Edman |
| JEC171 | MATaade2 lys2 | J. Edman |
| WSC1 | MATα mfα1::ADE2 ura5 ade2 | This study |
| WSC13 | MATα mfα2,3::URA5 ura5 | This study |
| WSC18 | MATα mfα1::ADE2 mfα2,3::URA5 ade2 ura5 | This study |
| WSC50 | MATα mfα1::ADE2 mfα2,3::ura5 ade2 ura5 (FOAr) | This study |
| WSC51 | MATα mfα1::ADE2 mfα2,3::ura5 ade2 ura5 (FOAr) | This study |
| WSC56 | MATα mfα1::ADE2 mfα2,3::ura5 ade2 ura5 (FOAr) MFα1-URA5 | This study |
| WSC129 | MATα gpb1::URA5 ura5 | This study |
| WSC131 | MATα gpb1::ura5 ura5 (FOAr) | This study |
| RDC21-14 | MATα ste7::ADE2 ade2 ura5 | R. C. Davidson et al., unpublished data |
| RDC46-3 | MATα mfα1::ADE2 mfα2,3::URA5 ade2 ura5 | This study |
| RDC46-4 | MATα mfα1::ADE2 mfα2,3::URA5 ade2 ura5 | This study |
FOAr, resistant to 5-FOA.
Transformations.
Transformation by electroporation was performed by the method described by Edman and Kwon-Chung (20) by using a Bio-Rad gene pulser (480 mV, 25 μF, 800 Ω). This method was used for all pCnTel1-based plasmids, which were cleaved with the rare-cutting meganuclease enzyme I-SceI to reveal the telomeric ends prior to transformation. Biolistic transformations were performed by the method previously described by Toffaletti and Perfect (58) by using a Bio-Rad Model PDS-1000/He Biolistic Particle Delivery System. This method was used for all non-telomere-based plasmids.
Pheromone gene deletions.
Deletion alleles were constructed by subcloning flanking DNA as PCR products or genomic subclones and replacing the coding regions with C. neoformans ADE2 or URA5 gene cassettes. The mfα1 pheromone gene deletion construct was made by overlap PCR (31). The C. neoformans ADE2 gene was cloned in place of the MFα1 coding region. The deletion fragment was released from the vector and biolistically transformed into the MATα ade2 ura5 strain JEC155. Transformants were selected on synthetic medium lacking adenine and containing 1 M sorbitol. Ade+ transformants were colony purified, and deletion mutants were identified by PCR analysis. One deletion strain was backcrossed to the MATa ade2 lys2 strain JEC171 and a MATα prototrophic mfα1 deletion strain selected for phenotypic characterization. The mfα2,3 pheromone gene deletion plasmid was made by inserting the URA5 gene between a 1.4-kb BamHI-XhoI PCR fragment downstream of the MFα3 gene and a 1-kb XhoI-KpnI PCR fragment downstream of the MFα2 gene to replace the MFα2,3 genomic region with the URA5 marker. The mfα2,3 deletion allele was released and biolistically transformed into the MATα ura5 strain JEC43 to create the mfα2,3 deletion strain or into the mfα1 ura5 deletion strain to create the mfα1,2,3 deletion strain (WSC18). Transformants were selected on synthetic medium lacking uracil and containing 1 M sorbitol. Transformants were screened by PCR and confirmed by Southern blot. The MFα1 gene linked to the URA5 marker was reintroduced into a ura5 derivative of the mfα1,2,3 triple mutant strain (WSC50) (generated by selection on 5-fluoroorotic acid [5-FOA] medium) to yield the mfα1,2,3 MFα1 strain (WSC56).
Northern blot analysis.
C. neoformans serotype D wild-type MATα and MATa strains and pheromone deletion strains were grown in liquid yeast extract-peptone-dextrose (YPD) medium overnight at 30°C. The cells were harvested, washed with sterile water, and resuspended in sterile water at 1 × 109 to 5 × 109 cells/ml. Then, 200 μl or 50 μl of MATα wild-type and pheromone deletion strains were spread on V8 or YPD medium. Parallel experiments were performed by spreading the strains mentioned above with the same amount of wild-type MATa cells as mating mixtures. Plates were incubated at 24°C for 28 h, and the cells were collected and lyophilized. Total RNA was isolated by using the RNeasy Mini Kit (Qiagen) and electrophoresed, transferred, and subjected to Northern hybridization by standard procedures. The MFα common open reading frame, the unique MFα3 3′-untranslated region, and the C. neoformans actin gene were used as probes.
Mating assays.
Strains to be subjected to mating assays were first grown on YPD medium for 2 days at 30°C and then cocultured with the wild-type MATa strain JEC20 on V8 mating medium at 24°C. Matings were scored daily for filamentation by using a Nikon Eclipse E400 microscope. Photomicroscopy was on representative sectors of mating mixes.
Quantative mating assay.
107 cells of the MATα prototrophic wild-type, mfα1,2,3 mutant, and mfα1,2,3 MFα1 complemented strains were mixed with 2 × 107 cells of the MATa lys1 ura5 strain JEC53 in 100 μl of H2O. Next, 10 μl of these mixtures was spotted in triplicate onto V8 agar medium, followed by incubation for 10 days. The agar plug containing the mating mixture was excised and completely resuspended in 1 ml of sterile water. The suspension was then serially diluted and plated on synthetic medium lacking lysine and containing 5-FOA, which is toxic to URA5 strains of C. neoformans, to select for recombinant basidiospores. The colonies were counted to determine the mating efficiency.
Cell fusion assays.
107 cells of the MATα mfα1,2,3 ura5 auxotrophic mutant strains WSC50 and WSC51 and the wild-type MATα ura5 auxotrophic strain JEC43 were each incubated separately with the MATa ade2 lys2 auxotrophic strain JEC171 or the MATa lys1 strain JEC30 on V8 medium at room temperature (∼24°C) for 1 to 4 days. An agar plug containing the entire spot of cells was cut out and vortexed vigorously in sterile H2O. The agar was allowed to settle and dilutions of the liquid cell mixture were plated to yeast nitrogen base (YNB) minimal medium or SD-Ura-Lys medium to select for heterokaryons, diploids, or recombinants that are prototrophic as a result of cell fusion. The number of colonies that grew after 3 days was counted and recorded.
Haploid fruiting assays.
Strains were cultured on YPD medium for 48 h, resuspended in sterile water, spotted onto filament agar, and incubated at 24°C for up to 4 weeks in the dark.
Plasmids.
The MFa1 plasmid (pRCD101) was made by PCR amplification from MATa genomic DNA with the primers JOHE6604 (5′-GAGCTCCGACAAGTCCGG GAA-3′) and JOHE6605 (5′-TTTTATATCTGACCCGGAAGC-3′) based on the published sequences (13). The PCR product was cloned into the pCR2.1 vector by using the Topo TA Cloning Kit (Invitrogen), which contains flanking EcoRI restriction sites to create plasmid pRCD99. The insert was subcloned with EcoRI into the telomere containing C. neoformans/Escherichia coli shuttle vector pCnTel1 marked with URA5 to create plasmid pRCD101. The MFα1 plasmid (pRCD17) was made by subcloning the 2.1-kb PCR-amplified MFα1 region from the pCR2.1-based plasmid pRCD1 (18) into the C. neoformans/E. coli URA5 marked shuttle vector pJMM97-3.
β-Galactosidase assays.
Samples were prepared by growing the indicated strains on solid medium, and cells were removed from the agar surface by scraping with a sterile glass rod and resuspending them in distilled water. Cells were then permeabilized by vortexing them for 5 min in 4% chloroform. Control experiments showed that the optical density at 600 nm (OD600) measurement of cultures at different densities corresponded to protein determinations in C. neoformans. β-Galactosidase assays were then performed by standard S. cerevisiae protocols (9). The Miller units for a sample were calculated by the following standard formula: activity = (1,000 × X)/(assay time × volume assayed × Y) and represent units of activityper minute per milliliter per OD. Value X was calculated by performing the standard β-galactosidase assay and measuring the OD595 of the resulting samples. Y is the OD600 of the culture sample. The results shown are representative of several experiments in which similar results were obtained.
GPB1 gene disruption.
The gpb1 gene deletion plasmid was made by inserting the URA5 gene between a 920-bp BamHI-EcoRV PCR fragment upstream of the GPB1 gene open reading frame and a 1,200-bp EcoRV-PstI PCR product downstream of the GPB1 gene to replace the GPB1 genomic region with the URA5 marker. This linear gpb1::URA5 allele was introduced into the MATα ura5 strain JEC43 by biolistic transformation and then confirmed by PCR and Southern analysis. A ura5 derivative of the resulting gpb1 mutant strain was isolated after selection on 5-FOA medium.
Virulence tests.
In vivo testing for virulence assays was conducted in 4- to 6-week-old female DBA mice (NCI/Charles River Laboratories) via tail vein injections. Ten mice were infected with 107 yeast cells of each strain in a volume of 100 μl via lateral tail vein injection as described previously (15, 25). The mice were fed ad libitum and monitored with twice-daily inspections. Mice that appeared moribund or in pain were sacrificed by using CO2 inhalation. Survival data from the mouse experiments were analyzed by a Kruskal-Wallis test.
RESULTS
Identification of the MFα1, MFα2, and MFα3 pheromone genes.
By sequence analysis of the cloned MATα locus, two additional MFα pheromone genes were identified in the well-characterized serotype D strain JEC21 (B-4500) and its congenic derivatives. The MFα2 pheromone gene (GenBank accession number AF305782) is located 18.5 kb away from the MFα1 gene (GenBank accession number S56460), and the two genes are transcribed in opposite orientations (see also reference 18). A third pheromone gene, MFα3, was also identified and is located immediately adjacent to and 613 bp upstream of the MFα2 gene; the closely linked MFα2 and MFα3 genes are divergently transcribed (GenBank accession number AF069982). Similar mapping results have recently been reported by others (32). No other pheromone genes are apparent in the shotgun sequence coverage (12 times the size of the genome) obtained by the C. neoformans serotype D genome sequencing project.
Sequence comparison of the coding and flanking genomic regions of the three pheromone genes reveals that the MFα1 and MFα2 genes are highly related, whereas the MFα3 gene is more divergent. The coding regions of the MFα1 and MFα2 genes are identical. The MFα3 gene has three nucleotide changes in the coding region compared to the MFα1 and MFα2 genes; one alteration results in the replacement of threonine 9 of the pheromone precursor with alanine. The promoter regions (526 bp) of the MFα1 and MFα2 genes share 94.9% identity, compared with only 83.2 and 83.4% identity between the MFα3 gene and the MFα1 and MFα2 genes, respectively. The MFα1 and MFα2 genes differ by only one nucleotide in the 200-bp 3′-untranslated region, whereas this region of the MFα3 gene is completely divergent.
Mutation of the MFα1, MFα2, and MFα3 pheromone genes.
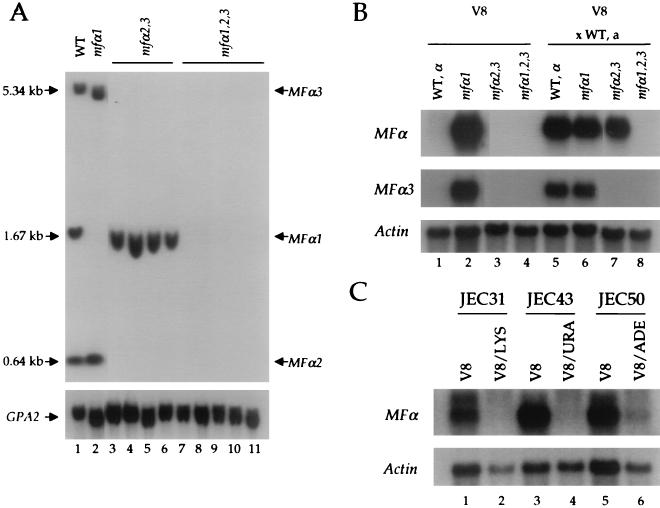
To determine the roles of the three MFα pheromone genes in C. neoformans var. neoformans, the genes were mutated individually and in combination in congenic serotype D strains. The MFα1 gene was replaced with the ADE2 gene, and the linked MFα2 and MFα3 genes were replaced with the URA5 gene (see Materials and Methods). The resulting mfα1Δ::ADE2 and mfα2,3Δ::URA5 deletion alleles were introduced by biolistic transformation. Ade+, Ura+, and Ade+ Ura+ isolates were screened by PCR, and deletion strains were verified by Southern analysis (Fig. 1A). In the mfα1, mfα2,3, and mfα1,2,3 deletion strains, the corresponding pheromone genes were deleted (Fig. 1A and Table 1). No additional pheromone genes that are homologous to MFα1-3 were detected in the mfα1,2,3 triple mutant strain by Southern blot analysis of total genomic DNA. Genetic crosses and meiotic segregation analysis confirmed that the mfα1Δ::ADE2 and mfα2,3Δ::URA5 mutations were linked to each other, to the MATα mating-type locus, and to a defect in mating and that no ectopic integrations of either marker gene had occurred (Table 2).
FIG. 1.
Disruption and characterization of the MFα pheromone genes. (A) The disruption alleles of the MFα1 and MFα2,3 genes were constructed as described in Materials and Methods. Genomic DNA was isolated from the MFα1,2,3 wild-type strain, one mfα1Δ::ADE2 mutant strain, four mfα2,3Δ::URA5 mutant strains, and five mfα1Δ::ADE2 mfα2,3Δ::URA5 mutant strains; digested with XhoI and HindIII; electrophoresed; and analyzed by Southern hybridization with the common open reading frame of the MFα genes as a probe. The GPA2 gene encoding a C. neoformans heterotrimeric G-protein α subunit was used as a control. (B) Total RNA was isolated from the MATα wild-type, mfα1, mfα2,3, and mfα1,2,3 strains grown on V8 agar medium in the presence or absence of the wild-type MATa strain JEC20. Then, 10 μg of total RNA was analyzed with the MFα common, MFα3 specific, and actin gene probes. (C) Total RNA was isolated from MATα lys1 (JEC31), MATα ura5 (JEC43), and MATα ade2 (JEC50) auxotrophic strains grown on V8 agar medium or V8 supplemented with 30 μg of lysine, 20 μg of uracil, or 20 μg of adenine/ml, respectively. Next, 10 μg of total RNA was analyzed with the MFα common and actin gene probes.
TABLE 2.
Segregation analysis of an mfα1,2,3 triple mutant straina
| Segregant | Markerb
|
MAT | Mating efficiencyc | ||
|---|---|---|---|---|---|
| Ade | Ura | Lys | |||
| 1 | − | − | − | a | Fertile |
| 2 | − | − | + | a | Fertile |
| 3 | − | − | + | a | Fertile |
| 4 | − | − | + | a | Fertile |
| 5 | + | + | − | α | Sterile |
| 6 | + | + | − | α | Sterile |
| 7 | − | − | + | a | Fertile |
| 8 | + | + | + | α | Sterile |
| 9 | + | + | + | α | Sterile |
| 10 | + | + | + | α | Sterile |
| 11 | + | + | − | α | Sterile |
| 12 | + | + | − | α | Sterile |
| 13 | + | + | − | α | Sterile |
| 14 | + | + | + | α | Sterile |
| WSC18 | + | + | + | α | Sterile |
| JEC169 | − | − | − | a | Fertile |
Parents: MATα mfα1::ADE2 mfα2,3::URA5 ade2 ura5 (WSC18) × MATa ade2 lys1 ura5 (JEC169).
Growth (+) or no growth (−) on culture medium lacking adenine (Ade), uracil (Ura), or lysine (Lys).
Fertile, wild-type mating; sterile, 1% mating equivalent to the mfα1,2,3 mutant.
Induction of MFα pheromone gene expression by nutrient limitation and MATa cells.
Expression of the pheromone genes was examined by Northern blot to confirm that the pheromone genes had been functionally mutated and to examine conditions regulating pheromone gene expression. Mating of C. neoformans is routinely conducted on V8 medium and does not occur on rich medium such as YPD medium. Cells were grown on V8 and YPD medium with or without congenic MATa mating partner cells. RNA was isolated and analyzed with probes to the open reading frame common to all three MFα genes, the unique 3′ region of the MFα3 gene, or the actin gene as a control (Fig. 1B). The pheromone genes were expressed at a low level when the wild-type MATα strain was grown on V8 medium alone (Fig. 1B). Pheromone gene expression was dramatically induced by the presence of MATa mating partner cells on V8 medium (Fig. 1B, compare lanes 1 and 5). The MFα1 pheromone gene was induced in response to mating partner in the mfα2,3 mutant strain (Fig. 1B, compare lanes 3 and 7), whereas pheromone gene expression was completely abolished in the mfα1,2,3 triple mutant strain (Fig. 1B). Importantly, the fact that the MFα3 gene has a unique 3′ sequence allowed us to show with an MFα3-specific probe that this pheromone gene is also induced by a mating partner (Fig. 1B, compare lanes 1 and 5). Furthermore, MFα3 is not expressed in the mfα2,3 and mfα1,2,3 mutant strains (Fig. 1B). These findings reveal that at least the MFα1 and MFα3 genes are induced during coculture with mating partner cells, that the MFα1-3 genes have been functionally deleted in the mutant strains, and that no other homologous pheromone genes are functionally expressed.
Pheromone gene expression was also found to be induced by nutritional limitation. First, a modest level of pheromone expression could be detected when MATα wild-type cells were cultured with MATa cells in YPD medium, but the level of expression was significantly lower than that observed on V8 medium (data not shown).
Second, surprisingly, expression of the MFα2 and MFα3 genes was readily detected when the mfα1 mutant cells were cultured on V8 medium in the absence or presence of MATa mating partner cells. In contrast to the wild-type and mfα2,3 and mfα1,2,3 mutant strains, the mfα1 mutant strain is a ura5 auxotroph, and V8 medium is limiting for uracil and additional nutrients. Similar findings were obtained with MATα lys1, MATα ura5, and MATα ade2 mutant strains: MFα expression was dramatically induced during culture on V8 medium alone, whereas supplementation with lysine, uracil, or adenine repressed MFα expression (Fig. 1C). These findings may explain the finding that auxotrophic C. neoformans strains mate with enhanced proficiency compared to prototrophic strains. In summary, MFα pheromone gene expression is induced by both nutrient limiting conditions and factors secreted by mating partner cells, such as MFa pheromone. These findings are in accord with our recent studies on MFα1 gene expression by using a reporter gene approach (18).
MFα pheromone promotes cell fusion and is important but not essential for mating.
The pheromone triple gene deletion mutants exhibited defects in mating when crossed with congenic wild-type serotype D MATa cells on V8 medium and the production of mating filaments was monitored by microscopy. In this assay, the mfα1 and mfα2,3 mutant strains mated with an efficiency similar to the wild-type MATα strain (data not shown). By comparison, mating of the mfα1,2,3 triple mutant strain was significantly reduced compared to the wild type, particularly at early time points (Fig. 2). Importantly, the pheromone triple mutant strain was not completely sterile, and some mating filaments, basidia, and basidiospores were still produced. When these filaments were stained with calcofluor white and ethidium bromide, both fused clamp connections and dikaryotic hyphal cells characteristic of mating were observed (data not shown). Because the microscopy-based assay used to monitor mating efficiency is qualitative, we employed a quantitative mating assay that monitors the production of ura5 LYS+ recombinant basidiospores (see Materials and Methods). In this assay, the efficiency of mating of the mfα1,2,3 pheromone mutant strain WSC18 with the MATa lys1 ura5 strain JEC53 was reduced ca. 100-fold compared to the congenic wild-type strain JEC21 (Table 3). Mating was restored to the wild-type level in both the filamentation and quantitative mating assays when the wild-type MFα1 gene was reintroduced into the mfα1,2,3 triple mutant by transformation (Table 3 and Fig. 2). Recombinant basidiospores that exhibited meiotic recombination for parental markers were also recovered from similar crosses (Table 2).
FIG. 2.
MFα pheromone promotes but is not essential for mating. The isogenic wild-type (JEC21), mfα1,2,3 (WSC18), and mfα1,2,3 plus MFα1 (WSC56) strains were coincubated with a MATa wild-type strain (JEC20) on V8 agar mating medium in room light for 2 days at 24°C. The edges of the mating mixtures were photographed at ×100 magnification.
TABLE 3.
Quantitative mating analysis
| Strain | Total no. of recombinants (dilution) | Calculated avg | % Wild-type mating |
|---|---|---|---|
| Wild type (JEC21) | 38 (1:36 dilution) | ||
| 56 (1:36 dilution) | 2,088 | 100 | |
| 80 (1:36 dilution) | |||
| mfα1,2,3 (WSC18) | 16 (undiluted) | ||
| 19 (undiluted) | 19 | 0.9 | |
| 21 (undiluted) | |||
| mfα1,2,3 MFα1 (WSC56) | 40 (1:36 dilution) | ||
| 72 (1:36 dilution) | 1,812 | 86.7 | |
| 39 (1:36 dilution) |
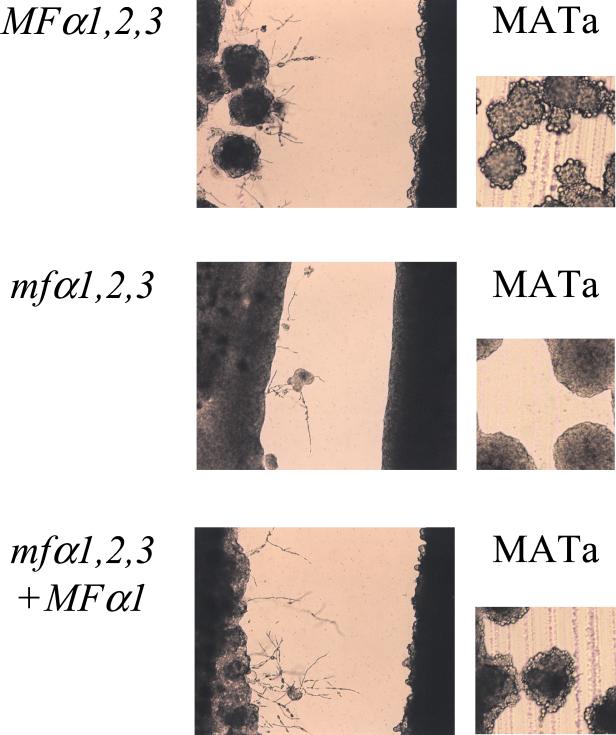
The role of the MFα pheromone in mating was analyzed in further detail by cell fusion and confrontation assays. The mfα1,2,3 ura5 mutant strains WSC50 and WSC51 exhibited a defect in cell fusion, and the production of prototrophic heterokaryons was reduced 50- to 100-fold compared to the wild-type MATα ura5 strain JEC43 coincubated with the MATa lys1 strain JEC30 or the MATa lys2 ade2 strain JEC171 after incubation on V8 medium for 1 to 4 days (see Materials and Methods and data not shown). Thus, the MFα mating pheromone plays a role in the initial cell fusion event during mating. The role of pheromone was also examined in confrontation assays. When wild-type MATα and MATa cells are grown as lines of confronting cells on filamentation agar, MATα cells produce conjugation tubes and haploid fruit (Fig. 3). In contrast, MATa cells produce fewer conjugation tubes and instead a large number of enlarged, round, refractile cells are observed (2, 18, 62). The mfα1,2,3 pheromone triple mutant strain failed to induce confronting MATa cells to produce either conjugation tubes or enlarged round cells (Fig. 3). Thus, the MFα pheromone controls morphological responses of MATa cells during mating. In addition, the mfα1,2,3 mutant cells responded more poorly to MATa cells and formed fewer conjugation tubes and haploid fruited to a more limited extent than did wild-type MATα cells (Fig. 3). These findings suggest that MFα pheromone is required to induce production of the MFa pheromone that then acts on MATα cells. Alternatively, as discussed further below, additional evidence indicates that the MFα pheromone also acts on MATα cells by autocrine signaling.
FIG. 3.
MFα pheromone regulates morphogenesis of confronting MATa cells. The MATα wild-type (JEC21), mfα1,2,3 (WSC18), and MFα1,2,3 MFα1 (WSC56) strains were incubated in close proximity in confrontation assays with the MATa strain JEC20 on filament agar and photographed at ×100 magnification (left panels) or at ×200 magnification after 120 h incubation at 24°C (right panels).
MFα pheromone promotes haploid fruiting of MATα cells.
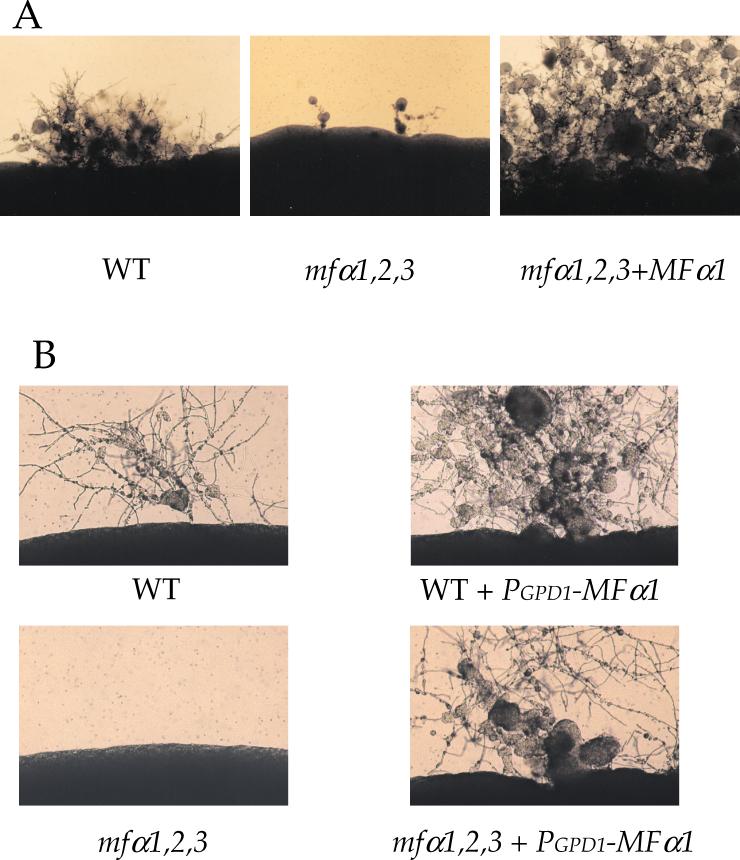
Surprisingly, the mfα1,2,3 triple mutant strain was found to have a significant defect in haploid fruiting in response to nitrogen limitation. Wild-type MATα and the isogenic mfα1,2,3 mutant strain were cultured on filamentation agar for 10 days in the absence of light to enhance haploid fruiting. As shown in Fig. 4A, the wild-type MATα strain haploid fruited under these conditions and produced filaments, basidia, and basidiospores. In comparison, the pheromone triple mutant strain largely failed to differentiate. Some haploid fruiting filaments were observed with the pheromoneless MATα mutant strain upon prolonged incubation but were decreased compared to the wild-type strain. After genetic crosses and isolation of recombinant basidiospores by micromanipulation, the haploid fruiting defect was found to cosegregate with the mfα1,2,3 mutations (data not shown). Finally, reintroduction of the wild-type MFα1 gene into the mfα1,2,3 mutant restored haploid fruiting (Fig. 4A).
FIG. 4.
MFα pheromone stimulates haploid fruiting of C. neoformans. (A) The isogenic wild-type (JEC21), mfα1,2,3 (WSC18), and mfα1,2,3 plus MFα 1 (WSC56) strains were incubated on filament agar for 10 days at 24°C in the dark. (B) The isogenic wild type (JEC21), the mfα1,2,3 mutant strain (WSC17), the MATα ura5 wild-type strain JEC43 transformed with the PGPD1-MFα1 gene fusion, and the mfα1,2,3 ura5 mutant strain (WSC50) transformed with the PGPD1-MFα1 gene fusion (strain WSC57) were incubated on filament agar for 10 days at 24°C in the dark. The edges of the colony were photographed at ×100 magnification.
Haploid fruiting was significantly enhanced in the mfα1,2,3 plus MFα1 reconstituted strain compared to the congenic wild-type strain (Fig. 4A). We considered two possible explainations. First, overexpression of the MFα1 pheromone itself might enhance haploid fruiting of MATα cells. Alternatively, regulatory sequence elements in the promoter of the MFα1 gene could titrate an inhibitory factor and enhance expression of other genes that control differentiation. To distinguish between these models, the MFα1 gene promoter was replaced with the constitutive promoter from the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene (GPD1). When the resulting PGPD1-MFα1 gene fusion was introduced into the wild-type and mfα1,2,3 mutant strains, haploid fruiting was restored in the mfα1,2,3 triple mutant and enhanced in wild-type cells by expression of the MFα1 pheromone from a heterologous promoter (Fig. 4B). These observations support the conclusion that the MFα mating pheromone itself signals differentiation of MATα cells in response to nitrogen-limiting conditions, possibly via an autocrine signaling pathway.
MFα pheromone directs mating and fruiting via a G-protein-mitogen-activated protein (MAP) kinase cascade.
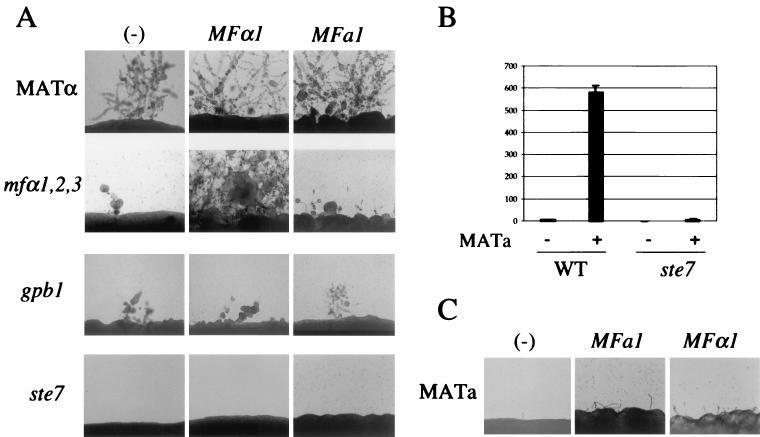
We tested whether overexpression of the MFα1 pheromone enhances haploid fruiting of MATα cells via known components of the pheromone response pathway. To this end, the G-protein β-subunit Gpb1 implicated in regulating mating by pheromone (62) was identified from the congenic serotype D strains JEC21 and JEC20 and replaced with a gpb1::URA5 mutant allele by biolistic transformation and homologous recombination in the MATα ura5 strain JEC43. Five transformants identified by PCR and confirmed by Southern blot contained the gpb1 mutant allele and lacked the wild-type locus (not shown). The MATα gpb1 mutant strains were sterile when coincubated with the MATa wild-type strain JEC20 on V8 mating medium (not shown). Haploid fruiting was reduced, but not completely abolished, in the MATα gpb1 mutant strains, similar to the mfα1,2,3 pheromoneless mutant (Fig. 5A). When the MFα1 pheromone was overexpressed from either the MFα1 or the GPD1 gene promoter in ura5 derivatives of the gpb1 mutant strains, the partial defect in haploid fruiting conferred by the gpb1 mutation was not suppressed (Fig. 5A and data not shown).
FIG. 5.
Pheromones signal haploid fruiting and mating associated gene activation via a conserved G-protein-activated MAP kinase pathway. (A) MFα and MFa pheromones enhance haploid fruiting of wild-type cells but not gpb1 or ste7 mutant cells. The isogenic MATα ura5 wild-type strain (JEC43) and the mfα1,2,3 (WSC50), gpb1 (WSC131), and ste7 (RDC21) mutant strains were biolistically transformed with the MFα1 (pRCD17) or MFa1 (pRCD101) expression plasmids as described in Materials and Methods. Transformants were incubated on filament agar for 5 days at room temperature and photographed at ×100 magnification. (B) The Ste7 MAP kinase is required for activation of MFα pheromone gene expression by MATa cells. The congenic MATα wild-type strain JEC43 and the ste7 mutant strain RDC21 were transformed with the MFα1-lacZ reporter plasmid pRCD41 (18). Transformants were grown on V8 mating medium for 24 h, and the β-galactosidase activity was measured as described in Materials and Methods and plotted here in Miller units. (C) Both MFa1 and MFα1 pheromones stimulate conjugation tube formation by MATa cells. The MATa ura5 strain JEC34 was transformed with the MFa1 (pRCD101) or the MFα1 (pRCD17) pheromone expression plasmid by biolistics as described in Materials and Methods, and Ura+ transformants were incubated on filament agar for 2 days at room temperature and photographed at ×100 magnification.
Because haploid fruiting induced by overexpression of the MFα pheromone required the G protein β subunit Gpb1, we next tested whether signaling occurs via a MAP kinase pathway. Members of a putative pathway regulating mating and filamentation have been identified in C. neoformans (R. C. Davidson et al., unpublished data), including a homolog of the gene enconding the MAP kinase kinase Ste7 that regulates mating and filamentation in S. cerevisiae. C. neoformans serotype D ste7 mutant strains exhibit a severe defect in mating and haploid fruiting (Davidson et al., unpublished data). When the MFα1 pheromone was overexpressed from either the MFα1 or the heterologous GPD1 gene promoter in ste7 mutant strains, the defect in haploid fruiting was not suppressed (Fig. 5A and data not shown).
Induced expression of the MFα1 pheromone gene in response to MFa pheromone or other factors produced by MATa cells was found to require the Ste7 kinase. Expression was monitored with an MFα1-lacZ reporter gene introduced into MATα STE7 wild-type and ste7 mutant cells that were cocultured with MATa cells. MFα1 gene expression was induced in wild-type MATα cells by MATa cells, but not in MATα ste7 mutant cells (Fig. 5B). We note that the basal level of MFα1 pheromone expression in nutrient-limited cells did not require either Gpb1 or Ste7. Thus, MATα gpb1 and MATα ste7 mutant cells induced morphological changes in confronting MATa cells, albeit not to the full extent of wild-type cells, indicating that a reduced level of MFα pheromone is expressed and secreted and is sufficient to mediate paracrine signaling responses in MATa cells (data not shown). Taken together, these findings indicate that elements of a conserved MAP kinase pathway are required for both induced expression of mating pheromone and the autocrine effects of the MFα1 pheromone on MATα cells.
MFa pheromone stimulates fruiting of MATα cells and morphogenesis of MATa cells.
The MFa pheromone was also found to function in both paracrine and autocrine signaling fashions to regulate morphogenesis and development of both MATα and MATa cells. A 2.1-kb region spanning the MFa1 gene (13) was PCR amplified and cloned in the E. coli/C. neoformans URA5 shuttle plasmid pCnTel1. When the resulting MFa1 expression plasmid was introduced into the MATα ura5 strain JEC43, haploid fruiting was dramatically enhanced to a level comparable to MATα cells grown in confrontation with MATa cells (Fig. 5A). These findings provide further evidence that haploid fruiting of MATα cells is enhanced in a paracrine fashion by MFa pheromone secreted by MATa cells.
Most interestingly, when MATa cells were transformed with the MFa1 pheromone expression plasmid, the formation of conjugation tubes was stimulated (Fig. 5C). The autocrine response of MATa cells to MFa pheromone was somewhat less marked than that observed when the MFα1 pheromone gene was introduced into MATa cells (Fig. 5C; see also references 18 and 45). In summary, while cells of each mating type respond more dramatically to the pheromone produced by the opposite cell type, overexpression of the self pheromone (i.e., MFα in MATα or MFa in MATa) stimulates an autocrine pheromone response in C. neoformans.
The MFa pheromone may in part regulate haploid fruiting of MATα cells by inducing the MFα pheromone genes. When the MFa1 pheromone gene was overexpressed in the mfα1,2,3 pheromone triple mutant, haploid fruiting was partially but not completely restored (Fig. 5A). The mfα1,2,3 strain expressing the MFa1 plasmid formed abundant short filaments like the wild-type strain after 3 to 4 days (data not shown). However, after 10 days, the wild-type strain expressing the MFa1 plasmid formed abundant filaments whereas the response of the mfα1,2,3 strain was more modest (Fig. 5A). Thus, the MFα pheromone appears to play a role in filamentation that is in part distinct from that of the MFa pheromone.
The MFa pheromone may regulate haploid fruiting of MATα cells via elements of the conserved G-protein-activated MAP kinase cascade. When the MFa pheromone overexpression plasmid was introduced into the MATα gpb1 or ste7 mutant strains, no restoration of haploid fruiting was observed, providing additional evidence that both Gpb1 and Ste7 participate in sensing either pheromone during mating and haploid fruiting.
MFα pheromone modestly contributes to but is not essential for virulence.
In previous studies, the MATα locus has been linked to virulence of C. neoformans (39). We therefore tested whether the MFα mating pheromones contribute to C. neoformans virulence in a murine tail vein injection model (Fig. 6). A total of 107 cells of the wild-type strain, the mfα1,2,3 mutant strain, the mfα1,2,3 MFα1 reconstituted strain, and two independent mfα1,2,3 mutant strains obtained after a genetic cross were injected into groups of 10 mice each. DBA mice lacking the C5 component of complement and which are particularly susceptible to lethal infection by the congenic serotype D laboratory strains were used (15, 25, 50-52).
FIG. 6.
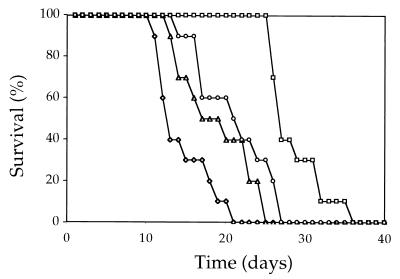
The MFα pheromone is not essential for virulence. A total of 107 cells of the wild-type strain (JEC21), the mfα1,2,3 mutant strain (WSC18), and two independent mfα1,2,3 meiotic segregants isolated after a genetic cross (strains RDC46-3 and RDC46-4) were each injected intravenously into groups of 10 C5 complement-deficient DBA mice. Survival was monitored for 40 days and plotted. Symbols: ◊, MATα; □, mfα1,2,3; ▵, mfα1,2,3-1; ○, mfα1,2,3-2.
Virulence of the original mfα1,2,3 mutant strain was modestly attenuated compared to the wild-type MATα strain JEC21 (P < 0.001). Two independent mfα1,2,3 mutant f1 meiotic segregants were also modestly attentuated for virulence compared to the wild-type strain JEC21 (P = 0.024 and P = 0.006), although not to the same extent as the original mfα1,2,3 mutant strain. The two independent mfα1,2,3 mutant f1 segregants were equally virulent (P = 0.24), both were more virulent than the original mfα1,2,3 deletion strain (P < 0.001 and P = 0.003), and both were less virulent than the wild type. These observations suggest that the virulence defect of the original mfα1,2,3 mutant strain is attributable to the mfα1,2,3 mutations and other events that occurred during the construction of this strain. In accord with this interpretation, virulence was not fully restored in the mfα1,2,3 MFα1 reconstituted strain (data not shown). In summary, we conclude that the MFα pheromone is not essential for virulence, since three independent triple pheromone gene deletion mutant strains were capable of causing 100% lethal infection. This said, infection by three mfα1,2,3 mutant strains resulted in slower progression to lethal infection compared to the wild type, providing evidence that the MFα pheromone makes a modest contribution to virulence.
DISCUSSION
C. neoformans has a bipolar mating-type system in which haploid MATα and MATa cells fuse to produce a filamentous heterokaryon. Like the model yeasts S. cerevisiae and Schizosaccharomyces pombe, the mating-type locus directs cells fusion in C. neoformans and cells with opposite mating type readily fuse, whereas cells with the same mating type rarely do so (C. Hull and J. Heitman, unpublished results). Our findings indicate that the MFα pheromone controls cell fusion in a way that is similar to the role of mating pheromones in the model yeasts. Our findings also reveal that C. neoformans mutants lacking the MFα pheromone exhibit a mating defect but are not absolutely sterile. This is in marked contrast to budding and fission yeasts, in which mating pheromones are absolutely required for cell fusion and mating. On the other hand, these findings are more similar to complex basidiomycetes with multiple sexes, such as S. commune and C. cineurus, in which cell fusion is completely promiscuous and the pheromones only function later in regulating nuclear migration and clamp cell fusion. In these organisms, because there are literally thousands of mating types, virtually every cell encounter is of two different mating types, and stringent regulation of fusion to prevent like pairings is unnecessary (11, 34, 59). Our findings in C. neoformans are most similar to previous studies in the basidiomycetous maize pathogen U. maydis in which pheromones function to promote conjugation tube formation and cell fusion during the earliest steps in mating (4, 56).
In all of these basidiomycetes, pheromones also likely function during later steps in mating to mediate clamp or hook cell fusion and ensure the stability of the dikaryon. We found no dramatic defect in filament morphology in matings involving an mfα1,2,3 mutant strain and wild-type MATa cells. One possible explanation is that MFa pheromone produced locally by the MATa nucleus may suffice to drive clamp cell fusion by signaling to the MFa pheromone receptor expressed by the mfα1,2,3 mutant.
A more surprising finding was that the MFα pheromone stimulates MATα cells to differentiate and undergo monokaryotic fruiting, which involves filamentation and sporulation in response to nitrogen limitation. Haploid fruiting of C. neoformans was thought to be analogous to pseudohyphal differentiation of S. cerevisiae diploid cells in response to nitrogen limitation (26, 64). However, although S. cerevisiae pseudohyphal growth is controlled by elements of the MAP kinase cascade that also functions in mating of haploid yeast cells, the pheromones, their receptors, and the coupled G protein are not expressed in diploid cells and do not participate in pseudohyphal growth (42). In contrast, haploid fruiting of C. neoformans occurs in haploid MATα cells (64), is activated by the G-protein β-subunit Gpb1 involved in pheromone detection (62), is stimulated by MFα mating pheromone in an autocrine fashion (this study), and can be dramatically enhanced by factors secreted by MATa cells (62) or expression of the MFa pheromone (this study). These findings suggest that haploid fruiting is normally activated by mating pheromones and plays a role in the early stages of mating. Recent findings reveal filamentous differentiation of haploid S. cerevisiae cells is also stimulated by mating pheromones, suggesting pheromone induced filamentation and mating are conserved in divergent ascomycetous and basidiomycetous yeasts (21, 53, 62).
Our findings support a model in which the MFα pheromone enhances differentiation by an autocrine signaling loop that activates the pheromone response pathway. This is surprising because in other fungus mating pheromones act only on cells of the opposite mating type. How might MATα cells respond to their own MFα pheromone? One hypothesis is that the MFα pheromone acts as a partial agonist for the MFa pheromone receptor encoded by the STE3α/CPR1α gene. The MFα and MFa pheromones are farnesylated carboxymethylated peptides and also share some amino acid identity, particularly at the predicted amino terminus of the mature pheromone (MFα1 [QEAHPGGMTLC*] and MFa1 [EEAYGSGQGPTYSC*]) (13, 18, 45). Thus, the MFα pheromone may bind to the MFa pheromone binding pocket on a common receptor. Similarly, the MFa pheromone acts on both MATα cells by paracrine signaling and on MATa cells by autocrine signaling. The Ste3a/Cpr1a pheromone receptor expressed by MATa cells and the Ste3α/Cpr1α receptor expressed by MATα cells share significant amino acid sequence identity (∼33%) and may therefore share similar related ligand specificities that contribute to recognition of both self and nonself pheromone ligands. Further studies with synthetic pheromones and heterologous expression of the pheromone receptor genes in S. cerevisiae, as has recently been applied to C. cineurus and S. commune (24, 29, 46), should allow this model to be tested in further detail.
An alternative hypothesis might be that intracellular expression of the mating pheromone precursors alters the processing of other farnesylated proteins involved in signaling. The most likely candidates would be homologs of Ras or the G-protein γ subunit. However, previous studies indicate that these components play or are likely to play positive roles during mating and fruiting (1, 3, 16, 62). Thus, inhibition of their processing should inhibit differentiation in contrast to the stimulation observed upon overexpression of the MFα or MFa pheromone. A second alternative hypothesis is that C. neoformans expresses homologs of the mammalian RAMP proteins, which bind to and alter the ligand specificity of G-protein-coupled receptors (43). As yet, no RAMP homolog has been identified in the ongoing genomic sequence project.
Our studies on autocrine pheromone signaling are analogous to previous studies of self-compatible C. cineurus mutant strains. In the basidiomycete C. cineurus, the unlinked A and B loci determine mating-type compatibility. The B loci encode pheromones and pheromone receptors, and in wild-type strains the pheromones produced by any given B locus are not ligands for the receptor encoded by the same locus (28, 29, 48, 60, 63). Olesnicky et al. recently characterized several unusual self-compatible strains with mutations at the B locus (47). In one case, a single amino change (R96H) in the second intracellular loop of the Rcb36 pheromone receptor enables the receptor to be activated by a normally incompatible pheromone ligand (47). In another case, a single amino acid substitution in the Phb3.26 pheromone (F67W) allows the mutant pheromone to activate a normally incompatible receptor (47). Similar studies have been recently reported in the basidiomycete S. commune. In this case, mutations in a pheromone ligand caused a change in the cognate receptor, and a two-residue deletion in a receptor prevented recognition of one but not other pheromone ligands (23). Mutations that constitutively activate pheromone receptor signaling, or novel chimeric receptor genes, also confer self-compatibility (27, 46). These studies illustrate how subtle amino acid substitutions in either a pheromone receptor or a pheromone can profoundly alter the ligand-receptor interaction. Our findings in C. neoformans suggest the possibility that changes in either the MFa receptor, the MFα pheromone, or both lead to autocrine interactions between the pheromone and receptor that enable MATα cells to sense and differentiate in response to their own mating pheromone.
Our studies on autocrine pheromone signaling are also analogous to previous studies on the regulation of mitogenesis and mating in ciliates. The cosmopolitan soil protozoan ciliate E. raikovi exists in several different mating types, and cell-cell interactions during mating are regulated by secreted peptide mating pheromones (reviewed in reference 6). Importantly, the mating pheromones also regulate mitogenesis in an autocrine signaling fashion in which the secreted pheromone acts on the producing cell (61). Recent studies reveal that the pheromones are expressed in both a membrane-bound and a secreted form, and pheromone recognition involves homotypic interactions between the two forms (49). These findings suggest that the ciliate mating pheromones are similar to mammalian growth factors that drive cell division in an autocrine fashion. Thus, while fungal and ciliate mating pheromones act via distinct signaling mechanisms, there is striking similarity between the autocrine signaling events described in ciliates and our findings in the fungal pathogen C. neoformans.
We propose two possible evolutionary scenarios that gave rise to these autocrine signaling mechanisms. In the first model, pheromone signaling originally evolved as an autocrine signaling response that promoted growth, filamentation, and cell fusion of a primordial fungus with a single receptor and a single lipid modified pheromone. During evolution, some fungi evolved cell type signaling specificity in which pheromones came to act on only the receptors expressed by cells of opposite mating type, whereas other organisms retained autocrine signaling. In the second model, pheromones and their receptors evolved early on to act on only opposite mating partners, but in some fungi mutational events occurred to enable autocrine signaling. Mutations of this type have been readily isolated in C. cinereus and may explain the autocrine signaling we observe with both MFα and MFa pheromones in C. neoformans.
Mating pheromone autocrine signaling may function like the autoinducer factors that control quorum sensing in bacteria. During quorum sensing, bacteria produce and respond to local gradients of small molecules, often homoserine lactone derivatives, in response to population density. The MFα and MFa mating pheromones are hydrophobic farnesylated peptides that likely remain preferentially bound to the membrane of the producing cell. This would provide a mechanism to locally concentrate the pheromone and promote autocrine signaling in response to population density increases in a growing colony. The MFα pheromone genes are induced by nutrient deprivation, which occurs as the colony expands and increasing numbers of cells exhaust the nutrient supply. Autocrine signaling would then stimulate haploid filamentation and sporulation, producing spores that could escape from the colony to forage for nutrients or mating partners.
Our studies also addressed the role of the MFα mating pheromones encoded by the MATα locus in virulence of C. neoformans. Several independent mutants lacking the MFα pheromone were capable of causing 100% lethal infections in a murine inhalation model, and thus the MFα pheromone is not strictly essential for virulence. In accord with this finding, the mfα1,2,3 triple mutant strain had no overt defect in capsule or melanin production and grew normally at 37°C on minimal medium. The virulence potential of the mfα1,2,3 mutant strains was modestly attenuated compared to the wild type, a finding which is consistent with a minor role for the pheromones in virulence. The MFα1 gene was previously found to be induced in vivo at 2 to 3 weeks after infection of the central nervous system in a rabbit model of cryptococcal meningitis with a pathogenic serotype A clinical isolate (19). Thus, the MFα1 gene may respond to nutrient-limiting conditions present in the host, similar to what we observed in our in vitro expression studies. The production of MFα pheromone may subtly alter virulence by acting on the host, on the fungal producing cells by autocrine signaling, or via both mechanisms. We note that, whereas MFα pheromone promotes filamentous differentiation in vitro, during infection C. neoformans cells are found virtually exclusively as budding yeast cells (2). Thus, the MFα pheromone might promote other types of autocrine responses in the host that contribute to virulence.
Acknowledgments
We thank members of the Heitman lab for discussions and John Perfect for his enthusiastic support and encouragement.
This work was supported by NIAID R01 grants AI39115 and AI42159 (to J.H.), and P01 award AI44975 from NIAID to the Duke University Mycology Research Unit. Gary Cox is a Burroughs Wellcome New Investigator in Molecular Pathogenic Mycology. Joseph Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an associate investigator of the Howard Hughes Medical Institute.
W.-C.S. and R.C.D. contributed equally to this study.
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Alspaugh, J. A., R. C. Davidson, and J. Heitman. 2000. Morphogenesis of Cryptococcus neoformans, p. 217-238. In J. F. Ernst and A. Schmidt (ed.), Dimorphism in human pathogenic and apathogenic yeasts, vol. 5. Karger, Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 3.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11:3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakkeren, G., and J. W. Kronstad. 1996. The pheromone cell signaling components of the Ustilago a mating-type loci determine intercompatibility between species. Genetics 143:1601-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banuett, F. 1998. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 62:249-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beale, G. 1990. Self and non-self recognition in the ciliate protozoan Euplotes. Trends Genet. 6:137-139. [DOI] [PubMed] [Google Scholar]
- 7.Bölker, M., M. Urban, and R. Kahmann. 1992. The a mating type locus of U. maydis specifies cell signaling components. Cell 68:441-450. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell, G. A., S. H. Wang, C. B. Xue, Y. Jiang, H. F. Lu, F. Naider, and J. M. Becker. 1994. Molecular determinants of bioactivity of the Saccharomyces cerevisiae lipopeptide mating pheromone. J. Biol. Chem. 269:19817-19826. [PubMed] [Google Scholar]
- 9.Cardenas, M. E., C. Hemenway, R. S. Muir, R. Ye, D. Fiorentino, and J. Heitman. 1994. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 13:5944-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 11.Casselton, L. A., and N. S. Olesnicky. 1998. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62:55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturvedi, S., B. Rodeghier, J. Fan, C. M. McClelland, B. L. Wickes, and V. Chaturvedi. 2000. Direct PCR of Cryptococcus neoformans MATα and MATa pheromones to determine mating type, ploidy, and variety: a tool for epidemiological and molecular pathogenesis studies. J. Clin. Microbiol. 38:2007-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, P., S. K. Sapperstein, J. D. Choi, and S. Michaelis. 1997. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J. Cell Biol. 136:251-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz, M. C., R. A. L. Sia, M. Olson, G. M. Cox, and J. Heitman. 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 68:982-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Souza, C. A., J. A. Alspaugh, C. Yue, T. Harashima, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21:3179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davey, J. 1998. Fusion of a fission yeast. Yeast 14:1529-1566. [DOI] [PubMed] [Google Scholar]
- 18.Davidson, R. C., T. D. E. Moore, A. R. Odom, and J. Heitman. 2000. Characterization of the MFα pheromone of the human fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 39:1-12. [DOI] [PubMed] [Google Scholar]
- 19.Del Poeta, M., D. L. Toffaletti, T. H. Rude, S. D. Sparks, J. Heitman, and J. R. Perfect. 1999. Cryptococcus neoformans differential gene expression detected in vitro and in vivo with green fluorescent protein. Infect. Immun. 67:1812-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edman, J. C., and K. J. Kwon-Chung. 1990. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol. Cell. Biol. 10:4538-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdman, S., and M. Snyder. 2001. A filamentous growth response mediated by the yeast mating pathway. Genetics 159:919-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler, T. J., M. F. Mitton, L. J. Vaillancourt, and C. A. Raper. 2001. Changes in mate recognition through alterations of pheromones and receptors in the multisexual mushroom fungus Schizophyllum commune. Genetics 158:1491-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler, T. J., S. M. DeSimone, M. F. Mitton, J. Kurjan, and C. A. Raper. 1999. Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol. Biol. Cell 10:2559-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox, D. S., M. C. Cruz, R. A. L. Sia, H. Ke, G. M. Cox, M. E. Cardenas, and J. Heitman. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39:835-849. [DOI] [PubMed] [Google Scholar]
- 26.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 27.Gola, S., J. Hegner, and E. Kothe. 2000. Chimeric pheromone receptors in the basidiomycete Schizophyllum commune. Fungal Genet. Biol. 30:191-196. [DOI] [PubMed] [Google Scholar]
- 28.Halsall, J. R., M. J. Milner, and L. A. Casselton. 2000. Three subfamilies of pheromone and receptor genes generate multiple B mating specificities in the mushroom Coprinus cinereus. Genetics 154:1115-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegner, J., C. Siebert-Bartholmei, and E. Kothe. 1999. Ligand recognition in multiallelic pheromone receptors from the basidiomycete Schizophyllum commune studied in yeast. Fungal Genet. Biol. 26:190-197. [DOI] [PubMed] [Google Scholar]
- 30.Heitman, J., B. Allen, J. A. Alspaugh, and K. J. Kwon-Chung. 1999. On the origins of the congenic MATα and MATa strains of pathogenic yeast Cryptococcus neoformans. Fungal Genet. Biol. 28:1-5. [DOI] [PubMed] [Google Scholar]
- 31.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 32.Karos, M., Y. C. Chang, C. M. McClelland, D. L. Clarke, J. Fu, B. L. Wickes, and K. J. Kwon-Chung. 2000. Mapping of the Cryptococcus neoformans MATα locus: presence of mating type-specific mitogen-activated protein kinase cascade homologs. J. Bacteriol. 182:6222-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothe, E. 1999. Mating types and pheromone recognition in the homobasidiomycete Schizophyllum commune. Fungal Genet. Biol. 27:146-152. [DOI] [PubMed] [Google Scholar]
- 34.Kothe, E. 1996. Tetrapolar fungal mating types: sexes by the thousands. FEMS Microbiol. Rev. 18:65-87. [DOI] [PubMed] [Google Scholar]
- 35.Kronstad, J. W., and C. Staben. 1997. Mating type in filamentous fungi. Annu. Rev. Genet. 31:245-276. [DOI] [PubMed] [Google Scholar]
- 36.Kues, U. 2000. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol. Mol. Biol. Rev. 64:316-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 38.Kwon-Chung, K. J., and J. E. Bennett. 1978. Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am. J. Epidemiol. 108:337-340. [DOI] [PubMed] [Google Scholar]
- 39.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon-Chung, K. J., I. Polacheck, and T. J. Popkin. 1982. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 150:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon-Chung, K. J., and J. C. Rhodes. 1986. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect. Immun. 51:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, H., C. A. Styles, and G. R. Fink. 1993. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262:1741-1744. [DOI] [PubMed] [Google Scholar]
- 43.McLatchie, L. M., N. J. Fraser, M. J. Main, A. Wise, J. Brown, N. Thompson, R. Solari, M. G. Lee, and S. M. Foord. 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333-339. [DOI] [PubMed] [Google Scholar]
- 44.Michaelis, S., and I. Herskowitz. 1988. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol. Cell. Biol. 8:1309-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, T. D. E., and J. C. Edman. 1993. The α-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 13:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olesnicky, N. S., A. J. Brown, S. J. Dowell, and L. A. Casselton. 1999. A constitutively active G-protein-coupled receptor causes mating self-compatibility in the mushroom Coprinus. EMBO J. 18:2756-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olesnicky, N. S., A. J. Brown, Y. Honda, S. L. Dyos, S. J. Dowell, and L. A. Casselton. 2000. Self-compatible B mutants in coprinus with altered pheromone-receptor specificities. Genetics 156:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Shea, S. F., P. T. Chaure, J. R. Halsall, N. S. Olesnicky, A. Leibbrandt, I. F. Connerton, and L. A. Casselton. 1998. A large pheromone and receptor gene complex determines multiple B mating type specificities in Coprinus cinereus. Genetics 148:1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortenzi, C., C. Alimenti, A. Vallesi, B. D. Pretoro, A. L. Terza, and P. Luporini. 2000. The autocrine mitogenic loop of the ciliate Euplotes raikovi: the pheromone membrane-bound forms are the cell binding sites and potential signaling receptors of soluble pheromones. Mol. Biol. Cell 11:1445-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhodes, J. C. 1985. Contribution of complement component C5 to the pathogenesis of experimental murine cryptococcosis. Sabouraudia 23:225-234. [DOI] [PubMed] [Google Scholar]
- 51.Rhodes, J. C., and D. H. Howard. 1980. Isolation and characterization of arginine auxotrophs of Cryptococcus neoformans. Infect. Immun. 27:910-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhodes, J. C., L. S. Wicker, and W. J. Urba. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect. Immun. 29:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts, C. J., B. Nelson, M. J. Marton, R. Stoughton, M. R. Meyer, H. A. Bennett, Y. D. He, H. Dai, W. L. Walker, T. R. Hughes, M. Tyers, C. Boone, and S. H. Friend. 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287:873-880. [DOI] [PubMed] [Google Scholar]
- 54.Salas, S. D., J. E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanfelice, F. 1894. Contributo alla morfologia e biologia dei blastomiceti che si svilup/pano nei succhi di alcuni frutti. Ann. Igien. 4:463-495. [Google Scholar]
- 56.Spellig, T., M. Bolker, F. Lottspeich, R. W. Frank, and R. Kahmann. 1994. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 13:1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sprague, G. F., Jr., and J. W. Thorner. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae, p. 657-744. In E. W. Jones, J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces: gene expression, vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 58.Toffaletti, D. L., and J. R. Perfect. 1994. Biolistic DNA delivery for Cryptococcus neoformans transformation, p. 303-308. In B. Maresca, and G. S. Kobayashi (ed.), Molecular biology of pathogenic fungi: a laboratory manual. Telos Press, New York, N.Y.
- 59.Vaillancourt, L. J., and C. A. Raper. 1996. Pheromones and pheromone receptors as mating-type determinants in basidiomycetes. Genet. Eng. 18:219-247. [DOI] [PubMed] [Google Scholar]
- 60.Vaillancourt, L. J., M. Raudaskoski, C. A. Specht, and C. A. Raper. 1997. Multiple genes encoding pheromones and a pheromone receptor define the Bβ1 mating-type specificity in Schizophyllum commune. Genetics 146:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vallesi, A., G. Giuli, R. A. Bradshaw, and P. Luporini. 1995. Autocrine mitogenic activity of pheromones produced by the protozoan ciliate Euplotes raikovi. Nature 376:522-524. [DOI] [PubMed] [Google Scholar]
- 62.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein β subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wendland, J., L. J. Vaillancourt, J. Hegner, K. B. Lengeler, K. J. Laddison, C. A. Raper, and E. Kothe. 1995. The mating-type locus Bα1 of Schizophyllum commune contains a pheromone receptor gene and putative pheromone genes. EMBO J. 14:5271-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto, M., Y. Imai, and Y. Watanabe. 1997. Mating and sporulation in Schizosaccharomyces pombe, p. 1037-1106. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]