Abstract
Purpose
The expression of eukaryotic translation initiation factor-2 subunit 3 (EIF2S3) in patients with non-small cell lung and colorectal cancer is lower than that in healthy individuals. However, the functions of EIF2S3 remain unclear, and its study in leukemia has not been reported. The article aims to explore the role of EIF2S3 in AML (acute myeloid leukemia) and its underlying mechanism.
Methods
Reverse transcription-quantitative PCR was performed to evaluate the expression levels of EIF2S3, and its association with patient prognosis was determined. Inducible HEL-EIF2S3 and HL-60-EIF2S3 cell lines were established by retrovirus infection. Cellular proliferation and the cell cycle were analyzed using Cell Counting Kit-8 and flow cytometric analyses. Tumorigenic ability was evaluated using xenograft nude mouse model. Gene expression profiles were analyzed in HL-60-EIF2S3 cells by next-generation sequencing, and WB analysis was performed to detect the expression of related proteins.
Results
The expression of EIF2S3 in patients with AML was lower than that experiencing CR (P = 0.02). Furthermore, EIF2S3 overexpression inhibited cellular proliferation, halted G0/1 to S phase cell cycle progression, and inhibited tumorigenicity (P = 0.015). 479 differentially expressed genes were identified between HL60-EIF2S3 DOX (−) and HL60-EIF2S3 DOX ( +) cells via NGS and several of them involved in MAPK/ERK signaling pathway. The phosphorylation levels of ERK decreased when EIF2S3 was overexpressed (P < 0.050).
Conclusion
EIF2S3 overexpression may result in a decrease in cellular proliferation, cell cycle arrest, and tumorigenic inhibition via the MAPK/ERK signaling pathway in AML cells.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03712-5.
Keywords: EIF2S3, Cellular proliferation, The cell cycle, Prognosis, Acute myeloid leukemia, MAPK/ERK signaling pathway
Introduction
Acute myeloid leukemia (AML) is a myeloid malignancy characterized by the aberrant proliferation of immature and abnormal primitive cells, and the disruption of hematopoiesis (Narayanan and Weinberg 2020). As a common type of leukemia, the American Cancer Society estimated 19,520 new cases of AML in the United States, accounting for 1.2% of all new cancer cases in 2019 (Siegel et al. 2019). In China, leukemia was one of the top ten malignancies with a high mortality rate, with an increasing incidence rate from 2000 to 2011 (Chen et al. 2016). With the rapid development of cytogenetics and molecular biological techniques, constant progress has been made in the diagnosis and treatment of AML in recent years. In addition to the traditional treatments of chemotherapy and hematopoietic stem cell transplantation, various novel molecular-targeted agents have been identified, including histone deacetylase and receptor-type tyrosine-protein kinase FLT3 inhibitors (Coombs et al. 2016). The Surveillance, Epidemiology, and End Results Program have demonstrated that the relative 5-year survival rate increased from 7.41 to 29.77% in patients with AML, though this is still a relatively low rate in general (Siegel et al. 2019). Furthermore, relapse remained the primary reason for this poor prognosis (Bose et al. 2017). Therefore, the focus of AML therapy is to identify novel biomarkers for improving treatment options and patient prognosis.
Eukaryotic translation initiation factor-2 subunit 3 (EIF2S3/eIF2γ) is located in the Xp22.11 region of the X chromosome, and comprises 472 amino acids. EIF2S3 is a protein subunit that contains a GTP-binding pocket. As the core of the heterotrimer, EIF2S3 constitutes the eukaryotic translation initiation factor-2 (eIF2) with the other two subunits, EIF2S1 and EIF2S2 (Hinnebusch 2011, 2014; Hinnebusch and Lorsch 2012). eIF2 plays an important role in eukaryotic translation, binding GTP through eIF2γ, and initiator methionyl-tRNA (Met-tRNAiMet) to form a stable ternary complex, and identifying the translation initiation site (Hinnebusch 2011; Young-Baird et al. 2019). Previous studies have reported that EIF2S3 mutations cause X-linked intellectual disabilities (XLID; also known as mental retardation, epileptic seizures, hypogenitalism, microcephaly, and obesity syndrome, based on its associated characteristics), characterized by mental deficiency, epilepsy, hypogenitalism, microcephaly and obesity (Stanik et al. 2018; Skopkova et al. 2017; Moortgat et al. 2016; Borck et al. 2012). In tumor-related studies, Chian et al. (2016) illustrated that the level of peripheral blood EIF2S3 mRNA expression was lower in patients with non-small cell lung cancer than in healthy subjects. Chang et al. (2014) observed a similar outcome in colorectal cancer. However, the functions and mechanisms underlying these observations remain unclear, and there are currently no relevant reports surrounding EIF2S3 expression in AML.
To the best of our knowledge, the present study was the first to discover that EIF2S3 is significantly associated with clinicopathological parameters and a positive prognosis in patients with AML. EIF2S3 overexpression resulted in a decrease in cellular proliferation, cell cycle arrest, and tumorigenic inhibition via the MAPK/ERK signaling pathway.
Materials and methods
Patients and samples
A total of 61 bone marrow (BM) samples were acquired from patients with AML at the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China), who were diagnosed between October 2012 and March 2017. Diagnosis was established following clinical, morphological, cytochemical, flow cytometric, and cytogenetic analysis, which conformed to the World Health Organization (WHO) diagnostic criteria (Dohner et al. 2017; Arber et al. 2016). A marrow or blood blast count ≥ 20% was considered as diagnostic of AML, except for patients with t(15;17), t(8;21), inv(16) or t(16;16). Complete remission (CR) was defined as follows: i) A BM blast count < 5%; ii) absence of blasts with Auer rods; iii) absence of extramedullary disease; iv) absolute neutrophil counts > 1.0 × 109/l; v) platelet counts > 100 × 109/l; and vi) independence of red cell transfusions (Dohner et al. 2010). According to the French–American–British (FAB) classification for the diagnosis and classification of AML (Arber et al. 2016), 41 samples were classified as primary AML, including one sample with M0, three samples with M1, 12 samples with M2, six samples with M3, seven samples with M4, seven samples with M5, one sample with M6, and four unclassified samples. A further 20 samples were obtained from patients with CR. The follow-up time ended in July 2018. Besides, we also collect nine peripheral blood samples from primary AML patients and eight healthy people as control, comparing the expression level of EIF2S3. The study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University, and each patient gave written informed consent for the use of their specimens for medical research, conforming to the tenets of the Declaration of Helsinki.
Cell culture
AML cell lines (HL-60 and HEL) and 293FT cells were purchased from the cell library of the Chinese Academy of Sciences, and separately cultured in RPMI 1640 medium (HL-60 and HEL cells) and DMEM (293FT cells) (both HyClone; Cytiva) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The cells were maintained in an incubator at 37 °C with 5% CO2 and 95% air.
Vector construction
Cells overexpressing EIF2S3 were generated using the Retro-X Tet-Off Advanced doxycycline (DOX) inducible expression system per the manufacturer’s protocol (TaKara Biotechnology Co., Ltd.). The targeted gene (EIF2S3) was suppressed in the presence of 100 ng/ml DOX (Sigma-Aldrich; Merck KGaA). The full-length coding sequence of the EIF2S3 gene was determined using the NCBI (https://www.ncbi.nlm.nih.gov/gene/) and amplified using KOD DNA polymerase (Toyobo Life Science) with the following primers: EIF2S3 forward, 5ʹ-ATAAGAATGCGGCCGCATGGCGGGCGGAGAA-3ʹ and reverse, 5ʹ-GGAATTCTCAGTCATCATCTACTGTTGGC-3ʹ. The pRetroX-Tight-Pur vector was linearized using Not I and EcoR I and then purified on a 1% agarose gel to remove the fragment. The EIF2S3 gene fragment was cloned into the linearized vector (designated pRetroX-Tight-EIF2S3) using the Ligation high kit (Toyobo Life Science).
Transformation, transfection, and stable cell line generation
Competent DH5α cells (TransGen Biotech Co., Ltd.) were transformed with the recombinant pRetroX-Tight-EIF2S3 vector. The cells were cultured on amp-agarose plates overnight (16–24 h) at 37 °C. Colonies were then picked and cultured in ampicillin broth for an additional 16–24 h. Positive clones were confirmed by digestion with Xho I and Not I followed by sequencing. pRetroX-Tight-EIF2S3, pRetroX-Tight-Pur, pRetroX Tet-Off Advanced and the packing plasmid pCL-Ampho were co-transfected into 293FT cells using TransIntro™ EL Transfection Reagent (TransGen Biotech Co., Ltd) per the manufacturer’s protocol. At 48 h post-transfection, the culture media were clarified using a 0.45-μm filter and the viral supernatant was harvested; HL-60 and HEL cells were infected following the addition of 4 μg/ml polybrene. After 6 h, the media were replaced, and the cells were incubated for a further 24 h. Stably transfected cells were selected with puromycin (1 μg/ml for 72 h). Cultured cells were induced with DOX (100 ng/ml) for 2 days and protein expression was assessed by western blotting. Cells were induced for a further 4 days prior to use in experiments.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Mononuclear cells were isolated from BM samples using lymphocyte separation medium (TBDscience Co., Ltd.) and total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. Complementary DNA was synthesized using the PrimeScript™ RT reagent Kit (Takara Biotechnology Co., Ltd.), and qPCR was conducted using iQTM SYBR® Green Supermix with the CFX384 Real-Time System (both Bio-Rad Laboratories, Inc.). GAPDH was used as the internal control. Melting curves were detected with only one peak and corresponded to the annealing temperature. The relative expression ratio was calculated using the 2−ΔΔCq method, and the primer sequences were as follows: EIF2S3 forward, 5ʹ-ATCCCTGTCAACAGGAGGGA-3ʹ and reverse, 5ʹ-GGGGCCACATAAAAGGGAGAA-3ʹ; and GAPDH forward, 5ʹ-TGTTGCCATCAATGACCCCTT-3ʹ and reverse, 5-CTCCACGACGTACTCAGCG-3ʹ.
Western blot analysis
Total protein was extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology) and the concentration was determined by the BCA method. Then, approximately 25 μg proteins were boiled at 100 °C for 5 min and separated on 10% SDS-PAGE gels (Bio-Rad Laboratories, Inc.). The proteins were transferred to PVDF membranes (EMD Millipore), which were then blocked with 5% non-fat milk in TBST for 1 h at room temperature, and subsequently incubated overnight with antibodies against EIF2S3, ERK, p-ERK, p38, p-p38, JNK, and p-JNK (all Cell signaling Technology, Inc.). GAPDH (TransGen Biotech Co., Ltd.) was used as the loading control. The membranes were washed and then incubated with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (TransGen Biotech Co., Ltd.) for 1 h at room temperature. The protein bands were visualized with ECL solution (Beyotime Institute of Biotechnology).
Cellular proliferation assay
The Cell Counting Kit-8 (CCK-8; TransGen Biotech Co., Ltd.) was used to assess the cellular proliferation rate, according to the manufacturer’s instructions. The cells were seed into 96-well plates (1 × 103 cells/well) and assessed at the indicated timepoints (0, 24, 48, 72, 96, 120, and 144 h post-culture). The absorbance was measured at 450 nm and the proliferation rates were calculated.
Cellular invasion assay
A cell invasion assay was performed using 8-μm pore size Transwell plates (Costar; Corning, Inc.) precoated with Matrigel at a dilution 1:9 (BD Biosciences). A total of 1 × 104 cells/well in 200 μl serum-free medium were seeded into the upper chambers, while the bottom chamber was filled with 500 μl complete culture medium (10% FBS). After incubation for 24 h at 37 °C, the numbers of migratory cells in the bottom chamber were counted under a microscope.
Flow cytometric analysis
For cell cycle analysis, cells were washed twice with PBS and then fixed with cold 70% ethanol at 4 °C overnight. The fixed cells were then centrifuged and stained with propidium iodide (PI) solution (50 μg/ml; 100 μg/ml RNase A; and 0.5% Triton X-100) for 30 min at 37 °C in the dark. The stained cells were analyzed using an FACSCalibur flow cytometer (Becton, Dickinson and Company) and Flowjo software.
Mouse human tumor xenograft model
Following sodium pentobarbital anesthesia (50 mg/kg), HL-60-EIF2S3 cells (1 × 106 cells/100ul/mouse) were subcutaneously transplanted into 4–6-week-old BALB/C nude mice (Animal experimental center, Shanghai, China). The mice were divided into two groups (n = 6 each): one group was fed with standard water and the other was administered water supplemented with DOX (100 ng/ml). Tumor volumes were evaluated every day using the following formula: Tumor volume (mm3) = (length × width2)/2. The mice were euthanized by CO2 inhalation after 18 days, and the volume of the cage displaced per minute was 20%. The tumors were then removed and weighed. The study was performed according to the guidelines approved by the Animal Experimentation Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University. The experiments were approved on December 10, 2019 and performed between January 2020 and April 2020.
Next-generation sequencing (NGS)
Cells from the experimental group [HL60-EIF2S3 DOX (−)] and the control group [HL60-EIF2S3 DOX ( +)] were assessed using mRNA NGS (Sinotech Genomics). And the data were uploaded to GEO database (GSE163683). The R package Edge was used to identify differentially expressed genes (DEGs) between the cells overexpressing EIF2S3 and the control cells. The cut-off criteria were P value < 0.05 and |log2 fold change (FC) |≥ 2. Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed on the identified DEGs.
Bioinformatics analysis
RNA-seq data from patients with AML were acquired from The Cancer Genome Atlas (TCGA) database, as well as clinical information including sex, age, and FAB classification. The associations between EIF2S3 expression, clinical characteristics, and AML prognosis were evaluated using UALCAN (http://ualcan.path.uab.edu/) and OncoLnc (http://www.oncolnc.org/).
Statistical analysis
All data were analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc.) and SPSS 22.0 (IBM Corp). The experiments were repeated three times and are presented as the mean ± standard deviation (X ± SD). Differences between the means of two or more experimental groups were assessed using the Mann–Whitney test, t test, or the analysis of variance (ANOVA) test. The log-rank test was used to assess the statistical significance of the Kaplan–Meier survival plots. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. P < 0.05 was considered to indicate a statistically significant difference.
Results
TCGA bioinformatics analysis of the role of EIF2S3 in patients with AML
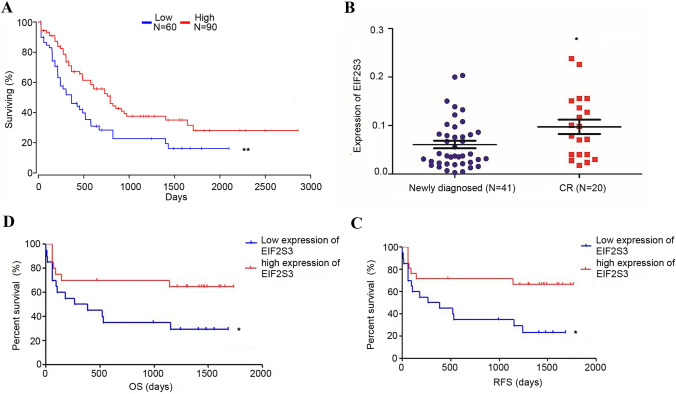
UALCAN was used to assess EIF2S3 mRNA expression levels in AML patients with different clinical characteristics. The results indicated that EIF2S3 expression may by associated with sex, age, and FAB type. (Fig. S1). However, there were no obvious differences in EIF2S3 expression between different races or genetic abnormities (FLT3 mutation, PML–RAR gene fusion, and Ras activation; Fig. S2). Furthermore, OncoLnc we used to evaluate the prognostic value of EIF2S3 in patients with AML from TCGA database. The results demonstrated that low EIF2S3 expression was significantly associated with poor prognosis (P = 0.003; Fig. 1A).
Fig. 1.
Association between EIF2S3 mRNA expression levels in patients with primary AML or CR and survival analysis. A A total of 61 samples (BM) were collected from patients with AML, and reverse transcription-quantitative PCR was used to detect EIF2S3 mRNA expression in newly diagnosed (n = 41) and CR (n = 20) patients (P = 0.02). B According to the differences in EIF2S3 expression level, patients from The Cancer Genome Atlas with the top 60% expression levels were defined as the high-expression group, and the remaining patients were classified as the low-expression group. Univariate survival analysis was used to investigate the prognostic effects of EIF2S3 in AML (P = 0.0037). C Univariate survival analysis of OS in 41 enrolled patients with primary AML. High EIF2S3 expression was detected in 21 samples (51.22%) and low EIF2S3 expression was detected in 20 samples (48.78%) (P = 0.023). D Univariate survival analysis of RFS in 41 patients with primary AML (P = 0.013). EIF2S3, eukaryotic translation initiation factor-2 subunit 3; AML acute myeloid leukemia; CR complete remission; OS overall survival; RFS relapse-free survival
Expression levels of EIF2S3 in patients with primary AML and CR
EIF2S3 mRNA expression levels were evaluated by RT-qPCR. A total of 61 AML samples (BM) were collected for experimentation. The results indicated that the expression levels of EIF2S3 in CR patients (n = 20) were higher than those in newly diagnosed patients (n = 41) (P = 0.020) (Fig. 1B). Furthermore, comparing with health controls, EIF2S3 expression was downregulated in primary AML patients (PB) (P = 0.009, Fig. S3).
Association between EIF2S3 expression and clinicopathological parameters
To further evaluate the impact of EIF2S3 in AML, the association between EIF2S3 expression and patient clinicopathological parameters was investigated. In total, 41 primary AML patient samples were divided into two groups according to the expression levels of EIF2S3 mRNA. High EIF2S3 expression was detected in 21 samples (51.22%) and low expression was detected in 20 samples (48.78%; Table 1). EIF2S3 expression was found to be correlated with patient CR (P = 0.012) and the outcome at study cut-off, whether alive or dead (P = 0.029). However, no significant association with patient age, sex, white blood cell (WBC) count, hemoglobin (HGB) level, platelet (PLT) count, blasts in the BM, FAB type, or receipt of treatment was determined (Table 1).
Table 1.
Association between EIF2S3 expression and clinicopathological parameters in 41 primary AML patients
| Clinicopathological variables | Cases (41) | EIF2S3 expression value | P value | |
|---|---|---|---|---|
| low (20) | High (21) | |||
| Age (years) | ||||
| ≤ 60 | 31 | 15 | 16 | 1.000 |
| > 60 | 10 | 5 | 5 | |
| Gender | ||||
| Male | 18 | 8 | 10 | 0.756 |
| Female | 23 | 12 | 11 | |
| WBCa) (× 109/L) | ||||
| < 10 | 19 | 8 | 11 | 0.536 |
| ≥ 10 | 22 | 13 | 9 | |
| HGBb) (g/L) | ||||
| < 80 | 21 | 13 | 8 | 0.121 |
| ≥ 80 | 20 | 7 | 13 | |
| PLTc) (× 109/L) | ||||
| < 50 | 22 | 9 | 13 | 0.354 |
| ≥ 50 | 19 | 11 | 8 | |
| Blast in BMd) | ||||
| < 50% | 14 | 6 | 8 | 0.737 |
| ≥ 50% | 24 | 13 | 11 | |
| FAB type | ||||
|
M0 M1 M2 M3 M4 M5 M6 |
1 3 12 6 7 7 1 |
1 2 6 1 2 6 0 |
0 1 6 5 5 1 1 |
0.132 |
| Complete Remissionf) | ||||
| Yes | 19 | 5 | 14 | 0.012 |
| No | 22 | 15 | 7 | |
| Treatment | ||||
| Chemotherapy | 28 | 13 | 15 | 0.172 |
| Transplantation | 10 | 4 | 6 | |
| None | 3 | 3 | 0 | |
| Outcome at study cut-off | 0.029 | |||
| Alive | 20 | 6 | 14 | |
| Dead | 21 | 14 | 7 | |
*P < 0.05, the difference was statistically significant
a) WBC white blood cell, b) HGB hemoglobin, c) PLT Platelets, d) Blast in BM blast percentage in bone marrow, f) CR complete remission
Association between EIF2S3 expression and prognosis in AML
Kaplan–Meier survival analysis demonstrated that high-EIF2S3 expression resulted in increased overall survival (OS; P = 0.023) and relapse-free survival (RFS; P = 0.013) times (Fig. 1C, D). Furthermore, multivariate Cox-regression analysis illustrated that EIF2S3 was an independent prognostic factor for OS (P = 0.013) and RFS (P = 0.008) (Table 2). In addition, univariate survival analysis indicated that age (P = 0.021) and treatment (P = 0.030) were also associated with RFS in patients with AML (Table2).
Table 2.
Cox-regression analysis of parameters associated with OS and RFS of AML patients
| Factors | OSa) | RFSb) | ||||||
|---|---|---|---|---|---|---|---|---|
| U variate | Multivariate | U variate | Multivariate | |||||
| P | HRc) | 95% CI | P | P | HR | 95% CI | P | |
| Age, years (≤ 60/ > 60) | 0.059 | 2.921 | 1.134–7.526 | 0.026 | 0.021 | 3.260 | 1.302–8.160 | 0.012 |
| Gender(male/female) | 0.743 | – | – | – | 0.887 | – | – | – |
| WBCd) (< 10/ ≥ 1 × 109/L) | 0.862 | – | – | – | 0.994 | – | – | – |
| HGBe) (< 80/ ≥ 80, g/L) | 0.142 | – | – | – | 0.200 | – | – | – |
| PLTf) (< 50/ ≥ 50, × 109/L) | 0.198 | – | – | – | 0.123 | – | – | – |
| Blast in BMg) (< 50%/ ≥ 50%) | 0.400 | – | – | – | 0.483 | – | – | – |
| EIF2S3 expression (high/low) | 0.023 | 0.299 | 0.115–0.772 | 0.013 | 0.011 | 0.277 | 0.108–0.716 | 0.008 |
| Treatment(chemotherapy/transplantation/none) | 0.132 | 0.618 | 0.299–1.281 | 0.196 | 0.030 | 0.717 | 0.370–1.387 | 1.387 |
*P < 0.05, the difference was statistically significant
a) OS overall survival, b) RFS relapse-free survival, c) HR hazard ratio, d) WBC white blood cell, e) HGB hemoglobin, f) PLT Platelets, g) Blast in BM blast percentage in bone marrow
EIF2S3 overexpression suppresses the proliferation of HEL and HL-60 cells
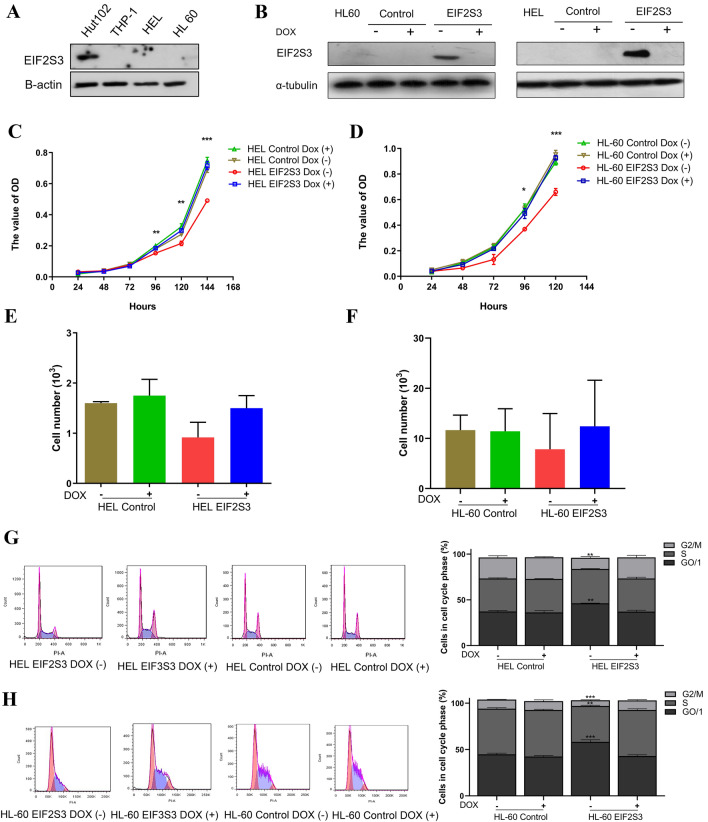
Western blotting was used to determine the EIF2S3 protein levels in AML and acute lymphocytic leukemia (ALL) cell lines, including THP-1, HEL, HL-60, and Hut102 cells (Fig. 2A). HEL and HL-60 cells overexpressing EIF2S3 were generated by inducible retrovirus infection. Empty vector-transfected cells were used as the control. Successful generation of HEL-EIF2S3, HEL control, HL-60-EIF2S3, and HL-60 control cells was confirmed by western blotting (Fig. 2B). In the overexpressing cells, EIF2S3 expression was suppressed in the presence of DOX. CCK-8 assays were performed to detect cellular proliferation, which revealed that upregulated EIF2S3 expression in HEL and HL-60 cells markedly suppressed proliferation rate compared with the control cells (Fig. 2C, D). The effects of the EIF2S3 gene on invasiveness were also evaluated, but no significant differences were observed between DOX (−) and DOX ( +) cells (Fig. 2E, F).
Fig. 2.
Overexpression of EIF2S3 suppresses cellular proliferation and G1/S transition in HL-60 and HEL cells. A Western blot analysis of EIF2S3 protein levels in AML (THP-1, HEL, and HL-60) and ALL (Hut102) cell lines. B Western blotting was used to confirm the successful overexpression of EIF2S3 in AML and the control cells. C Proliferation of HEL-EIF2S3 and HEL control cells was assessed at 24, 48, 72, 96 (P = 0.001), 120 (P = 0.001), and 144 h (P < 0.001). D Proliferation of HL-60-EIF2S3 and HL-60 control cells was evaluated at 24, 48, 72, 96 (P = 0.006), and 120 h (P < 0.001). E and F Cellular invasion analysis showed no significant differences between DOX (−) and DOX ( +) cells (P > 0.05). G and H Flow cytometry was used to investigate the cell cycle distribution of HEL-EIF2S3, HEL control, HL-60-EIF2S3 and HL-60 control cells. Compared with HEL-EIF2S3 DOX ( +) and control cells, HEL-EIF2S3 DOX (−) cells in the G0/1 phase (P = 0.001) were increased, and decreased in the G2/M phase (P = 0.002). Compared with HL-60-EIF2S3 DOX ( +) and the control cells, HL-60-EIF2S3 DOX (−) cells in the G0/1 (P < 0.001) and S phase (P = 0.003) were increased, and those in the G2/M phase (P < 0.001) were decreased. Results of three independent experiments are shown. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001. EIF2S3 eukaryotic translation initiation factor-2 subunit 3; AML acute myeloid leukemia; ALL acute lymphocytic leukemia; DOX doxycycline
EIF2S3 regulates the cell cycle in HEL and HL-60 cells
To further confirm the function of EIF2S3-mediated proliferation, flow cytometry was used to evaluate the effects of EIF2S3 overexpression on the cell cycle. The ratio of HEL-EIF2S3 DOX (−) to HL-60-EIF2S3 DOX (−) cells in the G0/1 phase was significantly increased compared with the DOX ( +) and control cells (Fig. 2G, H). By contrast, the number of HEL-EIF2S3 DOX (−) cells in the G2/M phase was decreased (Fig. 2G), as was the number of HL-60-EIF2S3 DOX (−) cells in the S and G2/M phases (Fig. 2H). There was no significant difference between the control and DOX ( +) cells. These results indicated that upregulated EIF2S3 expression resulted in G0/1 cell cycle arrest and inhibited G1/S transition in AML cells.
Upregulation of EIF2S3 inhibits the tumorigenicity of AML in vivo
Considering that EIF2S3 overexpression inhibited AML cell proliferation in vitro, its influence on tumor formation was subsequently investigated in vivo. Due to ethics considerations and the results of the aforementioned assays (showing that DOX did not influence the experimental results), 12 mice were then divided into two groups inoculated with HL-60 EIF2S3 cells. A group of mice were fed with pure water and the other group mice were received water supplemented with DOX (100 ng/ml) as the control. Consistent with the proliferation assay results, tumors with excessive EIF2S3 expression grew significantly slower than those of the control animals 1 week after xenografting (P = 0.004; Fig. 3A). After a further 18 days, the tumors were removed and the tumor weight and size recorded (Fig. 3B). The mean tumor weight and size were lower in mice with EIF2S3 overexpression compared with the control mice (P = 0.015; Fig. 3C). Therefore, the results suggested that EIF2S3 may play an important role in the inhibition of acute leukemia cell growth in vivo.
Fig. 3.
Upregulation of EIF2S3 expression inhibits the tumorigenicity of AML in model mice. A Tumors were removed from mice after inoculation for 18 days. B Growth curve (P = 0.004) and C weight (P = 0.015) of xenografted tumors showed that EIF2S3 overexpression decreased tumor growth and size. EIF2S3 eukaryotic translation initiation factor-2 subunit 3; AML acute myeloid leukemia; DOX doxycycline
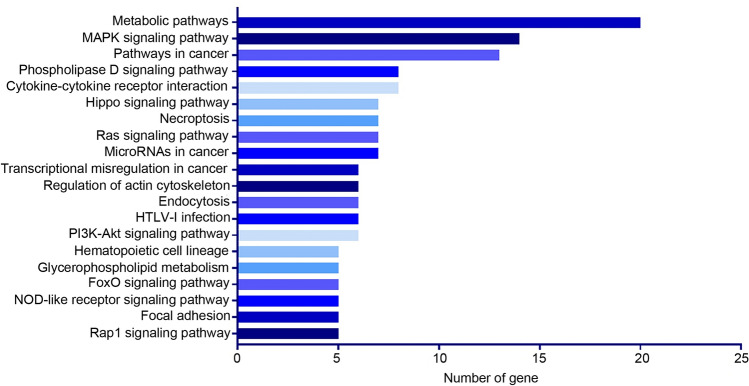
Identification of DEGs, GO functional analysis, and KEGG pathway enrichment
To investigate the mechanisms underlying the proliferative inhibition of HEL-EIF2S3 and HL-60-EIF2S3 cells, mRNA NGS was conducted using HL60-EIF2S3 DOX (−) and HL60-EIF2S3 DOX ( +) cells (GSE163683). In total, 479 DEGs (including 271 upregulated and 208 downregulated genes) were identified from NGS, using P < 0.05 and |log2FC|≥ 2 as the cut-off criteria. A volcano plot of DEGs and heatmap of the top 30 DEGs are presented in Fig. S4 A and B. GO functional analysis highlights relevant biological processes associated with DEGs, which showed that the identified DEGs were primarily involved in ‘leukocyte homeostasis’, ‘leukocyte apoptotic process’, ‘cell cycle arrest’, ‘regulation of transcription factor imported into nucleus’, ‘regulation of protein processing’, and ‘regulation of response to DNA damage stimulus’ (Fig. S5). KEGG pathway enrichment analysis identified the relevant signaling pathways associated with these DEGs. In order of the number of DEGs involved in the pathway, the results revealed that the second most enriched pathway was the MAPK signaling pathway, which is a common tumor signaling pathway (Fig. 4). After filtering the pathways (Fig. 4), the DEGs involved in five common cell signaling pathways (including those of MAPK, Ras, PI3K-Akt, FoxO, and Rap1) were further investigated (heatmap, Fig. 5). Furthermore, the top 30 enriched pathways also revealed that the DEGs were primarily enriched in the MAPK pathway (based on the rich factor; Fig. S6). Combining the number of DEGs involved in the pathways and the rich factor, these results suggested that the function of EIF2S3 may be associated with MAPK signaling. The expression of 15 DEGs in the MAPK signaling pathway was then confirmed by RT-qPCR, and the expression of 11 genes was markedly different between EIF2S3 overexpressing cells and the controls, which verified the reliability of the sequencing results (Fig. S7).
Fig. 4.
Identification of KEGG-enriched pathways in HL60-EIF2S3 DOX (−) and HL60-EIF2S3 DOX ( +) cells by next-generation sequencing. KEGG pathway enrichment analysis described the relevant signaling pathways in order of number of DEGs. KEGG Kyoto Encyclopedia of Genes and Genomes; DEG differentially expressed genes; DOX doxycycline
Fig. 5.
Analysis of five cell signaling pathways based on the results of KEGG analysis. A A total of five cell signaling pathways were chosen from Fig. 4 to further investigate the involved DEGs. B Heatmap of 20 DEGs; the closer the color to the ends of the color column, the greater the difference. KEGG Kyoto Encyclopedia of Genes and Genomes; DEG differentially expressed gene; DOX doxycycline
Relevant proteins and their phosphorylation levels in the MAPK signaling pathway
The results of NGS revealed that EIF2S3 may inhibit AML cell proliferation and G1/S transition via the MAPK signaling pathway. Western blotting was used to detect relevant pathway proteins and their phosphorylation levels in AML cells, including ERK, p-ERK, P38, p-P38, JNK1/2/3, and p-JNK1/2/3. The results showed that the levels of p-ERK were notably decreased in HEL-EIF2S3 DOX (−) and HL-60-EIF2S3 DOX (−) cells compared with their DOX ( +) counterparts (Fig. 6; P < 0.001). Collectively, the results suggested that differences in EIF2S3 expression altered the levels of ERK phosphorylation in AML cells, the core protein in the MAPK/ERK signaling pathway.
Fig. 6.
Relevant proteins of the MAPK signaling pathway and their phosphorylation levels in HEL-EIF2S3 and HL-60-EIF2S3 cells. A Western blot analysis of the relevant proteins and their phosphorylation levels in the MAPK signaling pathway. B Grayscale analysis of relative protein expression. Data are presented as the mean ± SEM. ***P < 0.001. DOX doxycycline
Discussion
AML is a common hematological malignancy with high incidence and mortality rates (Siegel et al. 2020). With developments in cytogenetics and molecular biology, chromosomal translocations in AML have been revealed, including t(8;21) (q22;q22), inv(16)(p13q22b), and t(16;16)(p13;q22). Gene fusions have also been discovered (such as BCR-ABL1, MLLT1-ETO, and CBFβ-MYH11), as well as mutations in the NPM1, NRAS, and RUNX1. These alterations were significantly correlated with clinical prognosis (Grimwade et al. 2010). As such, various novel molecular-targeted therapies have emerged, improving on traditional treatments such as chemotherapy and hematopoietic stem cell transplantation.
With its GTP-binding domain, EIF2S3 is one of the core subunits of EIF2, and plays an important role in eukaryotic translation initiation (Hinnebusch 2011, 2014; Hinnebusch and Lorsch 2012; Young-Baird et al. 2019). However, relevant studies surrounding the function and mechanism of EIF2S3 in AML are limited. In the present study, the results demonstrated that EIF2S3 expression levels were higher in patients with CR than in those with primary AML (BM). And comparing with health controls, EIF2S3 expression was downregulated in primary AML patients (PB). Survival analyses form database and enrolled patients both showed that high-EIF2S3 expression suggested improved prognosis. And EIF2S3 was an independent prognostic factor for OS and RFS.
Subsequently, the function and mechanism of EIF2S3 in AML were investigated using in vitro and in vivo experimentation. In vitro, EIF2S3 overexpression suppressed the proliferation rates and inhibited G0/1 to S phase transition in AML cells. Moreover, EIF2S3 inhibited the tumorigenicity of HL-60 cells in mice models. To investigate the mechanisms of EIF2S3 in AML, mRNA NGS with HL60-EIF2S3 DOX (−) and HL60-EIF2S3 DOX ( +) cells highlighted 479 DEGs (including 271 upregulated and 208 downregulated genes). GO functional analysis showed that these DEGs were primarily involved in the biological functions ‘‘leukocyte homeostasis’, ‘leukocyte apoptotic process’, ‘cell cycle arrest’, ‘regulation of transcription factor imported into nucleus’, ‘regulation of protein processing’, and ‘regulation of response to DNA damage stimulus’, suggesting that EIF3S3 may affects AML via these processes (Fig. S5). Among above biological processes, our results have proved that EIF2S3 overexpression resulted in G0/1 cell cycle arrest. Besides, cell apoptosis and DNA damage played vital roles in the pathogenesis of many diseases. Cancers was one of the situations where too little apoptosis occurs, leading to malignant cells will not die (Wong 2011). DNA damage can drive genomic instability and ultimately the disease process. Recent researches emphasized increased DNA damage and abnormalities in the DNA damage response as a critical characteristic of AML cells (Lord and Ashworth 2012; Dávila-Rodríguez et al. 2017; Pennisi et al. 2018). Further functional experiments will be carried out to explore the relationship between EIF2S3 and apoptosis and DNA damage in AML.
In order of the number of DEGs involved in the signaling pathway, KEGG pathway enrichment analysis revealed that the second most enriched pathway was the MAPK signaling pathway, which is commonly associated with tumor formation. This indicates that the function of EIF2S3 may be associated with the MAPK signaling pathway. In addition, western blot analysis revealed that the protein levels of p-ERK were markedly decreased in HEL-EIF2S3 DOX (−) and HL-60-EIF2S3 DOX (−) cells compared with the associated controls. Hence, the differences in EIF2S3 expression in AML cells were found to alter the phosphorylation levels of ERK, which is one of the fundamental proteins in the MAPK/ERK signaling pathway.
Eukaryotic cells receive external signals which are then transmitted to critical targets within the cell to achieve the appropriate biological response. These signal transduction processes are often initiated by receptor tyrosine kinases (RTKs); an essential effector cascade required for most RTK functions is the MAPK cascade, comprised of the Raf, MEK, and ERK kinases. The ERK1/2 signaling pathway has been widely researched (Lavoie and Therrien 2015; Guo et al. 2020). Previous studies have demonstrated that ERK1/2 signaling is closely associated with cellular proliferation, the cell cycle, and apoptosis (Saez-Rodriguez et al. 2015). ERK activation induces the cell cycle and inhibits negative regulators of the cell cycle, which is necessary for G1/S transition (Yamamoto et al. 2006). ERK activation also allowed successful G1 phase progression by inducing the assembly of cyclin D and the cyclin D-CDK4 complex, mediating Myc protein stability, regulating the expression of p21 and p27, and downregulating the expression of antiproliferative genes (Meloche and Pouyssegur 2007; Vasjari et al. 2019). The MAPK/ERK pathway was associated with various cancers, including colorectal cancer, ovarian cancer, melanoma, non-small cell lung cancer, and so on. In leukemia, the MAPK signaling pathway (including the ERK1/2 pathway) was found to be abnormally activated in progenitor cells and suppressing ERK activity inhibited the proliferation of primary acute myeloid leukemia cells and induced apoptosis (Lee and McCubrey 2002; Su et al. 2018). Our result demonstrated that EIF2S3 overexpression suppressed the ERK activation, suggesting that EIF2S3 may medicated the MAPK/ERK pathway in the proliferation and cell cycle in AML.
To the best of our knowledge, the present study is the first to investigate the function and mechanism of EIF2S3 in leukemia; therefore, some deficiencies and limitations are worth reporting. First, according to the 2016 WHO classification, AML is classified by morphology, genetic abnormalities, and chromosome translocation. However, genetic and chromosomal data from the enrolled patients were incomplete. Therefore, the association between EIF2S3 and genetic or chromosomal abnormalities could not be accurately analyzed, which may have affected the conclusions. Second, a limited number of patients were enrolled in the present study. Therefore, more samples and clinical data must be collected in the future to confirm the findings of the present study.
In conclusion, EIF2S3 expression may inhibit the cell cycle, cellular proliferation, and tumorigenicity in AML by inhibiting the activation of the MAPK/ERK signaling pathway, suggesting that EIF2S3 may be a critical biomarker and therapeutic target of AML.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Fig. S1 EIF2S3 mRNA expression level in AML patients of TCGA based on classification of clinical characteristics. A The difference of EIF2S3 mRNA expression based on FBA types. EIF2S3 expression in M3 was lower than M0 (P=0.019), M1 (P=0.001), M2 (P=0.004) and M4 (P=0.022) patients. EIF2S3 expression in M5 patients was lower than M0 (P=0.012), M1 (P<0.001), M2 (P<0.001) and M4 (P=0.003) patients. There were no significant differences between other types. B The difference of EIF2S3 mRNA expression based on gender (P=0.015). C The difference of EIF2S3 mRNA expression based on age. EIF2S3 expression in 21-40 years-old patient was higher than 81-100 years-old patients (P=0.048). There were no significant differences in other age groups. D The difference of EIF2S3 mRNA expression based on races (P>0.05) (TIF 634 KB)
Supplementary file2 Fig. S2 EIF2S3 mRNA expression level in AML patients of TCGA based on classification of genetic abnormality. A The difference of EIF2S3 mRNA expression based on FLT3 mutation or not (P>0.05). B The difference of EIF2S3 mRNA expression based on PML/RAR fusion or not (P>0.05). C The difference of EIF2S3 mRNA expression based on RAS activation or not (P>0.05) (TIF 14657 KB)
Supplementary file3 Fig. S3 EIF2S3 mRNA expression levels in patients with primary AML (PB) and healthy controls. Comparing with health controls, EIF2S3 expression was downregulated in primary AML patients (PB) (P =0.009). PB, peripheral blood (TIF 81 KB)
Supplementary file4 Fig. S4 Identify the DEGs in HL60-EIF2S3 DOX (-) and HL60-EIF2S3 DOX (+) by NGS. A Using P<0.05 and |log2FC|≥2 as cut-0ff criteria, the volcano plot of DEGs showed there were totally 479 DEGs, including 271 upregulated genes (red dots) and 208 downregulated genes (blue dots). B In the hot plot of top 30 DEGs, red represents upregulation and blue represent downregulation. The closer the color was to the ends of the color column, the greater the difference (TIF 9749 KB)
Supplementary file5 Fig. S5 GO enrichment analysis in HL60-EIF2S3 DOX (-) and HL60-EIF2S3 DOX (+). The top 30 of GO enrichment described relevant biological processes of DEGs. The color of the dots represents the significance of q value, and the size of the dots represents the number of DEGs involved in the GO domain (TIF 24273 KB)
Supplementary file6 Fig. S6 KEGG enrichment analysis in HL60-EIF2S3 DOX (-) and HL60-EIF2S3 DOX (+). The top 30 of pathway enrichment showed that DEGs primarily enriched in MAPK pathway. The color of the dots represents the significance of q value, and the size of the dots represents the number of DEGs involved in the different pathways (TIF 11516 KB)
Supplementary file7 Fig. S7 RT-qPCR showed the relative mRNA expression level of 13 DEGs in MAPK signaling pathway. *P < 0.05, **P < 0.01, and ***P < 0.001 (TIF 473 KB)
Author contributions
LX, YZ, and ZH conceived the study idea, supervised the project and designed the experiments; JL and SC performed the experiments and wrote the manuscript; LL, HT, BL, YC, and XZ collected the samples, performed the experiments, and analyzed the data.
Funding
The present study was supported by the National Natural Science Foundation of China (Grant no. 81672661, 81870113) and the Guangzhou Science and Technology Project (grant no. 201804010199).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The present study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University. All patients provided written informed consent for the use of their clinical specimens. Animals were purchased from the Animal experimental center, Shanghai, China, and the protocol was approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University. All mouse procedures were performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Guangzhou Medical University. Animal experiments were performed at Guangzhou Medical University and all surgeries were performed under sodium pentobarbital anesthesia; all efforts were made to minimize animal suffering.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jielun Lu and Shuyi Chen have contributed equally to this work.
Contributor Information
Yawei Zou, Email: zouyawei@gzhmu.edu.cn.
Lihua Xu, Email: xlhua@gzhmu.edu.cn.
References
- Arber DA, Orazi A, Hasserjian R et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405. 10.1182/blood-2016-08-733196 [DOI] [PubMed] [Google Scholar]
- Borck G, Shin BS, Stiller B et al (2012) eIF2gamma mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol Cell 48(4):641–646. 10.1016/j.molcel.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose P, Vachhani P, Cortes JE (2017) Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol 18(3):17. 10.1007/s11864-017-0456-2 [DOI] [PubMed] [Google Scholar]
- Chang YT, Huang CS, Yao CT et al (2014) Gene expression profile of peripheral blood in colorectal cancer. World J Gastroenterol 20(39):14463–14471. 10.3748/wjg.v20.i39.14463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- Chian CF, Hwang YT, Terng HJ et al (2016) Panels of tumor-derived RNA markers in peripheral blood of patients with non-small cell lung cancer: their dependence on age, gender and clinical stages. Oncotarget 7(31):50582–50595. 10.18632/oncotarget.10558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs CC, Tallman MS, Levine RL (2016) Molecular therapy for acute myeloid leukaemia. Nat Rev Clin Oncol 13(5):305–318. 10.1038/nrclinonc.2015.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila-Rodríguez MI, Cortés-Gutiérrez EI, Hernández-Valdés R, Guzmán-Cortés K, De León-Cantú RE, Cerda-Flores RM, Báez-De la Fuente E (2017) DNA damage in acute myeloid leukemia patients of Northern Mexico. Eur J Histochem 61(4):2851. 10.4081/ejh.2017.2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner H, Estey EH, Amadori S et al (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474. 10.1182/blood-2009-07-235358 [DOI] [PubMed] [Google Scholar]
- Dohner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwade D, Hills RK, Moorman AV et al (2010) Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116(3):354–365. 10.1182/blood-2009-11-254441 [DOI] [PubMed] [Google Scholar]
- Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL (2020) ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 19(3):1997–2007. 10.3892/etm.2020.8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG (2011) Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev 75(3):434–467. 10.1128/MMBR.00008-11 (first page of table of contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG (2014) The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem 83:779–812. 10.1146/annurev-biochem-060713-035802 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG, Lorsch JR (2012) The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a011544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H, Therrien M (2015) Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol 16(5):281–298. 10.1038/nrm3979 [DOI] [PubMed] [Google Scholar]
- Lee JT Jr, McCubrey JA (2002) The Raf/MEK/ERK signal transduction cascade as a target for chemotherapeutic intervention in leukemia. Leukemia 16(4):486–507. 10.1038/sj.leu.2402460 [DOI] [PubMed] [Google Scholar]
- Lord CJ, Ashworth A (2012) The DNA damage response and cancer therapy. Nature 481(7381):287–294. 10.1038/nature10760 [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J (2007) The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26(22):3227–3239. 10.1038/sj.onc.1210414 [DOI] [PubMed] [Google Scholar]
- Moortgat S, Desir J, Benoit V et al (2016) Two novel EIF2S3 mutations associated with syndromic intellectual disability with severe microcephaly, growth retardation, and epilepsy. Am J Med Genet A 170(11):2927–2933. 10.1002/ajmg.a.37792 [DOI] [PubMed] [Google Scholar]
- Narayanan D, Weinberg OK (2020) How I investigate acute myeloid leukemia. Int J Lab Hematol 42(1):3–15. 10.1111/ijlh.13135 [DOI] [PubMed] [Google Scholar]
- Pennisi R, Albanesi J, Ascenzi P, Nervi C, di Masi A (2018) Are DNA damage response kinases a target for the differentiation treatment of acute myeloid leukemia? IUBMB Life 70(11):1057–1066. 10.1002/iub.1918 [DOI] [PubMed] [Google Scholar]
- Saez-Rodriguez J, MacNamara A, Cook S (2015) Modeling signaling networks to advance new cancer therapies. Annu Rev Biomed Eng 17:143–163. 10.1146/annurev-bioeng-071813-104927 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Skopkova M, Hennig F, Shin BS et al (2017) EIF2S3 mutations associated with severe X-linked intellectual disability syndrome MEHMO. Hum Mutat 38(4):409–425. 10.1002/humu.23170 (Epub 2017 Jan 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanik J, Skopkova M, Stanikova D et al (2018) Neonatal hypoglycemia, early-onset diabetes and hypopituitarism due to the mutation in EIF2S3 gene causing MEHMO syndrome. Physiol Res 67(2):331–337. 10.33549/physiolres.933689 (Epub 2018 Jan 5) [DOI] [PubMed] [Google Scholar]
- Su Y, Li X, Ma J et al (2018) Targeting PI3K, mTOR, ERK, and Bcl-2 signaling network shows superior antileukemic activity against AML ex vivo. Biochem Pharmacol 148:13–26. 10.1016/j.bcp.2017.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasjari L, Bresan S, Biskup C, Pai G, Rubio I (2019) Ras signals principally via Erk in G1 but cooperates with PI3K/Akt for Cyclin D induction and S-phase entry. Cell Cycle 18(2):204–225. 10.1080/15384101.2018.1560205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30(1):87. 10.1186/1756-9966-30-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ebisuya M, Ashida F, Okamoto K, Yonehara S, Nishida E (2006) Continuous ERK activation downregulates antiproliferative genes throughout G1 phase to allow cell-cycle progression. Curr Biol 16(12):1171–1182. 10.1016/j.cub.2006.04.044 [DOI] [PubMed] [Google Scholar]
- Young-Baird SK, Shin BS, Dever TE (2019) MEHMO syndrome mutation EIF2S3-I259M impairs initiator Met-tRNAiMet binding to eukaryotic translation initiation factor eIF2. Nucleic Acids Res 47(2):855–867. 10.1093/nar/gky1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Fig. S1 EIF2S3 mRNA expression level in AML patients of TCGA based on classification of clinical characteristics. A The difference of EIF2S3 mRNA expression based on FBA types. EIF2S3 expression in M3 was lower than M0 (P=0.019), M1 (P=0.001), M2 (P=0.004) and M4 (P=0.022) patients. EIF2S3 expression in M5 patients was lower than M0 (P=0.012), M1 (P<0.001), M2 (P<0.001) and M4 (P=0.003) patients. There were no significant differences between other types. B The difference of EIF2S3 mRNA expression based on gender (P=0.015). C The difference of EIF2S3 mRNA expression based on age. EIF2S3 expression in 21-40 years-old patient was higher than 81-100 years-old patients (P=0.048). There were no significant differences in other age groups. D The difference of EIF2S3 mRNA expression based on races (P>0.05) (TIF 634 KB)
Supplementary file2 Fig. S2 EIF2S3 mRNA expression level in AML patients of TCGA based on classification of genetic abnormality. A The difference of EIF2S3 mRNA expression based on FLT3 mutation or not (P>0.05). B The difference of EIF2S3 mRNA expression based on PML/RAR fusion or not (P>0.05). C The difference of EIF2S3 mRNA expression based on RAS activation or not (P>0.05) (TIF 14657 KB)
Supplementary file3 Fig. S3 EIF2S3 mRNA expression levels in patients with primary AML (PB) and healthy controls. Comparing with health controls, EIF2S3 expression was downregulated in primary AML patients (PB) (P =0.009). PB, peripheral blood (TIF 81 KB)
Supplementary file4 Fig. S4 Identify the DEGs in HL60-EIF2S3 DOX (-) and HL60-EIF2S3 DOX (+) by NGS. A Using P<0.05 and |log2FC|≥2 as cut-0ff criteria, the volcano plot of DEGs showed there were totally 479 DEGs, including 271 upregulated genes (red dots) and 208 downregulated genes (blue dots). B In the hot plot of top 30 DEGs, red represents upregulation and blue represent downregulation. The closer the color was to the ends of the color column, the greater the difference (TIF 9749 KB)
Supplementary file5 Fig. S5 GO enrichment analysis in HL60-EIF2S3 DOX (-) and HL60-EIF2S3 DOX (+). The top 30 of GO enrichment described relevant biological processes of DEGs. The color of the dots represents the significance of q value, and the size of the dots represents the number of DEGs involved in the GO domain (TIF 24273 KB)
Supplementary file6 Fig. S6 KEGG enrichment analysis in HL60-EIF2S3 DOX (-) and HL60-EIF2S3 DOX (+). The top 30 of pathway enrichment showed that DEGs primarily enriched in MAPK pathway. The color of the dots represents the significance of q value, and the size of the dots represents the number of DEGs involved in the different pathways (TIF 11516 KB)
Supplementary file7 Fig. S7 RT-qPCR showed the relative mRNA expression level of 13 DEGs in MAPK signaling pathway. *P < 0.05, **P < 0.01, and ***P < 0.001 (TIF 473 KB)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.