Abstract
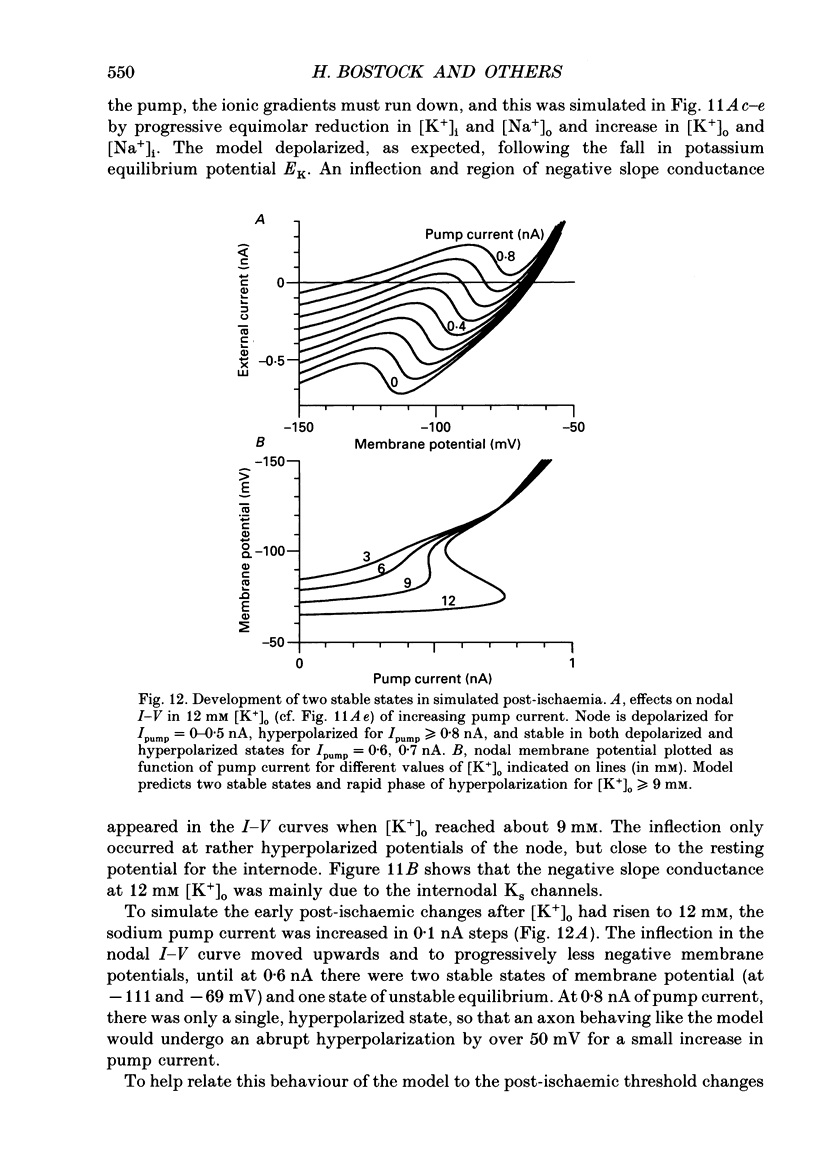
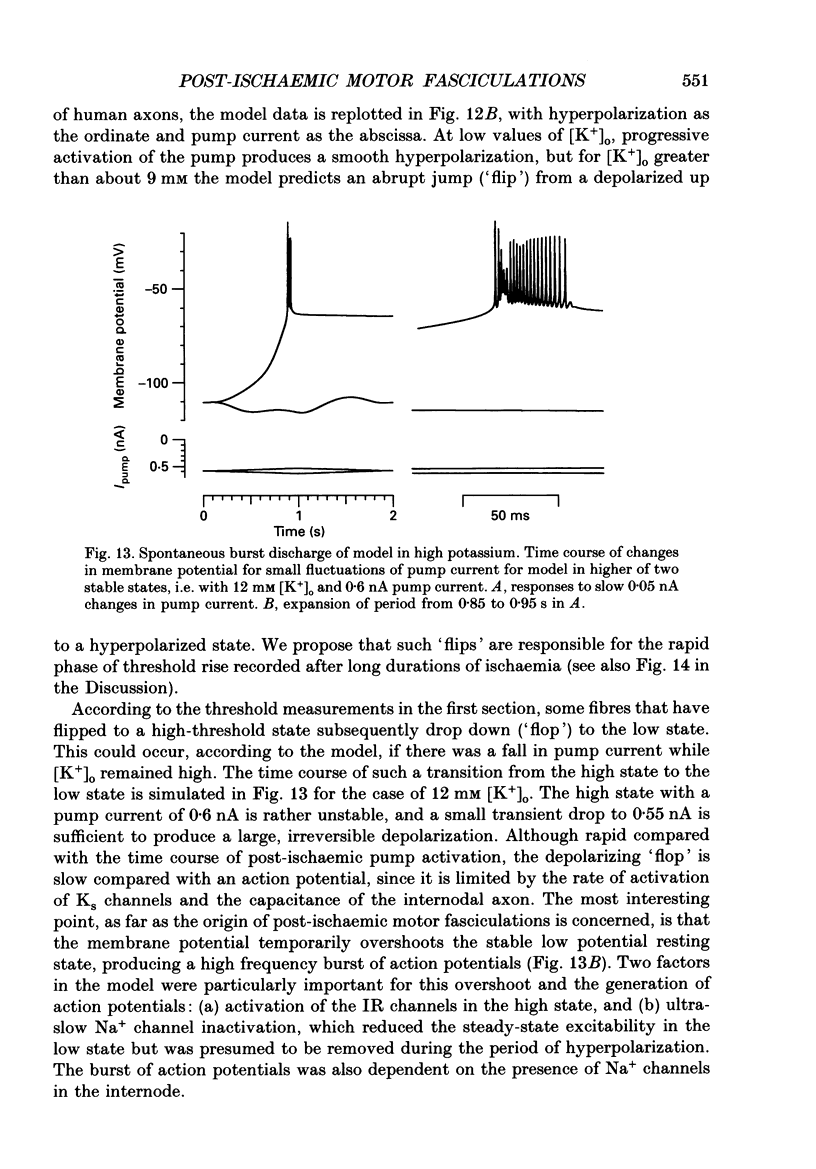
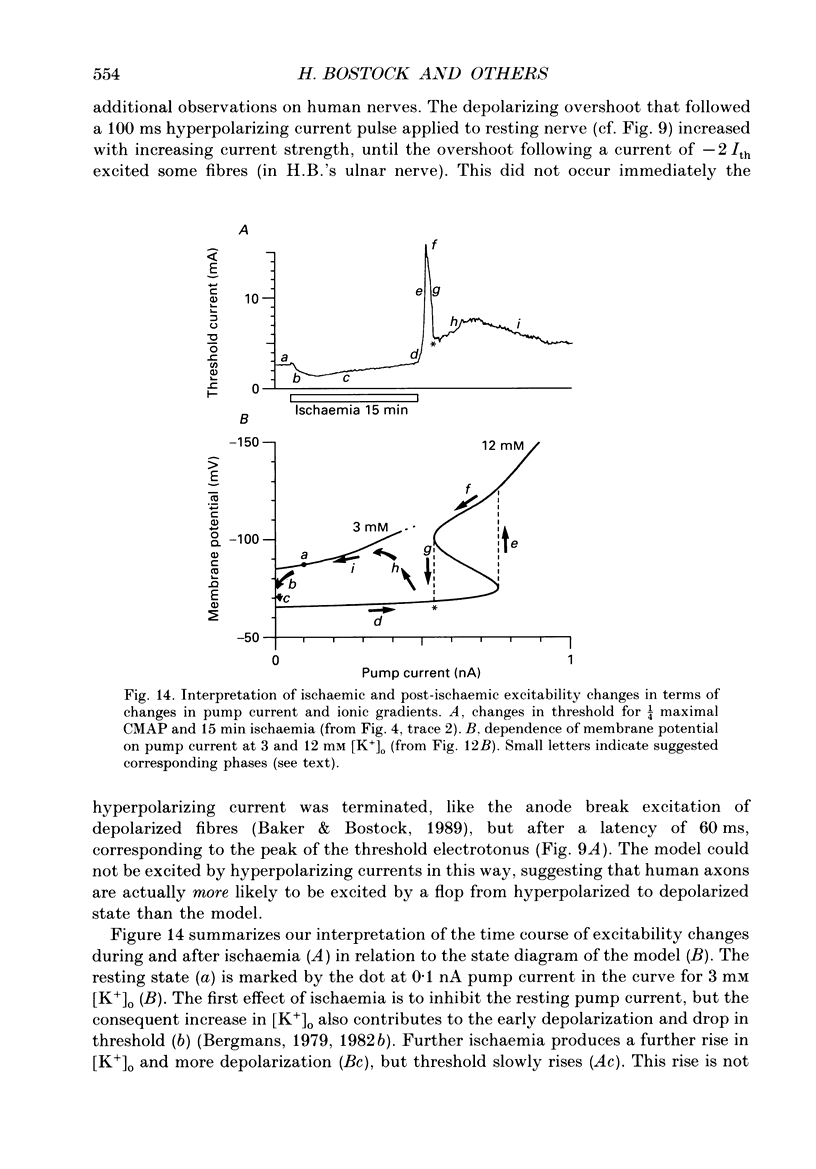
1. We have investigated the origin of post-ischaemic ectopic discharges in human nerve by recording changes in electrical excitability following periods of ischaemia (15-20 min) sufficient to induce spontaneous motor fasciculations. The ulnar nerve was stimulated beneath a pressure cuff on the upper arm, and compound motor action potentials recorded from abductor digiti minimi. 2. On releasing the cuff after 15 min of ischaemia, thresholds to short current pulses increased in two distinct phases: a slow phase followed by a rapid rise to a peak threshold. The rapid rise was too fast to track (i.e. 100% threshold increase in less than 4 s), and was sometimes followed after 30-40 s by an equally rapid fall. Small polarizing currents affected the timing of the rapid threshold increase, as if it was occurring at a particular membrane potential. 3. By recording complete stimulus-response curves every few seconds, we found that the rapid threshold changes were associated with a bimodal distribution of thresholds. Most fibres were found in either a high-threshold or low-threshold state, and these two states converged over a period of about 10 min. 4. Spontaneous motor fasciculations were only recorded after the rapid rise in threshold and when the fibres existed in two threshold states. The spontaneous activity was not responsible for inducing the two states, since they could also be recorded in its absence. 5. A computer model of a human motor axon node and internode was constructed, incorporating channel types demonstrated in other axons, and channel densities adjusted to match the responses of human axons to depolarizing and hyperpolarizing current pulses. An increase in extracellular potassium concentration produced a region of negative slope conductance in the current-voltage relationship of the model, and the appearance of two stable states with enhanced activity of the electrogenic sodium pump. 6. Transitions between the two stable states of the model could account qualitatively for the rapid threshold changes recorded from post-ischaemic axons. In the model, spontaneous action potentials occurred following some transitions from the high potential state to the low potential state. We suggest that post-ischaemic motor fasciculations in man also involve transitions between two equilibrium states, occurring in axons with high extracellular potassium and high electrogenic pump activity.
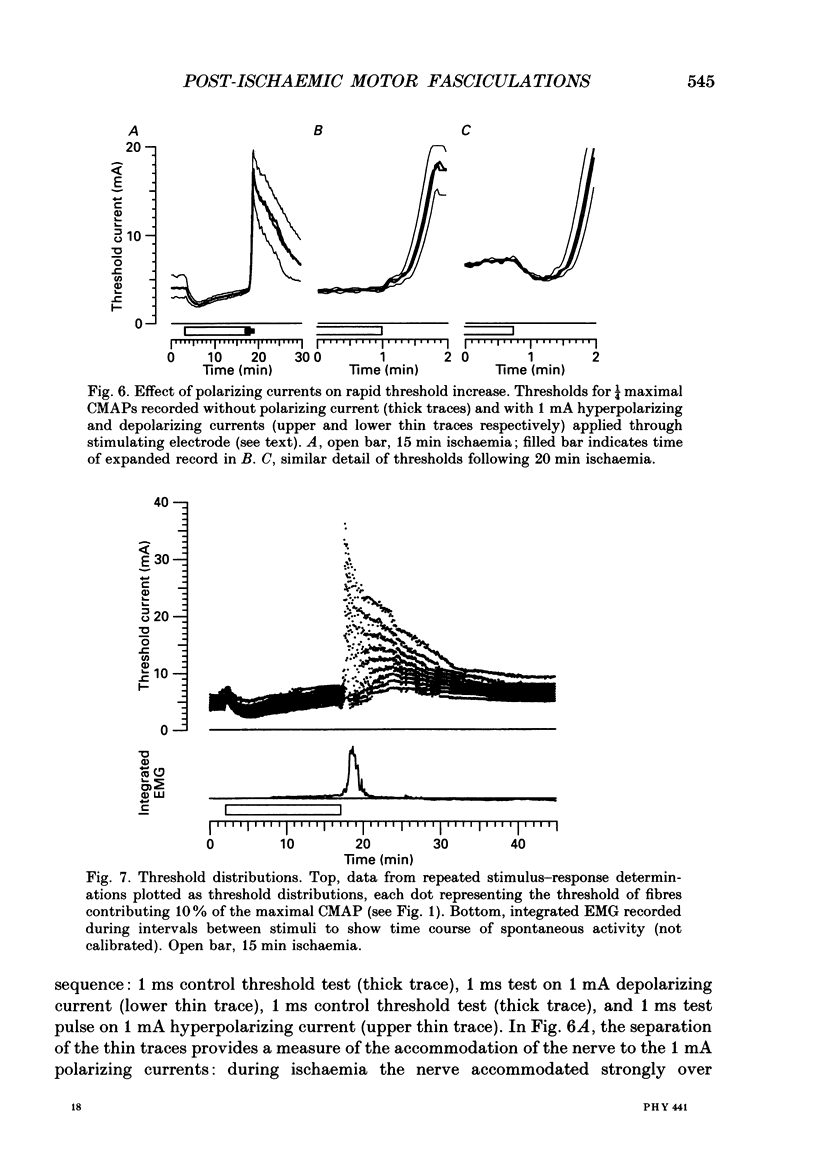
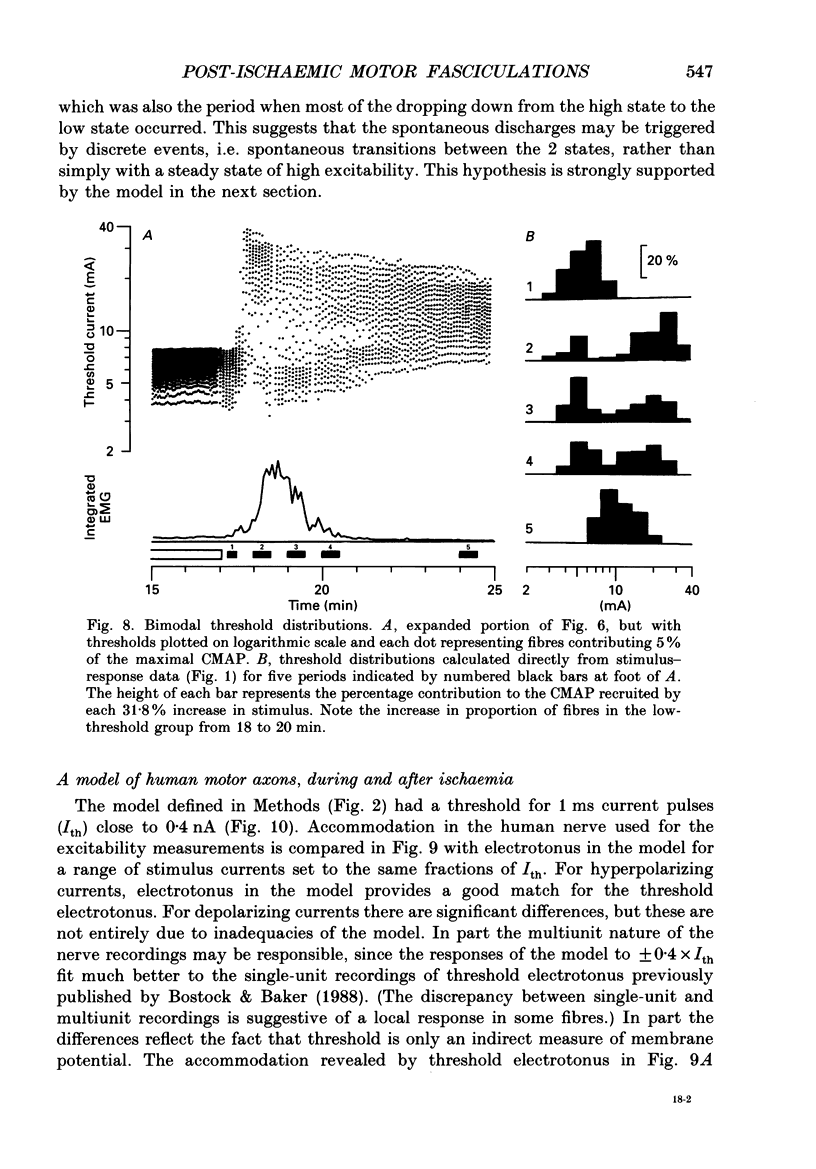
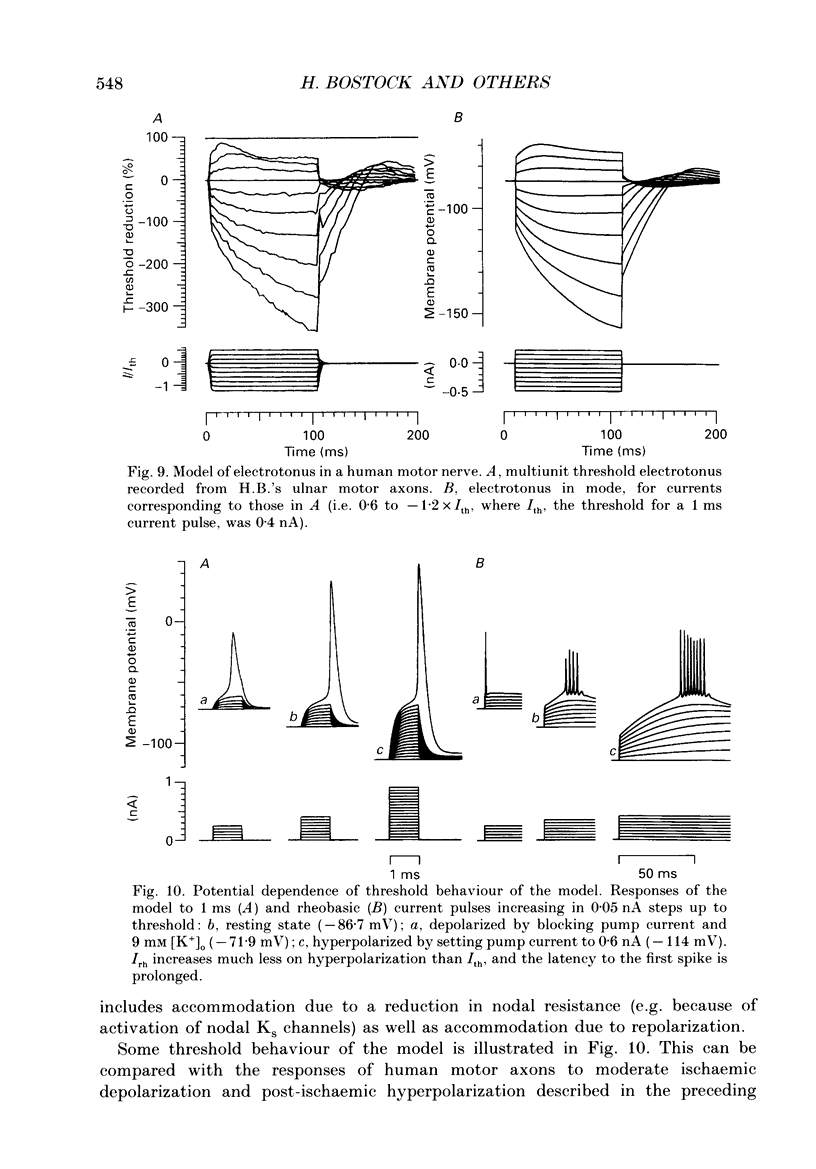
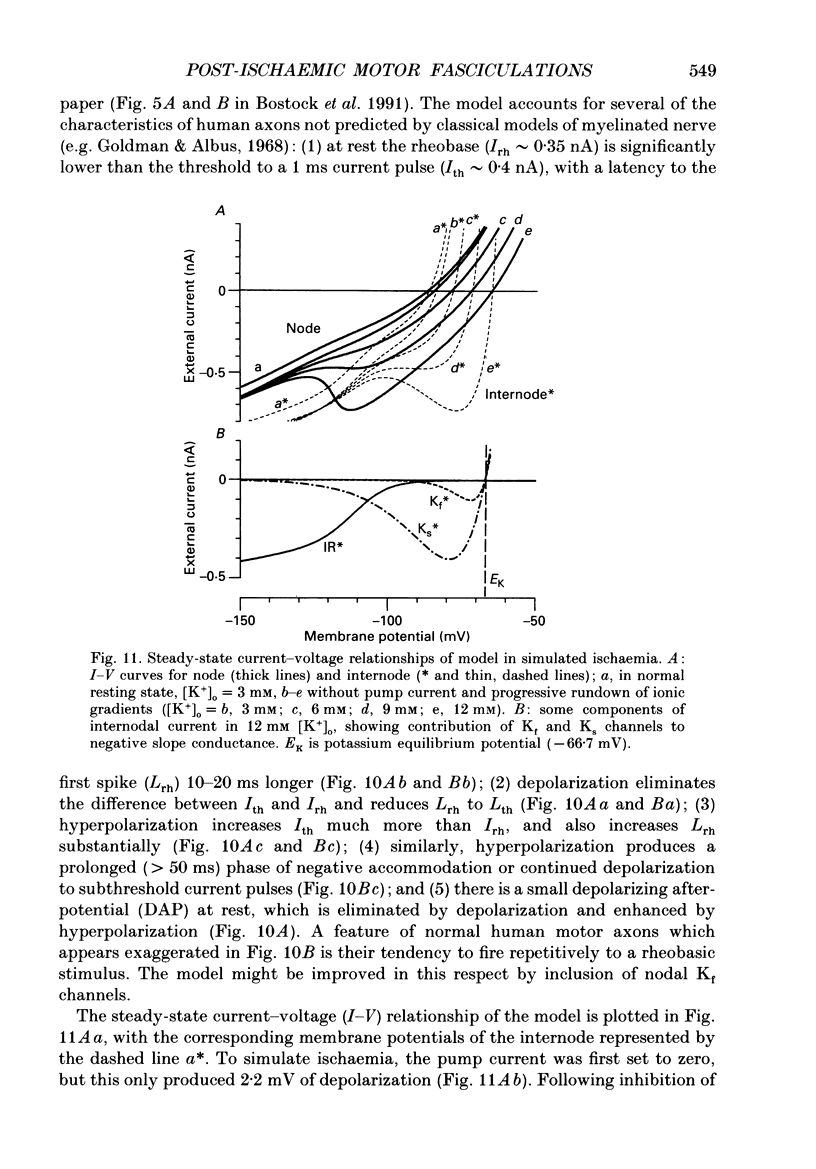
Full text
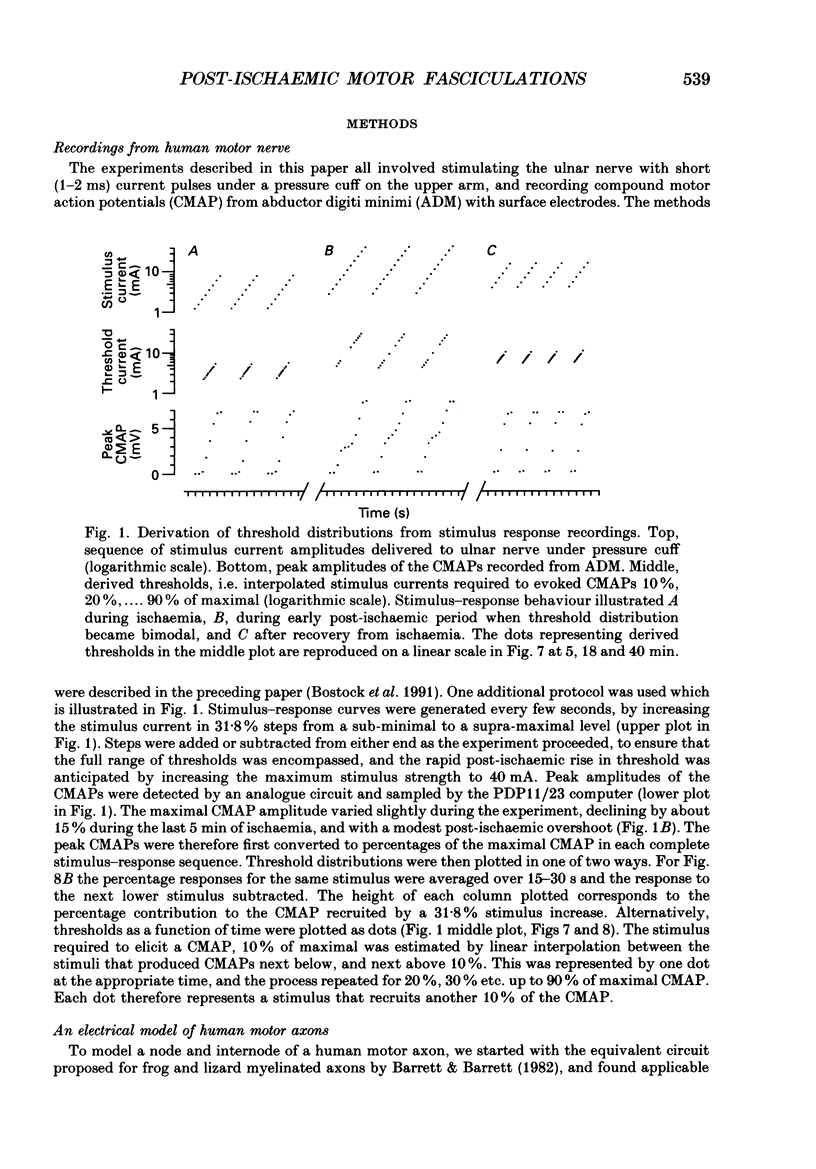
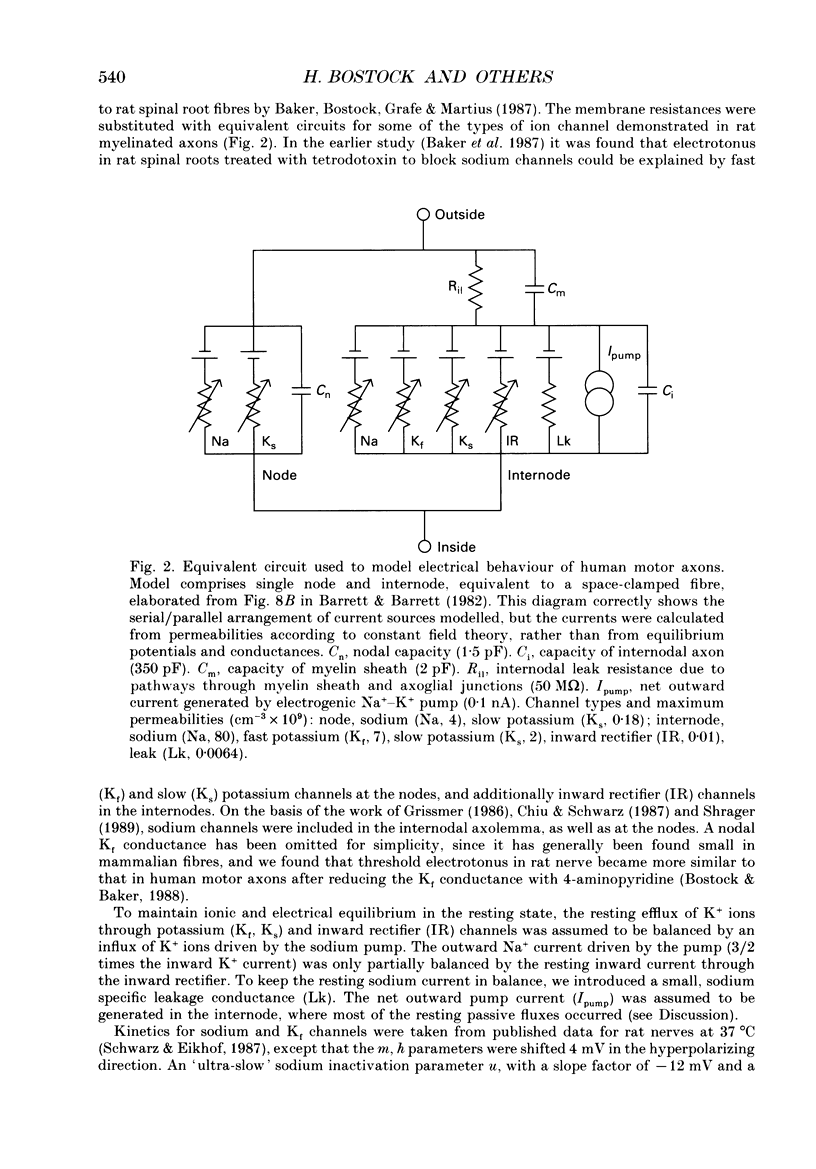
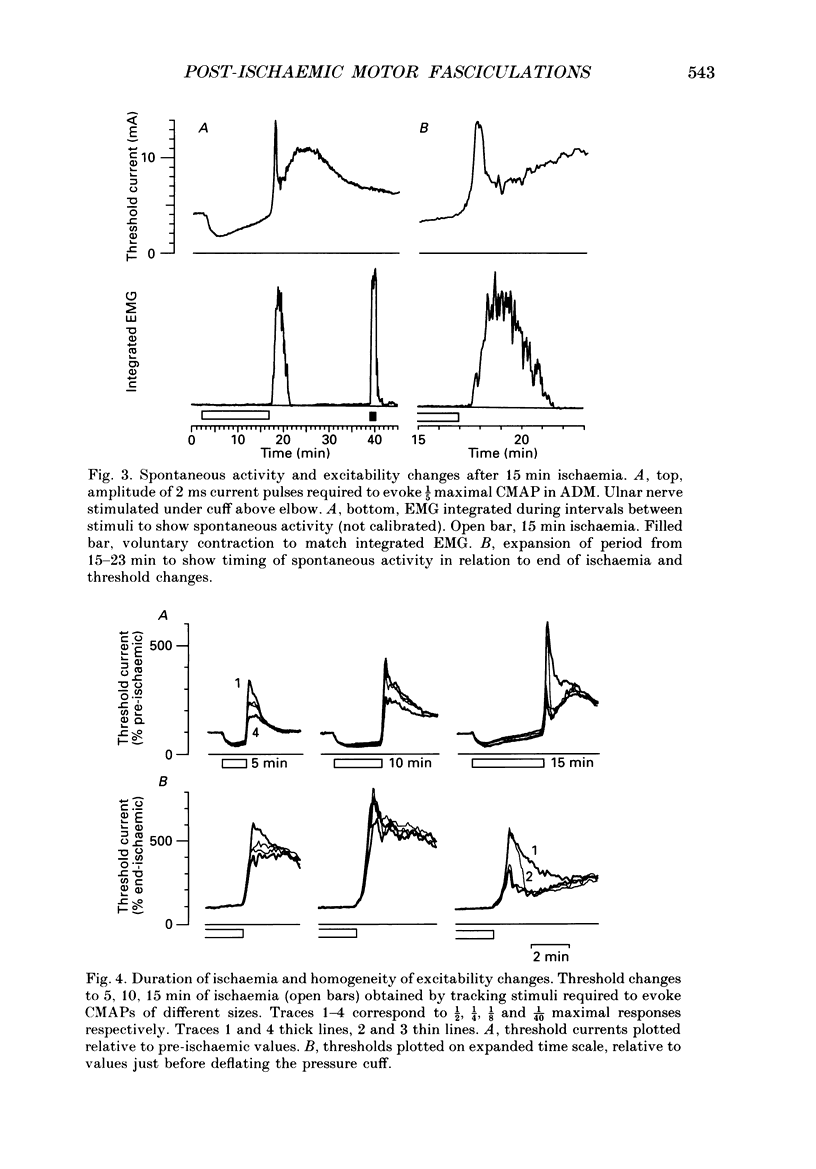
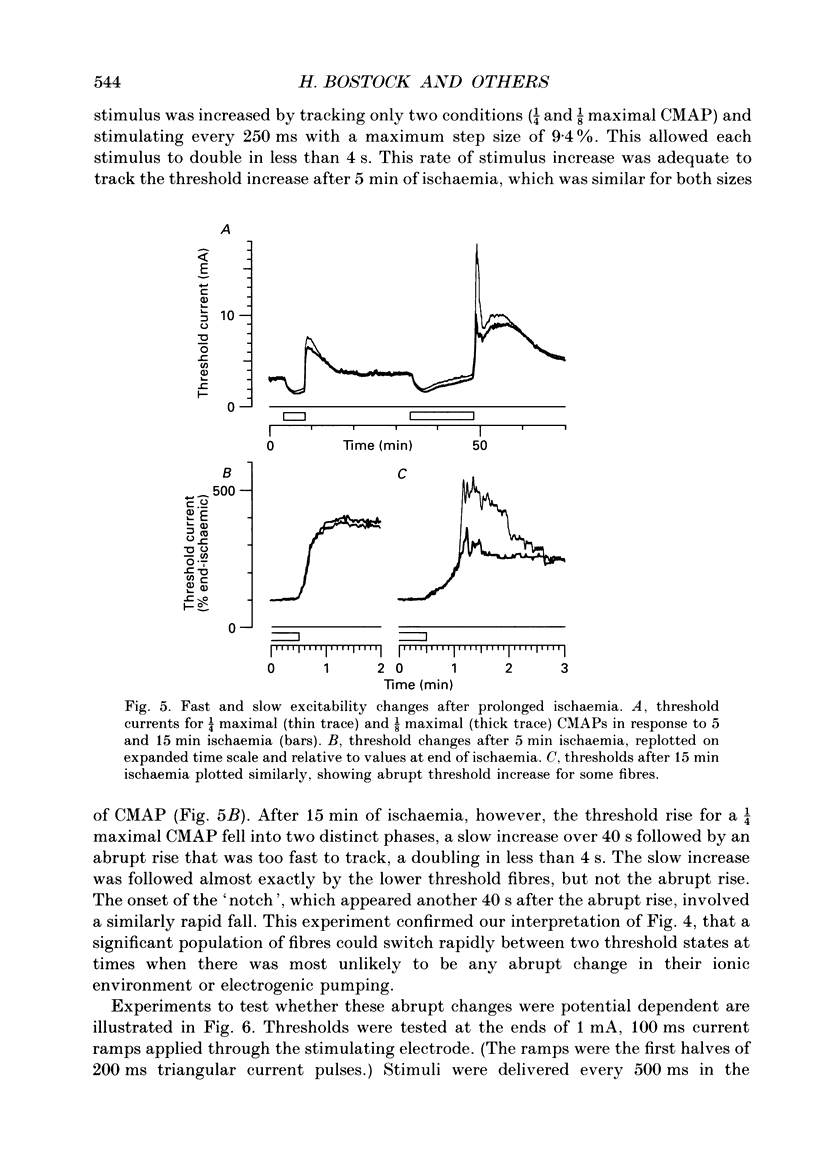
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate C., Burke D. Changes in excitability of human cutaneous afferents following prolonged high-frequency stimulation. Brain. 1989 Feb;112(Pt 1):147–164. doi: 10.1093/brain/112.1.147. [DOI] [PubMed] [Google Scholar]
- Ariyasu R. G., Nichol J. A., Ellisman M. H. Localization of sodium/potassium adenosine triphosphatase in multiple cell types of the murine nervous system with antibodies raised against the enzyme from kidney. J Neurosci. 1985 Oct;5(10):2581–2596. doi: 10.1523/JNEUROSCI.05-10-02581.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Bostock H. Depolarization changes the mechanism of accommodation in rat and human motor axons. J Physiol. 1989 Apr;411:545–561. doi: 10.1113/jphysiol.1989.sp017589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Bostock H., Grafe P., Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987 Feb;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982 Feb;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 1988 Oct 18;462(2):354–358. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H., Baker M., Grafe P., Reid G. Changes in excitability and accommodation of human motor axons following brief periods of ischaemia. J Physiol. 1991 Sep;441:513–535. doi: 10.1113/jphysiol.1991.sp018765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985 Aug;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Schwarz W. Sodium and potassium currents in acutely demyelinated internodes of rabbit sciatic nerves. J Physiol. 1987 Oct;391:631–649. doi: 10.1113/jphysiol.1987.sp016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. M. Ultra-slow inactivation of the ionic currents through the membrane of myelinated nerve. Biochim Biophys Acta. 1976 Mar 5;426(2):232–244. doi: 10.1016/0005-2736(76)90334-5. [DOI] [PubMed] [Google Scholar]
- Goldman L., Albus J. S. Computation of impulse conduction in myelinated fibers; theoretical basis of the velocity-diameter relation. Biophys J. 1968 May;8(5):596–607. doi: 10.1016/S0006-3495(68)86510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S. Properties of potassium and sodium channels in frog internode. J Physiol. 1986 Dec;381:119–134. doi: 10.1113/jphysiol.1986.sp016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- KUGELBERG E., COBB W. Repetitive discharges in human motor nerve fibers during the post-ischaemic state. J Neurol Neurosurg Psychiatry. 1951 May;14(2):88–94. doi: 10.1136/jnnp.14.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J. L., Torebjörk H. E. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980 Dec;103(4):835–853. doi: 10.1093/brain/103.4.835. [DOI] [PubMed] [Google Scholar]
- Röper J., Schwarz J. R. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. J Physiol. 1989 Sep;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL J. R. An anodal threshold phenomenon in the squid giant axon. Nature. 1958 Nov 15;182(4646):1370–1370. doi: 10.1038/1821370a0. [DOI] [PubMed] [Google Scholar]
- STAEMPFLI R. Is the resting potential of Ranvier nodes a potassium potential? Ann N Y Acad Sci. 1959 Aug 28;81:265–284. doi: 10.1111/j.1749-6632.1959.tb49313.x. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Eikhof G. Na currents and action potentials in rat myelinated nerve fibres at 20 and 37 degrees C. Pflugers Arch. 1987 Aug;409(6):569–577. doi: 10.1007/BF00584655. [DOI] [PubMed] [Google Scholar]
- Shrager P. Sodium channels in single demyelinated mammalian axons. Brain Res. 1989 Mar 27;483(1):149–154. doi: 10.1016/0006-8993(89)90046-2. [DOI] [PubMed] [Google Scholar]
- Strupp M., Bostock H., Weigl P., Piwernetz K., Renner R., Grafe P. Is resistance to ischaemia of motor axons in diabetic subjects due to membrane depolarization? J Neurol Sci. 1990 Nov;99(2-3):271–280. doi: 10.1016/0022-510x(90)90161-f. [DOI] [PubMed] [Google Scholar]
- TASAKI I. Demonstration of two stable states of the nerve membrane in potassium-rich media. J Physiol. 1959 Oct;148:306–331. doi: 10.1113/jphysiol.1959.sp006290. [DOI] [PMC free article] [PubMed] [Google Scholar]


