Abstract
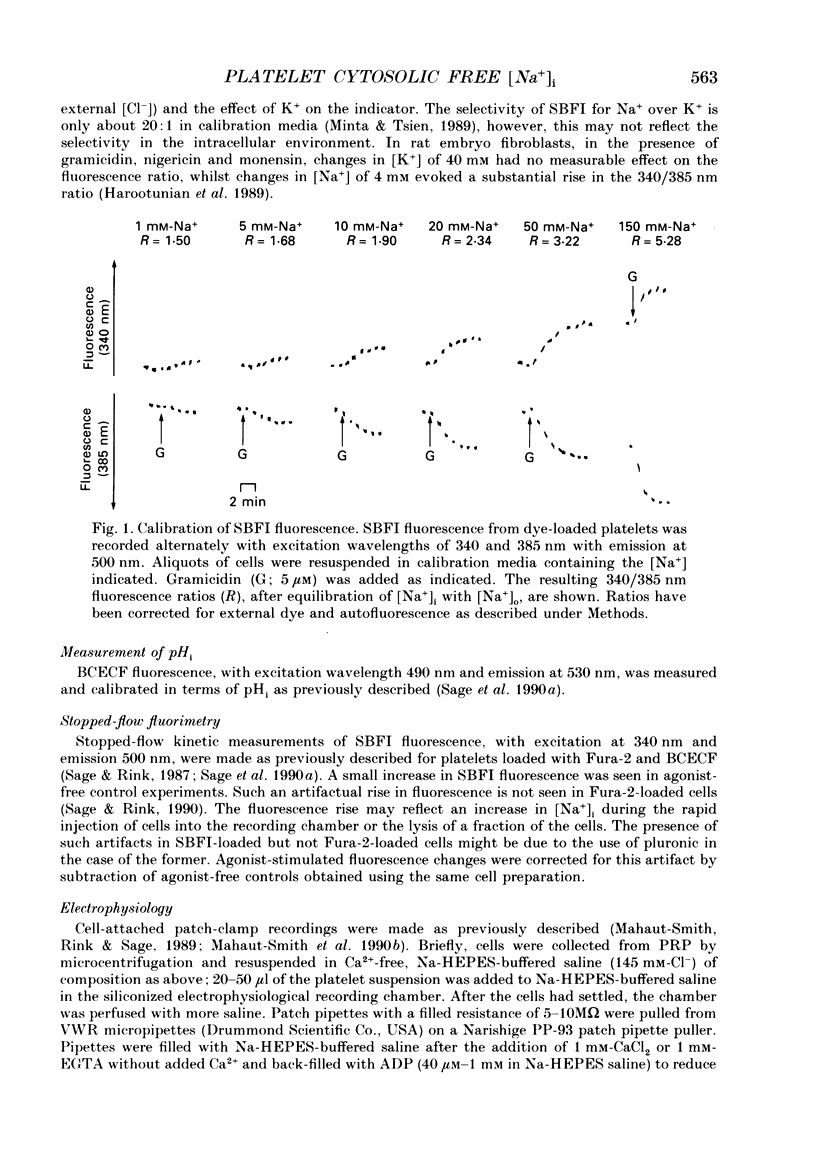
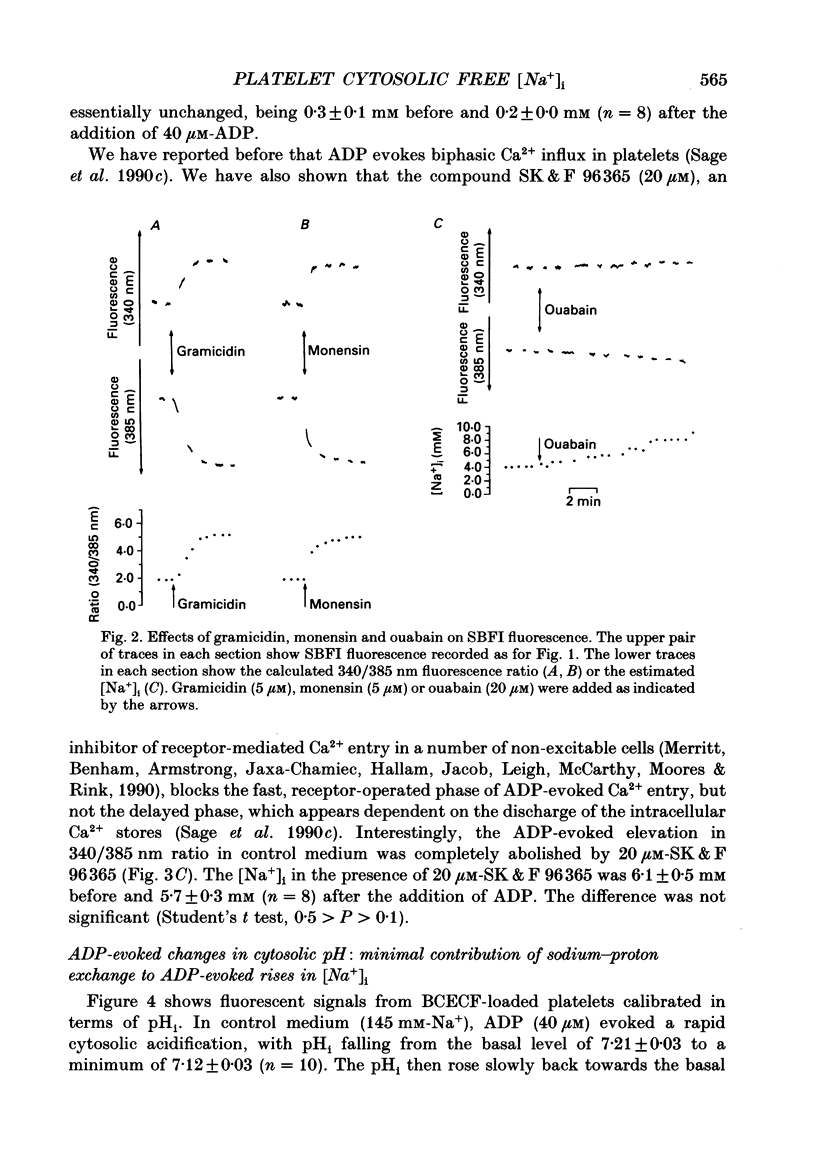
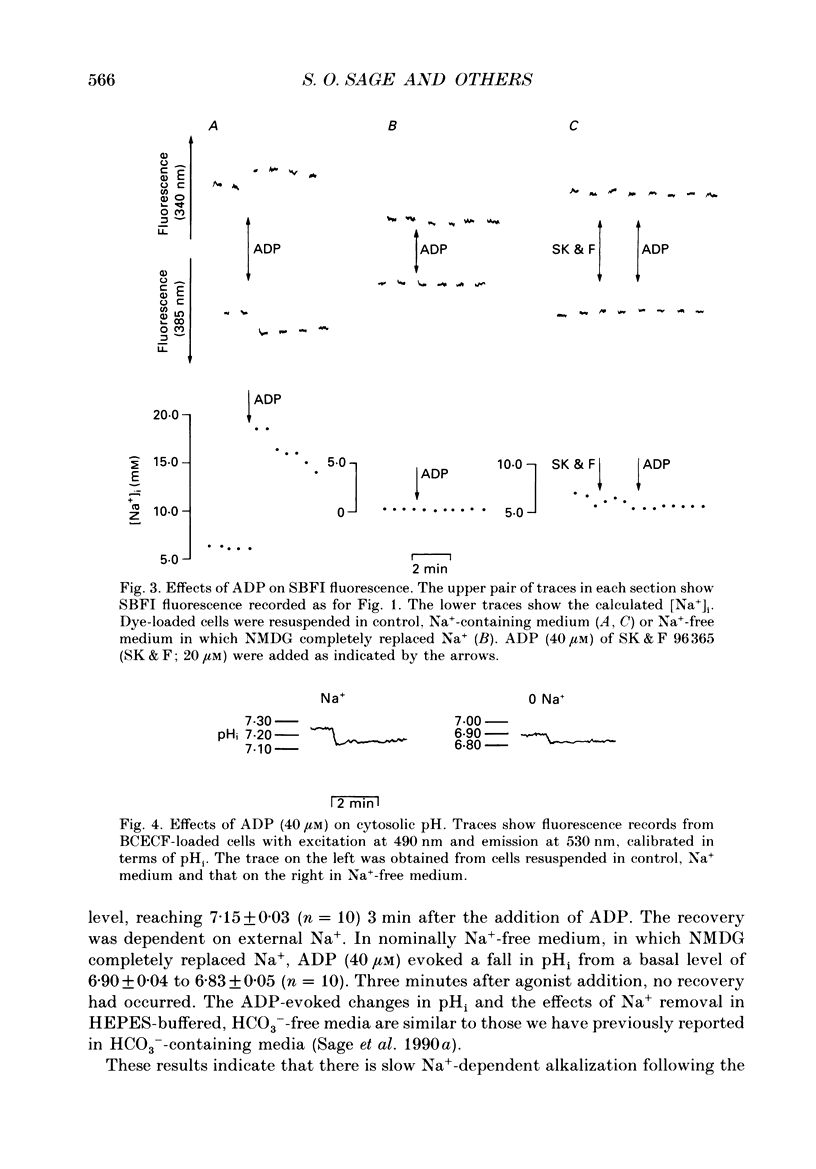
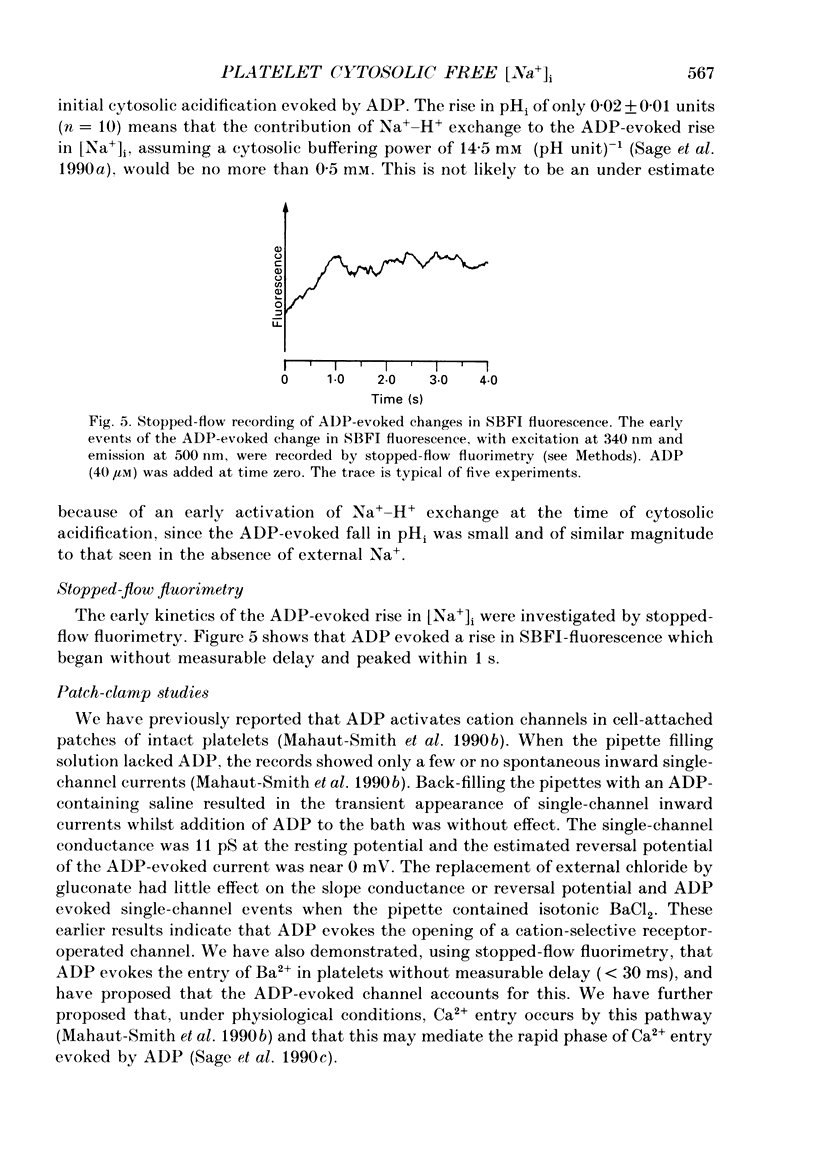
1. Cytosolic free Na+ concentration, [Na+]i, was investigated in human platelets loaded with the fluorescent indicator SBFI (sodium-binding benzofuran isophthalate). 2. SBFI fluorescence from platelet suspensions was measured at excitation wavelengths of 340 and 385 nm and the 340/385 nm fluorescence ratio was calibrated in terms of [Na+]i in situ. [Na+]i was set to known values by resuspending cells in media with various [Na+], in the presence of the Na(+)-K+ ionophore, gramicidin. 3. Basal free [Na+]i was 5.5 +/- 0.3 mM (n = 50). This is considerably lower than estimates of total platelet Na+, suggesting that much intracellular Na+ is sequestered or bound. 4. ADP (40 microM) evoked a rise in [Na+]i from 6.4 +/- 0.7 to 18.3 +/- 1.1 mM (n = 8). The ADP-evoked rise in [Na+]i was abolished when external Na+ was replaced with N-methyl-D-glucamine. This indicates that the rise in [Na+]i was due to Na+ entry. 5. In platelets loaded with the fluorescent pH indicator, BCECF, 40 microM-ADP was shown to evoke a fall in cytosolic pH (pHi) from 7.21 +/- 0.03 to 7.12 +/- 0.03 (n = 10). Three minutes after ADP addition pHi had only recovered to 7.15 +/- 0.03. The recovery was dependent on external Na+, suggesting it was mediated by Na(+)-H+ exchange. However, this would only account for an increase in [Na+]i of approximately 0.5 mM, indicating most of the ADP-evoked Na+ entry occurred by other mechanisms. 6. Stopped-flow fluorimetry showed that the ADP-evoked rise in [Na+]i commenced without measurable delay and peaked within 1 s. The initial kinetics were thus similar to those reported for ADP-evoked rises in [Ca2+]i. 7. Cell-attached patch-clamp recordings showed that ADP evoked single-channel inward currents when included in the pipette-filling solution. The currents were similar whether Ca2+ was present or absent from the pipette. The slope conductance was 11 pS in the presence of external Ca2+ and 10 pS in its absence. Current-voltage relationships were similar and the reversal potentials were close to 0 mV under both conditions. 8. SK & F 96,365 (20 microM), a blocker of receptor-mediated Ca2+ entry in several non-excitable cells, blocked the ADP-evoked rise in [Na+]i. This compound has been shown to only partly block the biphasic ADP-evoked rise in [Ca2+]i, being selective for the fast, receptor-operated phase of entry. 9. These data suggest that ADP rapidly activates a channel in that platelet plasma membrane which is permeable to Na+ and divalent cations.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borin M., Siffert W. Stimulation by thrombin increases the cytosolic free Na+ concentration in human platelets. Studies with the novel fluorescent cytosolic Na+ indicator sodium-binding benzofuran isophthalate. J Biol Chem. 1990 Nov 15;265(32):19543–19550. [PubMed] [Google Scholar]
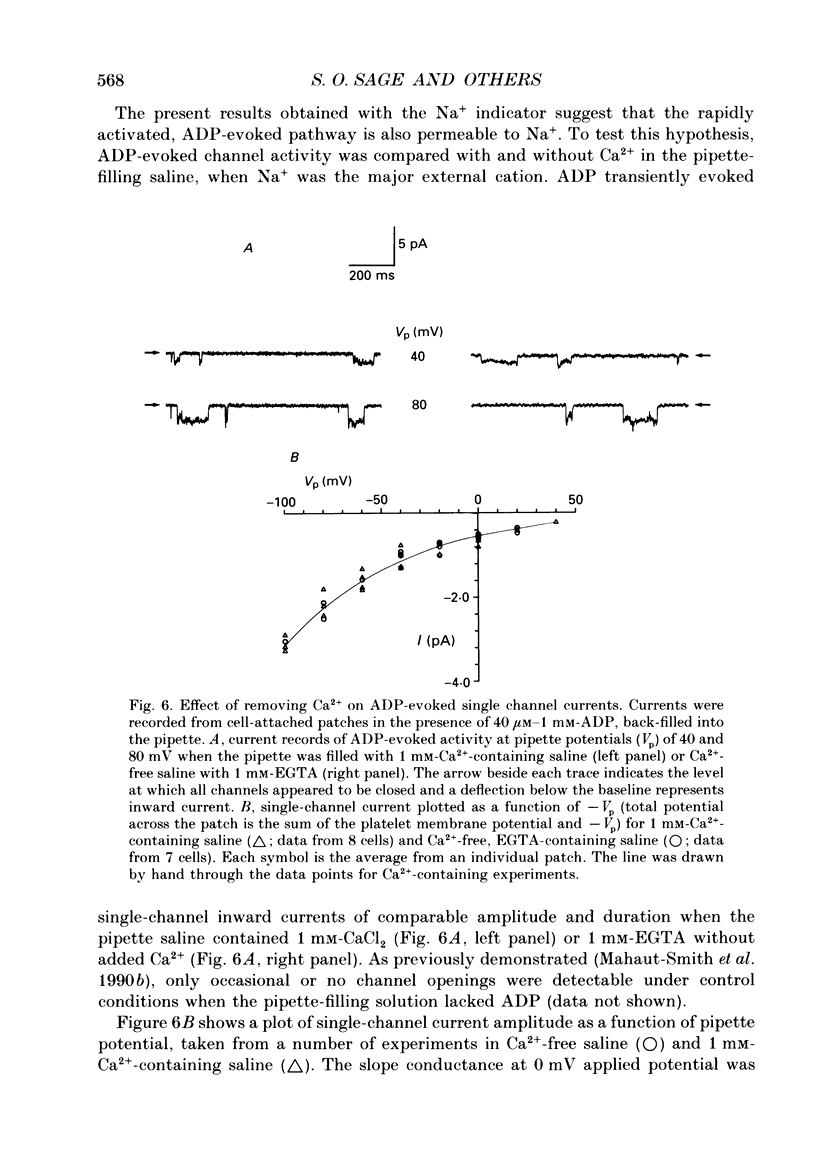
- Brass L. F. The effect of Na+ on Ca2+ homeostasis in unstimulated platelets. J Biol Chem. 1984 Oct 25;259(20):12571–12575. [PubMed] [Google Scholar]
- Feinberg H., Sandler W. C., Scorer M., Le Breton G. C., Grossman B., Born G. V. Movement of sodium into human platelets induced by ADP. Biochim Biophys Acta. 1977 Oct 17;470(2):317–324. doi: 10.1016/0005-2736(77)90109-2. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. Glass micro-electrodes for measuring intracellular activities of sodium and potassium. Nature. 1959 Oct 17;184(Suppl 16):1257–1258. doi: 10.1038/1841257a0. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Responses to adenosine diphosphate in human platelets loaded with the fluorescent calcium indicator quin2. J Physiol. 1985 Nov;368:131–146. doi: 10.1113/jphysiol.1985.sp015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Eckert B. K., Tsien R. Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989 Nov 15;264(32):19458–19467. [PubMed] [Google Scholar]
- Horowitz S. B., Paine P. L. Reference phase analysis of free and bound intracellular solutes. II. Isothermal and isotopic studies of cytoplasmic sodium, potassium, and water. Biophys J. 1979 Jan;25(1):45–62. doi: 10.1016/S0006-3495(79)85277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre D. E., Rink T. J. The role of platelet membrane potential in the initiation of platelet aggregation. Thromb Haemost. 1982 Feb 26;47(1):22–26. [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Rink T. J., Collins S. C., Sage S. O. Voltage-gated potassium channels and the control of membrane potential in human platelets. J Physiol. 1990 Sep;428:723–735. doi: 10.1113/jphysiol.1990.sp018237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Sage S. O., Rink T. J. Receptor-activated single channels in intact human platelets. J Biol Chem. 1990 Jun 25;265(18):10479–10483. [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Negulescu P. A., Harootunian A., Tsien R. Y., Machen T. E. Fluorescence measurements of cytosolic free Na concentration, influx and efflux in gastric cells. Cell Regul. 1990 Feb;1(3):259–268. doi: 10.1091/mbc.1.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palés J., Palacios-Araus L., López A., Gual A. Influence of sodium conductances on platelet activation. Biochim Biophys Acta. 1989 Mar 27;980(1):33–36. doi: 10.1016/0005-2736(89)90196-x. [DOI] [PubMed] [Google Scholar]
- Pipili E. Platelet membrane potential: simultaneous measurement of diSC3(5) fluorescence and optical density. Thromb Haemost. 1985 Oct 30;54(3):645–649. [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Hallam T. J. Calcium signalling in non-excitable cells: notes on oscillations and store refilling. Cell Calcium. 1989 Jul;10(5):385–395. doi: 10.1016/0143-4160(89)90064-x. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Stimulated calcium efflux from fura-2-loaded human platelets. J Physiol. 1987 Dec;393:513–524. doi: 10.1113/jphysiol.1987.sp016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Jobson T. M., Rink T. J. Agonist-evoked changes in cytosolic pH and calcium concentration in human platelets: studies in physiological bicarbonate. J Physiol. 1990 Jan;420:31–45. doi: 10.1113/jphysiol.1990.sp017900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Reast R., Rink T. J. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990 Feb 1;265(3):675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. Effects of ionic substitution on [Ca2+]i rises evoked by thrombin and PAF in human platelets. Eur J Pharmacol. 1986 Aug 22;128(1-2):99–107. doi: 10.1016/0014-2999(86)90563-7. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Sandler W. C., Le Breton G. C., Feinberg H. Movement of sodium into human platelets. Biochim Biophys Acta. 1980 Aug 4;600(2):448–455. doi: 10.1016/0005-2736(80)90447-2. [DOI] [PubMed] [Google Scholar]
- Siffert W., Akkerman J. W. Na+/H+ exchange as a modulator of platelet activation. Trends Biochem Sci. 1988 Apr;13(4):148–151. doi: 10.1016/0968-0004(88)90074-6. [DOI] [PubMed] [Google Scholar]
- Siffert W., Siffert G., Scheid P. Activation of Na+/H+ exchange in human platelets stimulated by thrombin and a phorbol ester. Biochem J. 1987 Jan 1;241(1):301–303. doi: 10.1042/bj2410301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Warner A. E., Warren R. L. The distribution of sodium and potassium in amphibian embryos during early development. J Physiol. 1973 Jul;232(2):297–312. doi: 10.1113/jphysiol.1973.sp010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. A., Morris P. G., Hesketh T. R., Metcalfe J. C. Design of an indicator of intracellular free Na+ concentration using 19F-NMR. Biochim Biophys Acta. 1986 Oct 31;889(1):72–83. doi: 10.1016/0167-4889(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Zavoico G. B., Cragoe E. J., Jr, Feinstein M. B. Regulation of intracellular pH in human platelets. Effects of thrombin, A23187, and ionomycin and evidence for activation of Na+/H+ exchange and its inhibition by amiloride analogs. J Biol Chem. 1986 Oct 5;261(28):13160–13167. [PubMed] [Google Scholar]


