Abstract
1. The modulation by Ca2+ of the activation by inositol 1,4,5-trisphosphate (IP3) of Ca2+ channels present in native sarcoplasmic reticulum membranes from frog skeletal muscle was studied after channel incorporation into planar phospholipid bilayers in the presence of Ca2+ or Ba2+ as current carrier species. 2. Channel activity expressed as fractional open time (Po) was low (less than or equal to 0.15) in the presence of varying free Ca2+ concentrations bathing the myoplasmic face of the channel (cis side), and did not increase significantly between 0.01 and 30 microM-Ca2+. 3. Channel activation mediated by IP3 could be elicited from free Ca2+ levels similar to those of resting skeletal muscle (about 0.1 microM) and was found to be strongly regulated by the free Ca2+ concentration present at the myoplasmic moiety of the channel. 4. Channel activation by 10 microM-IP3 depended on the Ca2+ concentration on the cis side. Po reached a maximum between pCa 7.0 and 6.0, but decreased at higher concentrations of free Ca2+. Thus, Ca2+ exerted a modulatory influence on IP3-mediated activation in a concentration range where the channel was insensitive to Ca2+. 5. The results indicate that Ca2+ ions act as modulators of IP3 efficacy to open the channel. This could arise from an interaction of Ca2+ with the channel gating mechanism or with the agonist binding site.
Full text
PDF



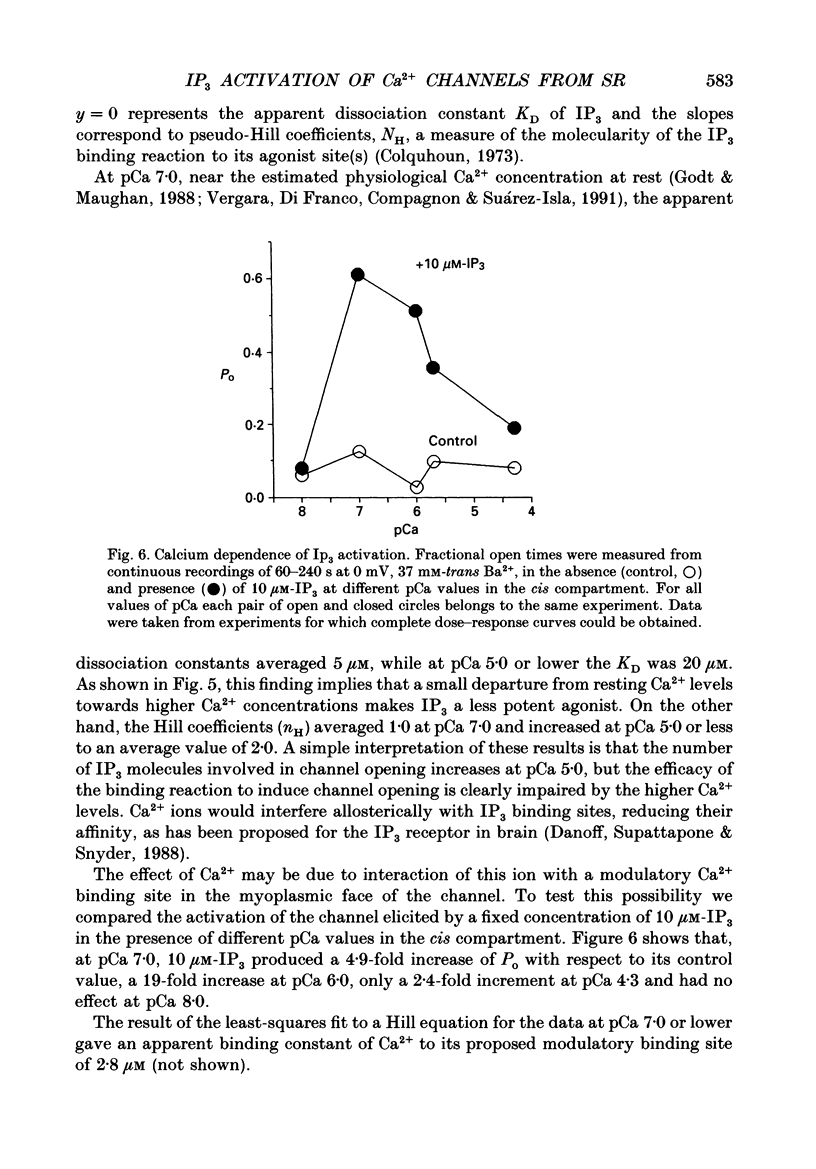
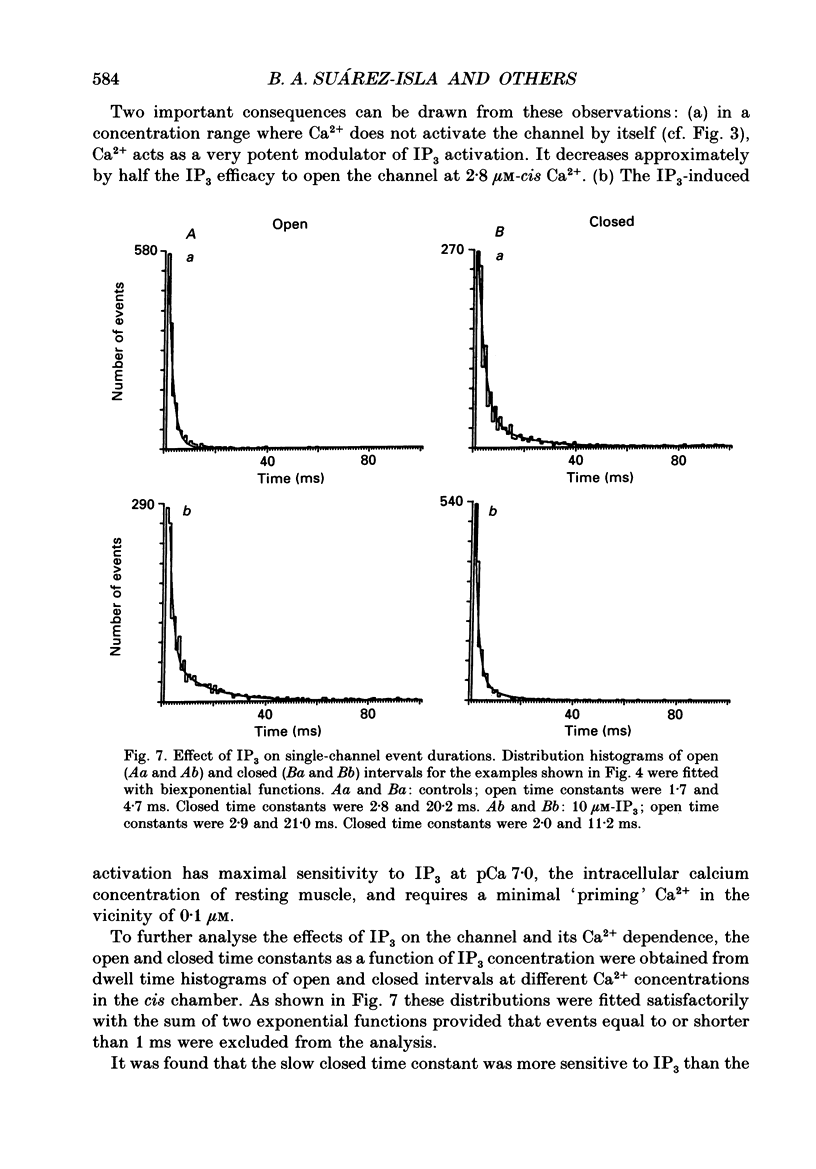
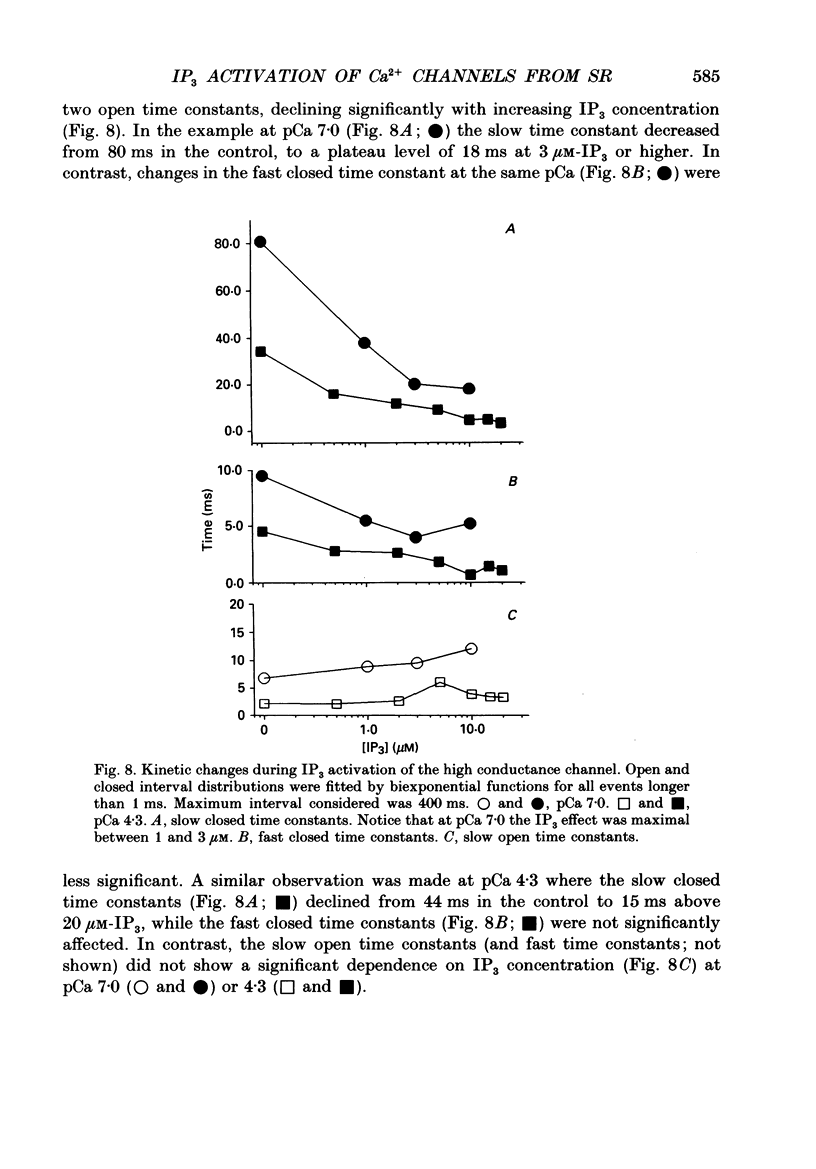






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Authi K. S., Crawford N. Inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ from highly purified human platelet intracellular membranes. Biochem J. 1985 Aug 15;230(1):247–253. doi: 10.1042/bj2300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J. The lipid environment of the nicotinic acetylcholine receptor in native and reconstituted membranes. Crit Rev Biochem Mol Biol. 1989;24(5):437–478. doi: 10.3109/10409238909086961. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bull R., Marengo J. J., Suárez-Isla B. A., Donoso P., Sutko J. L., Hidalgo C. Activation of calcium channels in sarcoplasmic reticulum from frog muscle by nanomolar concentrations of ryanodine. Biophys J. 1989 Oct;56(4):749–756. doi: 10.1016/S0006-3495(89)82722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueh S. H., Gill D. L. Inositol 1,4,5-trisphosphate and guanine nucleotides activate calcium release from endoplasmic reticulum via distinct mechanisms. J Biol Chem. 1986 Oct 25;261(30):13883–13886. [PubMed] [Google Scholar]
- Danoff S. K., Supattapone S., Snyder S. H. Characterization of a membrane protein from brain mediating the inhibition of inositol 1,4,5-trisphosphate receptor binding by calcium. Biochem J. 1988 Sep 15;254(3):701–705. doi: 10.1042/bj2540701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfert D. M., Hill S., Pershadsingh H. A., Sherman W. R., McDonald J. M. myo-Inositol 1,4,5-trisphosphate mobilizes Ca2+ from isolated adipocyte endoplasmic reticulum but not from plasma membranes. Biochem J. 1986 May 15;236(1):37–44. doi: 10.1042/bj2360037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Muñoz M., Hamilton S. L., Kaetzel M. A., Hazarika P., Dedman J. R. Modulation of Ca2+ release channel activity from sarcoplasmic reticulum by annexin VI (67-kDa calcimedin). J Biol Chem. 1990 Sep 15;265(26):15894–15899. [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Coronado R., Mickelson J. R., Vilven J., Ma J. J., Jacobson B. A., Louis C. F. Abnormal ryanodine receptor channels in malignant hyperthermia. Biophys J. 1990 Mar;57(3):471–475. doi: 10.1016/S0006-3495(90)82563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L., Burns K., MacLennan D. H., Reithmeier R. A., Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989 Dec 25;264(36):21522–21528. [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am J Physiol. 1988 May;254(5 Pt 1):C591–C604. doi: 10.1152/ajpcell.1988.254.5.C591. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Jaimovich E. Inositol trisphosphate and excitation-contraction coupling in skeletal muscle. J Bioenerg Biomembr. 1989 Apr;21(2):267–281. doi: 10.1007/BF00812072. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Parra C., Riquelme G., Jaimovich E. Transverse tubules from frog skeletal muscle. Purification and properties of vesicles sealed with the inside-out orientation. Biochim Biophys Acta. 1986 Feb 13;855(1):79–88. doi: 10.1016/0005-2736(86)90191-4. [DOI] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T., Klee C. B. Calcium modulation of inositol 1,4,5-trisphosphate-induced calcium release from neuroblastoma x glioma hybrid (NG108-15) microsomes. J Biol Chem. 1986 Dec 15;261(35):16414–16420. [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L., Williamson J. R. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J. 1989 Feb 15;258(1):261–265. doi: 10.1042/bj2580261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Inositol polyphosphates and intracellular calcium release. Arch Biochem Biophys. 1989 Aug 15;273(1):1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Moutin M. J., Dupont Y. Rapid filtration studies of Ca2+-induced Ca2+ release from skeletal sarcoplasmic reticulum. Role of monovalent ions. J Biol Chem. 1988 Mar 25;263(9):4228–4235. [PubMed] [Google Scholar]
- Nagasaki K., Kasai M. Calcium-induced calcium release from sarcoplasmic reticulum vesicles. J Biochem. 1981 Sep;90(3):749–755. doi: 10.1093/oxfordjournals.jbchem.a133529. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Ebashi S. Ca-releasing action of beta, gamma-methylene adenosine triphosphate on fragmented sarcoplasmic reticulum. J Biochem. 1976 Nov;80(5):1149–1157. doi: 10.1093/oxfordjournals.jbchem.a131370. [DOI] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Brunder D., Stein P., Hals G. Pharmacology of calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989 Apr;21(2):295–320. doi: 10.1007/BF00812074. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Corson D. W., Fein A., Berridge M. J. Excitation and adaptation of Limulus ventral photoreceptors by inositol 1,4,5 triphosphate result from a rise in intracellular calcium. J Gen Physiol. 1986 Jul;88(1):127–142. doi: 10.1085/jgp.88.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Walz B., Levy S., Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- Rojas C., Jaimovich E. Calcium release modulated by inositol trisphosphate in ruptured fibers from frog skeletal muscle. Pflugers Arch. 1990 May;416(3):296–304. doi: 10.1007/BF00392066. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Orozco C., Heller P. F., Froehlich J. P. Single calcium channels in native sarcoplasmic reticulum membranes from skeletal muscle. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7741–7745. doi: 10.1073/pnas.83.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Isla B. A., Irribarra V., Oberhauser A., Larralde L., Bull R., Hidalgo C., Jaimovich E. Inositol (1,4,5)-trisphosphate activates a calcium channel in isolated sarcoplasmic reticulum membranes. Biophys J. 1988 Oct;54(4):737–741. doi: 10.1016/S0006-3495(88)83009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Valdivia C., Valdivia H. H., Potter B. V., Coronado R. Ca2+ release by inositol-trisphosphorothioate in isolated triads of rabbit skeletal muscle. Biophys J. 1990 Jun;57(6):1233–1243. doi: 10.1016/S0006-3495(90)82642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J., DiFranco M., Compagnon D., Suarez-Isla B. A. Imaging of calcium transients in skeletal muscle fibers. Biophys J. 1991 Jan;59(1):12–24. doi: 10.1016/S0006-3495(91)82193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Salviati G., Di Virgilio F., Pozzan T. Inositol 1,4,5-trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature. 1985 Jul 25;316(6026):347–349. doi: 10.1038/316347a0. [DOI] [PubMed] [Google Scholar]
- Willems P. H., De Jong M. D., De Pont J. J., Van Os C. H. Ca2(+)-sensitivity of inositol 1,4,5-trisphosphate-mediated Ca2+ release in permeabilized pancreatic acinar cells. Biochem J. 1990 Feb 1;265(3):681–687. doi: 10.1042/bj2650681. [DOI] [PMC free article] [PubMed] [Google Scholar]


