Abstract
Cell wall mannoproteins are largely responsible for the adhesive properties and immunomodulation ability of the fungal pathogen Candida albicans. The outer chain extension of yeast mannoproteins occurs in the lumen of the Golgi apparatus. GDP-mannose must first be transported from the cytosol into the Golgi lumen, where mannose is transferred to mannans. GDP is hydrolyzed by a GDPase, encoded by GDA1, to GMP, which then exits the Golgi lumen in a coupled, equimolar exchange with cytosolic GDP-mannose. We isolated and disrupted the C. albicans homologue of the Saccharomyces cerevisiae GDA1 gene in order to investigate its role in protein mannosylation and pathogenesis. CaGda1p shares four apyrase conserved regions with other nucleoside diphosphatases. Membranes prepared from the C. albicans disrupted gda1/gda1 strain had a 90% decrease in the ability to hydrolyze GDP compared to wild type. The gda1/gda1 mutants showed a severe defect in O-mannosylation and reduced cell wall phosphate content. Other cell wall-related phenotypes are present, such as elevated chitin levels and increased susceptibility to attack by β-1,3-glucanases. Our results show that the C. albicans organism contains β-mannose at their nonreducing end, differing from S. cerevisiae, which has only α-linked mannose residues in its O-glycans. Mutants lacking both alleles of GDA1 grow at the same rate as the wild type but are partially blocked in hyphal formation in Lee solid medium and during induction in liquid by changes in temperature and pH. However, the mutants still form normal hyphae in the presence of serum and N-acetylglucosamine and do not change their adherence to HeLa cells. Taken together, our data are in agreement with the hypothesis that several pathways regulate the yeast-hypha transition. Gda1/gda1 cells offer a model for discriminating among them.
Candida albicans, one of the most frequently isolated fungal human pathogens, can cause superficial and systemic infections. Several virulence factors have been described for this organism: the secretion of lytic enzymes (28), the ability to undergo the transition from yeast to hyphal growth known as dimorphism (12, 38, 16, 51), and the ability to adhere to host cells and to penetrate epithelial and endothelial cells. The adhesion capacity of C. albicans depends on mannoproteins present in its cell wall. Recent studies from several laboratories have shown that glycosylated outer cell wall mannoproteins form direct interactions with the host (14, 54) and therefore glycosylation defects are important for virulence (11, 56). In yeast, the first O-glycosylation step and the addition of a core N-linked carbohydrate occurs in the endoplasmic reticulum; and then the glycoproteins move to the Golgi apparatus, where elongation of O-linked sugar chains and processing of complex N-linked oligosaccharide structures take place (22, 53). In Saccharomyces cerevisiae, many of the steps have been fully characterized at both the biochemical and genetic levels (22, 10). However, for C. albicans only a few genes—MNT1 (11), PMT1 (55), and PMT6 (56)—involved in the O-glycosylation pathway have been isolated. Deletion of any of the three showed strong alterations of virulence in animal models.
Terminal mannosylation of yeast proteins and lipids occurs in the lumen of the Golgi apparatus. The sugar donor for these reactions, GDP-mannose, must first be transported by a specific transporter from the cytosol, its site of synthesis, into the Golgi lumen, where mannose is transferred to mannans by specific mannosyltransferases (27). The other reaction product, GDP, is then hydrolyzed by a GDPase (Gda1p) to GMP, which then exits the Golgi lumen in a coupled, equimolar exchange with cytosolic GDP-mannose (2). This transport/antiport cycle was originally described in vitro with Golgi vesicles from rat liver (13). Evidence for its physiological relevance has been obtained in vivo and in vitro with yeast as well as with nematodes and mammals (4, 6). Recently the molecular defect causing the human disease leukocyte adhesion deficiency syndrome type II was localized to the gene encoding the Golgi GDP-fucose transporter (26).
S. cerevisiae Gda1p is very active toward GDP, moderately active toward UDP, and inactive toward ADP or any tri- or monophosphate (59). Deletion of this gene results in markedly reduced Golgi mannosylation of proteins and lipids in vivo and decreases fivefold the rate of GDP-mannose entry into Golgi vesicles compared to results with the wild type (5). The Kluyveromyces lactis GDA1 gene has also been isolated and characterized. Loss of function of the gene results in different defects in glycosylation, osmotic stability, and cell wall polymer composition in the two yeast species (35). Another Golgi nucleoside diphosphatase, encoded by the YND1 gene, was recently characterized for S. cerevisiae (21, 60). This phosphatase has a broader spectrum of specificity; nevertheless, it is partially redundant with Gda1p in function. The ynd1gda1 double mutant has more severe glycosylation phenotypes than any of the individual mutants (21). It is clear that there is regulation of the glycosylation process at the level of antiporter generation, but the precise relationship between Gda1p and Ynd1p is not yet understood.
The cell wall glycoproteins of fungal pathogens, such as C. albicans, are recognized during host invasion and modulate the immune response. Therefore, studying enzymes regulating the glycosylation process in these fungi could help in understanding mechanisms of host defense. To determine the in vivo role of C. albicans Golgi GDPase, the C. albicans GDA1 gene, encoding a protein highly similar to S. cerevisiae and K. lactis Gda1p, was cloned, and null mutants were constructed. The homozygous C. albicans gda1/gda1 strain was viable and showed drastically reduced in vitro membrane bound GDPase activity. We localized C. albicans Gda1p to the Golgi and demonstrated that it is implicated in cell wall biogenesis, hyphal formation, and O-mannosylation.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The yeast strains utilized in this study and their genotypes are listed in Table 1. Strains were grown in yeast extract-peptone-dextrose (YEPD) or synthetic dextrose medium (48), which for Ura− strains was supplemented with 50 μg of uridine/ml. Solid medium was obtained by adding agar (2%). Solid medium for inducing the yeast-hypha transition in C. albicans was Lee medium in which glucose was replaced by mannitol (1.25%). The dimorphic transition was induced in Lee medium by changing the temperature to 37°C, adding 4% bovine calf serum (GIBCO), or changing the carbon source (glucose was replaced by 1.25% N-acetylglucosamine [GlcNAc]).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype or description | Reference or source |
|---|---|---|
| C. albicans | ||
| SC5314 | Prototrophic | |
| CAI4 | Δura3::imm434/Δura3::imm434 | Fonzi and Irwin (20) |
| SS19-4 | ura3/ura3 Δmnn9::hisG/mnn9::hisG | Southard et al. (50) |
| BAB1 | ura3/ura3 GDA1/gda1::hisG-URA3-hisG | This work |
| BAB2 | ura3/ura3 GDA1/Δgda1::hisG | This work |
| BAB3 | ura3/ura3gda1::hisG-URA3-hisG/Δgda1::hisG | This work |
| BAB4 | ura3/ura3 Δgda1::hisG/Δgda1::hisG | This work |
| S. cerevisiae | ||
| G2-6 | MATα ura3-52 lys2-801 am ade2-101 oc trp1-Δ1 his3-Δ200 leu2-Δ1 | Abeijon et al. (2) |
| G2-7 | MATα ura3-52 lys2-801 am ade2-101 oc trp1-Δ1 his3-Δ200 leu2-Δ1 gda1::LEU2 | Abeijon et al. (2) |
Disruption of GDA1.
The plasmid pGDA1 contains the Candida GDA1 gene in a 4,120-bp fragment subcloned into pBluescript. The plasmid pGDA1 was used as a template for “divergent” PCR with the oligonucleotides AH1 (5′-AAAGAATGGTTAAAGAGCTCGAAGCTAGTG) and AH2 (5′-TTTTGGAAGATCTATTTTTCCAGTTGAATC). The oligonucleotide AH1 corresponds to nucleotides −667 to −638 relative to the ATG in the GDA1 sequence and also adds a SacI site to the PCR product, while the oligonucleotide AH2 corresponds to nucleotides −103 to −132 and adds a BglII site. The 456-bp PCR fragment was cut with SacI and BglII and ligated to the plasmid pMB7 (20), previously digested with SacI and BglII. The resulting plasmid was pGDAΔ1. The plasmid pGDA1 was used again as a template for divergent PCR with the oligonucleotides AH3 (5′-CGTATATGTCGACACAAGTTACATTTTAGA) and AH4 (5′-CGTTTGTGTGTGATTGGCAAGCTTTATTGT). The oligonucleotide AH3 correspond to nucleotides +31 to +60 relative to the stop codon in the GDA1 sequence and also adds a SalI site to the PCR product, while the oligonucleotide AH4 correspond to nucleotides +803 to +832 and adds a HindIII site. The 801-bp PCR fragment was cut with SalI and HindIII and ligated to the plasmid pGDAΔ1, previously digested with SalI and HindIII. From the resulting plasmid, pGDAΔ2, a 5.2-kb SacI-HindIII fragment was isolated and used to transform strain CAI4. Correct integration of the cassette into the GDA1 locus of the Ura+ transformants was verified by Southern blot analysis. Spontaneous Ura− derivatives of one of the heterozygous disruptants were selected on medium containing 5-fluoro-orotic acid (U.S. Biological). These clones were screened by Southern blot hybridization to identify those which had lost the URA3 gene via intrachromosomal recombination mediated by the hisG repeats. The procedure was then repeated to delete the remaining functional allele of GDA1. The GDA1 gene was then reintroduced into BAB4 by transforming this strain with the plasmid p1041GDA1. This plasmid was constructed by inserting the 4,120-bp BamHI fragment obtained from the plasmid pGDA1 into the BamHI site of the C. albicans p1041 plasmid (23). Although p1041 was described as an integrative plasmid, we strongly suspect that it integrated into the genome of the mutant gda1 strains because we could neither induce plasmid loss nor recover plasmid from the reconstituted strains.
Complementation analysis.
To express CaGDA1 in S. cerevisiae gda1Δ, the p426-CaGDA1 plasmid was constructed. The CaGDA1 open reading frame was amplified by utilizing pGDA1 as a template with S (5′-GGATTCGTTTCATAAATGATAAACCC) and A (5′-CGTAATATGTTTTGAAATTAAAACTTC) as primers. The amplified fragment was cloned into the pCRII-TOPO vector (Invitrogen), and the resulting plasmid, pTCaG, was sequenced. The CaGDA1 open reading frame was subcloned from pTCaG to p426 (39) using XhoI and KpnI restriction sites for 5′ and 3′ ends, respectively. The S. cerevisiae null mutant was then transformed with p426-CaGDA1 for functional analysis experiments.
Production of antibodies against CaGda1p.
The pMAL Protein Fusion and Purification system (New England Biolabs) was used for expressing and purifying a fragment of CaGda1p. The EcoRI fragment corresponding to nucleotides +579 to +1436 relative to CaGDA1 ATG was subcloned from pGDA1 to pMAL-c2X. This construction resulted in a fusion protein between the 5′ end of the malE gene and the fragment from CaGDA1. The resulting plasmid, pMALGDA1, was then used for production of recombinant protein (maltose binding protein) according to the manufacturers' instructions. About 2 mg of fusion protein was sent to Lampire Biologicals for the production of rabbit polyclonal antibodies.
Preparation of vesicle fractions.
Vesicle fractions were prepared from yeast cells (1.5 liters) grown to exponential phase in Lee Medium and from hypha cells induced by a change in the pH of Lee Medium over a 16-h period. After being washed with cold l0 mM sodium azide as previously described (2), cells were resuspended in spheroplast buffer (50 mM potassium phosphate [pH 7.5], 1.4 M sorbitol, 10 mM sodium azide, 0.3% β-mercaptoethanol) containing 0.5 mg of Zymolyase 100T (Seikagaku)/ml and incubated at 37°C for 40 min. The spheroplast suspension was centrifuged, and the pellet was washed with buffer 1 (10 mM triethanolamine acetic acid [pH 7.2], 0.8 M sorbitol, 1 mM EDTA, 1 μg of leupeptin/ml, 0.5 mM phenylmethylsulfonyl fluoride) and resuspended in the same buffer. Spheroplasts were then broken by passage through a narrow-bore 10-ml glass pipette, diluted in membrane buffer, and centrifuged at 1,000 × g for 10 min to obtain P1 “The supernatant was centrifuged at 100,000 × g for 30 min to obtain P100” Vesicles were stored at −70°C. Protein was measured using the BCA method (Bio-Rad).
Nucleotide diphosphate hydrolysis assay.
Hydrolysis of GDP, UDP, and ADP was measured in vesicle fractions as previously described (59) in buffer containing 0.2 M imidazole [pH 7.5], 10 mM CaC12 or 10 mM MnC12, 0.1% Triton X-100, and 2 mM GDP, UDP, or ADP. One hundred microliters of this solution, containing 5 to 10 μg of sample protein, was incubated at 30°C for 30 min. The reaction was stopped by transferring the tubes to ice and adding 10 μl of 10% sodium dodecyl sulfate (SDS). To determine the amount of phosphate released during hydrolysis, 200 μl of water and 700 μl of AMES reagent (1:6 mixture of 10% ascorbic acid and 0.42% ammonium molybdate in 1 N sulfuric acid) were added to each tube; following incubation at 45°C for 20 min, absorbance was measured at 660 nm.
α-1,2-mannosyltransferase assays.
Reactions were performed in a 50-μl final volume containing 2 to 4 μg of membrane protein, 50 mM HEPES (pH 7.2), 0.1% Triton X-100, 10 mM MnCl2, and 10 to 200 μM GDP- [3H]mannose (0.1 μCi). α-Methyl-d-mannonoside (10 mM) was added as an exogenous acceptor. After incubations at 30°C for 10 min, reactions were stopped by adding 0.5 ml of 5 mM EDTA. Radioactive substrates were separated from acceptors by binding of the former to a 1-ml Dowex-1 column; the radioactivity in the eluate (containing the acceptor) was measured.
Sucrose gradient fractionation.
Wild-type cells exponentially grown overnight in 500 ml of YEPD were used. Spheroplasts were prepared as described above and suspended in buffer 1. The P1 supernatant fraction was prepared as described previously, and aliquots (15 ml) were placed on top of two 30-ml preformed, 25 to 50% continuous sucrose gradients in Beckman SW28 centrifuge tubes. The sucrose solutions contained 1 mM MgCl2 and 10 mM triethanolamine acetic acid (pH 7.2). Gradients were centrifuged at 4°C for 90 min, at 27,000 rpm in an L8-90 Beckman Ultracentrifuge as described previously. Ten 2.5-ml fractions were collected from the top of each gradient, diluted, and centrifuged at 100,000 × g for 30 min; pellets were resuspended in buffer 1 and kept at −70°C. The α-1,2-mannosyltransferase assay was used to detect Golgi enrichment in fractions as reported previously, except that 5 to 10 μl of each fraction was used for the reactions. In order to measure endoplasmic reticulum (ER) enrichment in the fractions, NADPH cytochrome c reductase was assayed as described previously (32).
O-linked carbohydrate analysis.
The method of Haselbeck and Tanner (24) was followed for the isolation of total O-linked carbohydrates from 2-3H mannose (18 Ci/mmol; New England Nuclear [NEN]) radiolabeled cells. β-Elimination was achieved in 0.1 M NaOH for 24 h at room temperature, after which the reaction was stopped by addition of HCl to a final concentration of 0.15 M and the protein was removed by centrifugation. Radiolabeled species in the supernatant were subjected to thin-layer chromatography on Silica Gel G plates (Merck) with two ascents, in ethyl acetate-butanol-acetic acid-water (3:4:2.5:4). The thin-layer chromatograms were treated with EN3HANCE reagent (NEN) for fluorography and exposed to Kodak X-OMAT X-ray film at −70°C. Biogel-P4 chromatography was performed as described by Ferguson (18).
Assay for in situ acid phosphatase activity.
Acid phosphatase was analyzed as described by Schweingruber et al. (47) with some modifications. The cells were grown in 50 ml of Lee medium to log phase (optical density at 600 nm [OD600], 1.5), and acid phosphatase activity was induced by overnight incubation in the same medium, except that H2PO4 was replaced by KCl at the same concentration. Cells were collected by centrifugation, washed with water, and suspended in 240 μl of ice-cold lysis buffer (0.05 M sodium citrate [pH 5.5], 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride, 0.1 mM dithiothreitol, and 10% glycerol). Cell lysates were prepared by vortexing for 4 min with 0.5-mm-diameter glass beads at 4°C. After addition of 120 μl of lysis buffer, the lysates were recovered and centrifuged at 14,000 × g for 10 min (two times). Ten- to thirty-microliter aliquots were mixed with bromophenol blue (final concentration, 0.01%) and 15% glycerol and subjected to electrophoresis on a 5% native polyacrylamide gel. The upper running buffer contained 5.16 g of Tris and 3.48 g of glycine/liter, and the lower buffer contained 14.5 g of Tris and 60 ml of 1 M HCl/liter. Acid phosphatase activity in gels was detected as described previously (47). For enzymatic deglycosylation, 500 U of endoglycosidase H (Endo H) (New England Biolabs) was added to the samples (without heat or detergent) and incubated for 18 h at 37°C.
Zymolyase sensitivity phenotypic test.
Cultures of the wild-type (CAI4), heterozygous (BAB2), and homozygous (BAB4) C. albicans strains were grown in YEPD medium until the exponential phase. Cells were washed twice in water and resuspended in 10 mM Tris-HCl (pH 7.5)-0.3% β-mercaptoethanol. Approximately 2.5 × 107 cells were resuspended in the same buffer containing Zymolyase 100T at a concentration of 0.01 mg/ml. The optical density at 600 nm was measured at the start of the incubation and every 20 min thereafter. The decreased in optical density reflected the portion of cells that had lysed.
NaCl, Calcofluor White, caffeine, SDS, sodium orthovanadate, and hygromycin B sensitivities.
Methods for testing the C. albicans strains were similar for all the effectors. Cultures were grown in 100 ml of YEPD medium with 1% glucose until the exponential phase and diluted to an OD600 of 0.1. Four microliters of pure and 1/5 serial dilutions of each cell culture were spotted onto YEPD plates containing NaCl (0.5 to 1.5 M), Calcofluor White (10 to 25 μg/ml), caffeine (5 to 15 mM), SDS (0.005% to 0.05%), sodium orthovanadate (10 to 20 mM), and hygromycin B (100 to 300 μg/ml). Differences in growth were recorded after incubation of the plates at 28°C for 72 h.
Cell wall analysis.
For the analysis of cell wall carbohydrate, C. albicans yeast cells (100-ml cultures in 0.1% Glc YEPD) were labeled with 50 μCi of [U14C] glucose (310 mCi/mmol; NEN) during a 24-h period. Cell wall polysaccharides were fractionated and quantified as previously described (15). Chitin was measured enzymatically according to the method of Bulawa (9).
Alcian blue binding assay.
Alcian blue binding assays were carried out using the method of Odani et al. (41) with minor modifications. A standard curve was created by making a serial dilution of 0.1% alcian blue 8GX (Electron Microscopy Sciences) in 0.02 N HCl, measuring the absorbance at 600 nm, and plotting the OD600 versus micrograms of alcian blue. Five milliliters of cells at an OD600 of 1 (cells grown in YEPD until exponential phase) was washed once with 2 ml of 0.02 N HCl and resuspended in 1 ml of staining solution (0.005% alcian blue in 0.02 N HCl; a total of 50 μg in the staining solution). Cells were allowed to stand for 10 min in the tubes and then centrifuged for 3 min, and the OD600 of the supernatants was measured. The amount of dye (x μg) in the supernatant fluid was determined using the standard curve generated above. The amount of dye bound to the cells was calculated as 50 μg minus x μg.
Cell wall phosphate determination.
Phosphate determination assays were carried out using a published procedure (55). Cells grown until exponential phase in YEPD were centrifuged, resuspended in water, and broken with glass beads. The lysate was centrifuged at 900 × g for 10 min, and the cell wall pellet was washed twice with water. Approximately 15 mg (wet weight) of cell wall was resuspended in 300 μl of 10% Mg(NO3)2 · 6H20 in ethanol; the mixture was evaporated to dryness inside a 13- by 100-mm Pyrex test tube over a strong flame with rapid shaking and further heated in the flame until the brown fumes had disappeared. After the tube had cooled, 0.3 ml of 1 N HCl was added. The tube was capped with a marble and heated in a boiling bath for 15 min to hydrolyze to inorganic phosphate any pyrophosphate formed in the ashing procedure. The inorganic phosphate was then measured with the Ames method, as we described for the NDPase assay.
Adherence to epithelial cells.
Human cervical epithelial cells (HeLa) were grown to confluency in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 100 μg of ciprofloxacin/ml at 37°C (5% CO2). Monolayers established in six-well culture dishes were used for adhesion studies. Adhesion was determined according to the method of Timpel et al. (55). Briefly, monolayers were washed twice with 2 ml of Dulbecco's phosphate buffered saline (DPBS), overlaid with either 75 or 150 individual C. albicans cells in 1 ml of DPBS, and incubated at 37°C for 45 min in an atmosphere of air containing 5% CO2. Following the incubation, monolayers were washed twice with 2 ml of warm DPBS to remove nonadhering cells; the monolayer in each well was then covered by 2 ml of YEPD agar (1% agar). Yeast colonies appearing after 48 h of growth at 28°C were counted (each colony is assumed to be derived from a single cell). The exact amount of fungal cells (100%) applied to the monolayers was determined by direct plating on YEPD. Adherence was determined as the percentage of fungal cells attached to monolayers of HeLa cells.
Southern blot analysis.
Genomic DNA was prepared using the DNeasy Kit (QIAGEN) following the manufacturer's instructions. About 30 μg of genomic DNA was digested with BclI and electrophoresed through an 0.8% agarose gel. The fractionated DNA was transferred to positively charged nylon membranes (Hybond N+; Amersham), and the membranes were fixed by UV radiation. Prehybridization, hybridization, and labeling of the probe were done following the specifications of the DIG Luminescent labeling and detection kit (Roche).
Western blot analysis.
Protein fractions were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membranes (Bio-Rad). Proteins were visualized by standard procedures after reaction with the Renaissance chemiluminescence detection kit (NEN Life Science) according to the manufacturer's instructions. Polyclonal antibodies against S. cerevisiae chitinase and CPY were kindly provided by C. Specht and R. Gilmore and used at a final dilution of 1:3,000 and 1:2,000, respectively. Polyclonal anti-CaGda1p antibody was used at 1:3,000.
RESULTS
Isolation of a Golgi apyrase from C. albicans (CaGDA1).
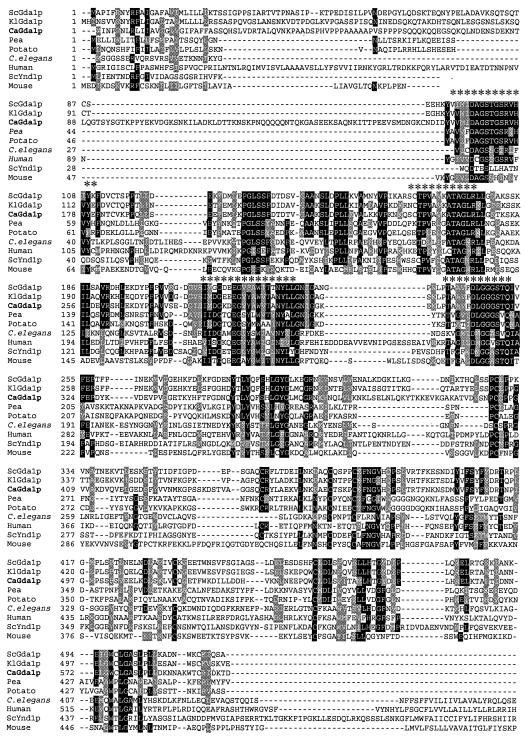
A 4.2-kb clone containing the complete CaGDA1 gene was obtained by hybridization of a partial BamHI library with a fragment of the CaGDA1 gene. This fragment was isolated by PCR using oligonucleotides designed on the basis of sequences present in the C. albicans database (http://www.sequence.stanford.edu/group/candida). The sequence of the CaGDA1 gene was determined using standard primers and sequence-specific oligonucleotides. This sequence was 100% identical to the CaGDA1 sequence that later appeared in the C. albicans genome project (and has EMBL accession No. AJ421721). Plasmid pGDA1 contained a single open reading frame of 1,800 bp. The predicted 599-amino-acid protein was 61% identical to S. cerevisiae ScGda1p. Interestingly, CaGda1p showed no potential N-glycosylation sites. The Kyte-Doolittle algorithm (not shown) revealed a single hydrophobic domain close to the amino terminus, consistent with a type II membrane protein, like the S. cerevisiae counterpart. The Candida GDPase contains a unique sequence in the stem region, between amino acids 60 and 100, predicted with high probability to adopt a coil-coil conformation that favors dimerization (44). In spite of not having that sequence, ScGda1p has been shown to be active as a homodimer in situ (5). Alignment of the CaGda1p with other proteins in the data bank showed structural similarity to a family of apyrases, sharing four conserved apyrase motifs (Fig. 1).
FIG. 1.
Multiple alignment of proteins related to Cagda1p. The BOXSHADE program was used to compare sequences of proteins, from fungi to mammals. Identical residues are shaded in black, and conserved residues are shaded in gray. Asterisks indicate apyrase conserved regions. GenBank accession numbers for the sequences used in the alignment are as follows: S. cerevisiae GDPase, NP_010872; K. lactis GDPase, AJ401304; C. albicans GDPase, AJ421721; Pea NTPase, BAA89275; potato ATPase, P80595; human CD39, NP_001767; mouse CD39, NP_033978; Caenorhabditis elegans (C. elegans), Q18411; S. cerevisiae YND1, NP_010920; human Golgi UDPase, AF016032.
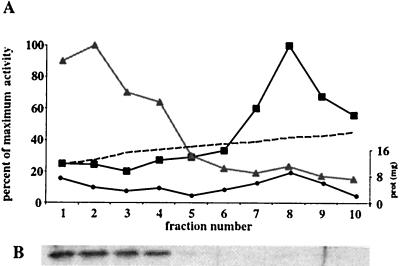
To determine whether the CaGda1p is localized to the Golgi apparatus, a mixed vesicle population was subjected to centrifugation through a continuous sucrose gradient, and its distribution was compared with that of ER and Golgi markers. Fractions enriched in an ER marker, NADPH cytochrome c reductase, migrated in the heavy end of the gradient (Fig. 2). CaGda1p was most abundant in lighter-density fractions migrating between 27 and 36% sucrose; the same fractions were enriched in α-1,2-mannosyltransferase, commonly used as Golgi marker (2).
FIG. 2.
CaGda1p is localized in the Golgi apparatus. (A) Enzyme activities were assayed after velocity sucrose gradient centrifugation as described in Materials and Methods. The maximum specific activity for NADPH cytochrome c reductase (ER marker) was 559 mmol/min, while the maximum activity for α-1,2-mannosyltransferase (Golgi marker) was 1.23 mmol/min. Dashed line, sucrose concentration; black dots, amount of protein; black squares, NADPH cytochrome c reductase; gray triangles, α-1,2-mannosyltransferase. (B) Western blotting against CaGda1p was performed as described in Materials and Methods.
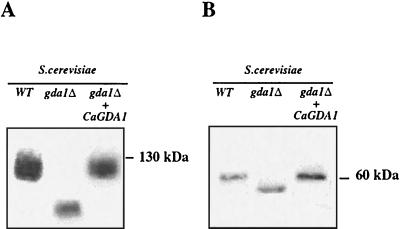
We next determined whether the CaGda1p was able to complement S. cerevisiae gda1Δ by transformation of this strain with the plasmid p426-CaGDA1. We previously showed with S. cerevisiae gda1Δ cells that the more rapid migration of chitinase on SDS gels, relative to the wild type, was the result of decreased O-mannosylation (2). In transformants carrying the CaGDA1 gene, the wild-type migration of chitinase was restored, demonstrating the functional homology between the two genes (Fig. 3A). CaGda1p was also able to fully complement the N-glycosylation defect of the S. cerevisiae gda1Δ strains as monitored by the electrophoretic migration of the N-glycosylated vacuolar glycoprotein CPY (Fig. 3B).
FIG. 3.
CaGDA1 complements S. cerevisiae glycosylation phenotypes. SDS-polyacrylamide gel electrophoresis separation and Western blotting with antibodies against secreted chitinase, an O-glycosylation reporter (A), and carboxypeptidase-Y, an N-glycosylation reporter (B).
Disruption of GDA1 alleles.
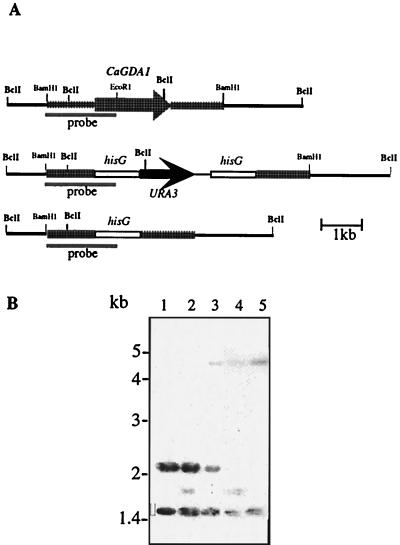
The Ura-blaster technique (20) was used to disrupt both alleles of CaGDA1 in the C. albicans GDA1/GDA1 strain CAI4. Chromosomal DNA of the wild type and transformants was digested with BclI and analyzed by Southern hybridization using a 1.7-kb BamHI-EcoRI-specific probe. The wild-type GDA1 allele displayed 1.5- and 2.1-kb bands (Fig. 4B, lane 1). The 1.5-kb band originated with the hybridization of the probe to sequences upstream of the GDA1 locus; that area was expected to remain unchanged during the disruption process (Fig. 4B, lanes 1 to 5). A new 1.8-kb band appeared in transformants with the Ura blaster integrated into one allele (Fig. 4B, lane 2). After selection on 5-fluoroorotic acid-containing medium, the loss of the URA3 gene and one copy of the hisG element resulted in a 4.8-kb band (Fig. 4B, lane 3). The remaining intact GDA1 allele was disrupted similarly, leading to homozygous gda1::hisG/gda1Δ::hisG-URA3-hisG strains (Fig. 4B, lane 4) and corresponding Ura− derivatives (Fig. 4B, lane 5). The phenotypes reported below were observed in at least two independently isolated disrupted or reconstituted strains.
FIG. 4.
Deletion of CaGDA1 alleles. (A) Structure of different alleles. The wild-type GDA1 gene and the alleles disrupted by the hisG-URA3-hisG cassette or by hisG alone are shown. (B) Southern blot analysis of genomic DNA was performed with the following strains digested with BclI. Lane 1, CAI4 (Δura3::imm434/ura3::imm434); lane 2, BAB1 (ura3/ura3 GDA1/gda1::hisG-URA3-hisG); lane 3, BAB2 (ura3/ura3 GDA1/Δgda1::hisG); lane 4, BAB3 (ura3/ura3gda1::hisG-URA3-hisG/Δgda1::hisG); and lane 5, BAB4 (ura3/ura3 Δgda1::hisG/Δgda1::hisG). The 1,727-bp BamHI-EcoRI fragment was used as a probe.
Cagda1 strains are defective in GDPase in vitro activity.
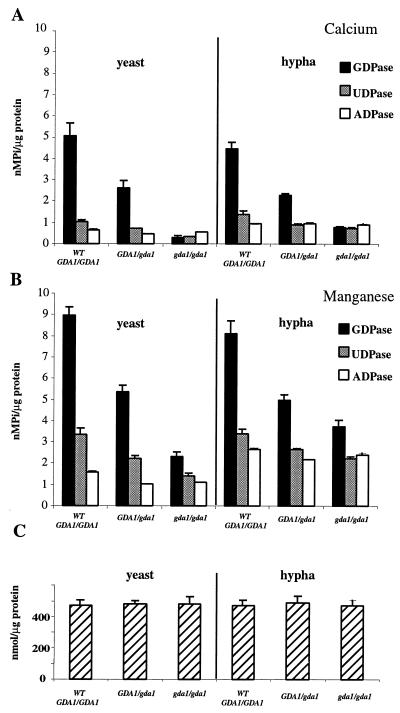
To determine whether CaGDA1 encodes a GDPase, we measured the abilities of membrane fractions isolated from the wild-type strain, CAI4, as well as heterozygous and homozygous mutant strains, BAB2 and BAB4, to hydrolyze GDP (Fig. 5). Membranes prepared from the homozygous mutant strains showed a 90% decrease in their ability to hydrolyze GDP in the presence of calcium with respect to wild-type strains (Fig. 5A). Activities of the hemizygous GDA1/gda1 strains were intermediate, demonstrating a clear gene dosage effect. Concomitantly, the UDPase activity also seemed to decrease while that of ADPase did not change, as we had previously found with S. cerevisiae gda1 strains (2) (Fig. 5). The GDPase activity in C. albicans doubled in the presence of manganese (Fig. 5B), differing from S. cerevisiae, where calcium is the preferred cation for GDP hydrolysis (59). Comparing the activities of yeast and hypha forms, we observed that the GDPase and UDPase activities are quite similar whereas the ADPase activity is slightly increased in the hypha. α-1,2-mannosyltranferase, a Golgi enzyme used as a control, was similar in all the membrane preparations (Fig. 5C).
FIG. 5.
GDPase, UDPase, and ADPase activities in membranes of yeast and hypha forms of C. albicans in the presence of Ca2+ (A) and Mn2+ (B). (C) α-1,2-mannosyltransferase activity. Results are averages of three independent determinations; bars indicate standard deviations.
CaGda1p has a role in O-glycosylation.
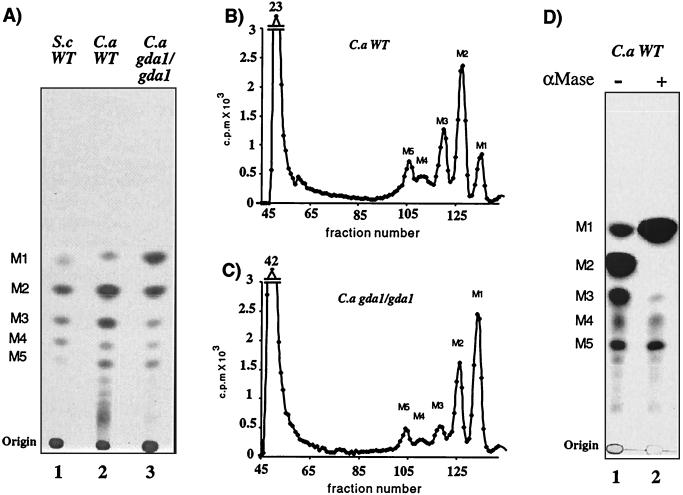
S. cerevisiae gda1 mutants have severe defects in mannosylation of O- and N-linked glycoproteins and glycosphingolipids (2). In order to determine whether CaGDA1 mutants have defects in O-glycosylation, we labeled wild-type and mutant cells with 2-3H mannose, released their O-linked carbohydrate chains by β-elimination, and analyzed them by thin-layer chromatography (Fig. 6A). The oligosaccharide pattern from the C. albicans wild-type strains showed five major species containing between one and five mannose residues (M1 to M5) (Fig. 6A, lane 2) as in S. cerevisiae (Fig. 6A, lane 1). Some minor slower-migrating species, not present in S. cerevisiae, were seen in C. albicans that could correspond to chains with six or seven mannose units according to Hayette et al. (25). These results differ from the trimannose O-linked mannan structure reported by Buurman et al. (11) and prompted us to look into other C. albicans wild-type strains. We observed the same pattern of 5- to 7-residue-long O-linked mannose chains not only for the CAI4 strain and its parental strain SC5314 but also for an unrelated wild-type strain, ATCC 10261 (data not shown), strongly suggesting that these chain lengths are a characteristic of C. albicans.
FIG. 6.
O-glycosylation: total alkali-releasable saccharides. Base-sensitive oligosaccharides from various strains radiolabeled with [2-3H]mannose were separated by thin-layer chromatography (butanol, ethanol, water [5:3:2]) and subjected to autoradiography (50,000 cpm/lane) (A and D) or Biogel P4 chromatography (0.8 × 106 and 1 × 106 cpm) (B and C). S.c WT is the S. cerevisiae wild-type strain G2-6; C.a WT is the C. albicans wild-type strain CAI4. C.a gda1/gda1 is C. albicans strain BAB4. αMase is Jack bean α-mannosidase.
C. albicans gda1/gda1 strains showed a partial block in their ability to extend O-linked mannan chains manifested by accumulation of M1, slight reduction of M2, and a drastic reduction of M3, while the amount M4 and M5 were quite similar to amounts with the wild type. Further analysis of products using a Biogel-P4 column allowed us to fractionate by size five clear peaks in wild-type and mutant strains and quantify the relative abundance of each O-liked mannose chain (Fig. 6B and C). While in the wild-type M1 represents 13% of the total O-linked pool (M1 to M5), in the gda1/gda1 mutant M1 represents 42%, showing that a high proportion of the initiated chains cannot be further extended. The most severe reduction was found in chains with three mannoses (M3), with gda1/gda1 strains having less than half of wild-type levels. The high-molecular-weight material remaining at the origin with thin-layer chromatography and appearing at the void volume of Biogel-P4 columns (Fig. 6A, B, and C) might represent pir-like proteins that are covalently attached to the cell wall and can be released by mild alkali treatment (30).
C. albicans O-linked chains were treated with Jack bean α-mannosidase in order to determine the glycosydic linkage between the mannose units (Fig. 6D). All of the most abundant M2 chains, as well as most of the M3 chains, were converted to free mannose. Neither prolonged incubation nor addition of fresh α-mannosidase could convert the small remnant to mannose. These observation, together with the fact that more-abundant M2 chains were completely digested in the same incubation, strongly suggest that C. albicans has at least two kinds of O-linked chains. Surprisingly, M4 and M5 chains were resistant to the exomannosidase attack, indicating that the mannose at their nonreducing end is not in α-linkage. S. cerevisiae has only α-linked mannose residues in its O-glycans.
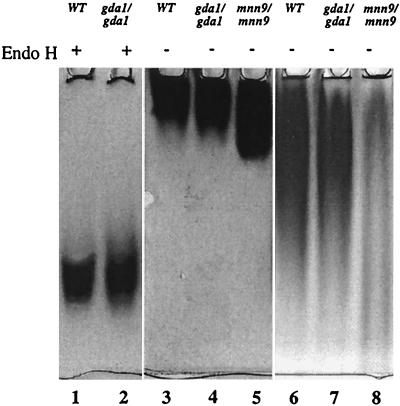
We took advantage of the fact that for C. albicans 88% of cell wall carbohydrates are N-linked (17), and we decided to analyze the amount of cell wall mannan in order to monitor possible N-glycosylation defects. Unlike the case with the S. cerevisiae gda1Δ strain, the amount of mannoproteins in the C. albicans gda1/gda1 mutants was unchanged from that in wild-type cells. In both cases mannoproteins constituted approximately 38% of total cell wall polymers. We also looked, by activity staining on native gels, at the size of the heavily N-glycosylated and secreted acid phosphatase (46, 47). We found no difference in the migration of acid phosphatase secreted by the wild type and by gda1 mutants (Fig. 7, lane 3 versus lane 4). Even when the gel was run for 20 h in order to allow detection of minor changes, no difference was found (Fig. 7, lane 6 versus lane 7). The MNN9 mutant, defective in outer chain glycosylation, was used as a positive control (Fig. 7, lanes 5 and 8). Acid phosphatase can be also used as a reporter of O-glycosylation after enzymatic removal of N-linked chains (40). We wanted to see if the general defect in O-glycosylation that we detected was evident also in this individual protein. After treatment with Endo H, no difference in the migration of the acid phosphatase produced by the wild type and by gda1 mutants was detected (Fig. 7, lane 1 versus lane 2). We see two possibilities: either the O-glycosylation of this particular reporter is not affected, or the N-deglycosylation done under native conditions was extensive but not total and small differences are masked. The absence of CaGda1p does not lead to severe truncation of large N-linked oligosaccharides, as reported for K. lactis gda1 mutants, which are affected in O- but not in N-glycosylation (35). Although Cagda1 mutants did not seem to display a defect in N-glycosylation, the CaGDA1 gene complements defects in N-glycosylation of S. cerevisiae gda1 null mutants (Fig. 3B).
FIG. 7.
N-glycosylation: activity staining of acid phosphatase. Twenty-microliter samples from C. albicans wild type (WT) (CAI4), gda1/gda1 (BAB4), and mnn9/mnn9 (SS19-4), prepared as described in Materials and Methods, were run in a 5% native gel for either 2 h (lanes 1 to 5) or 20 h (lanes 6 to 8) at 100 V. Endoglycosidase H (Endo H)-treated samples are indicated by +.
CaGda1p has a role in cell wall morphogenesis
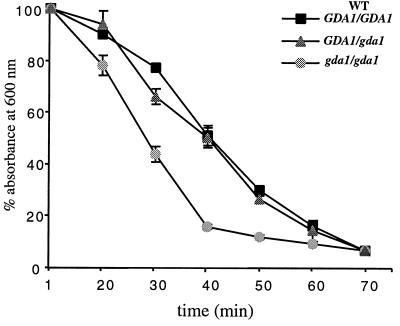
In order to determine whether the absence of CaGda1p affects cell wall morphogenesis in C. albicans, we initially compared the sensitivities of the wild type and mutants to different effectors. Cagda1/Cagda1 mutants showed only a very slight increase in sensitivity toward Calcofluor white and hygromycin B, but not toward NaCl or caffeine (data not shown). We also analyzed the sensitivity of the Cagda1/Cagda1 mutant toward cell lysis induced by a β-1,3-glucanase preparation (Zymolyase). Null mutant cells were more sensitive to enzymatic lysis than the wild type and the hemizygous strains when yeast cells were grown either at 23 oC (Fig. 8), or 37°C (data not shown). Analysis of the cell wall composition of wild-type and hemizygous strains showed that the amounts of glucan and mannoprotein in wild-type and homozygous mutant strains were similar, approximately 45% glucan and 38% mannoprotein. The only quantitative difference was observed at the level of chitin: in the gda1/gda1 mutant it was 8.71 ± 0.8 nmol GlcNAc/mg of cells (wt/wt), nearly double that of the wild type (3.77 ± 0.2 nmol GlcNAc/mg of cells [wt/wt]) and hemizygous strains (4.01 ± 0.5 nmol GlcNAc/mg of cells [wt/wt]).
FIG. 8.
C. albicans cell lysis sensitivity following treatment with β-1,3-glucanase. Cells were treated with Zymolyase for different times, and lysis was measured by the decrease in the OD600. Results are means ± standard deviations from three independent experiments.
CaGda1p affects cell surface charge and phosphate content.
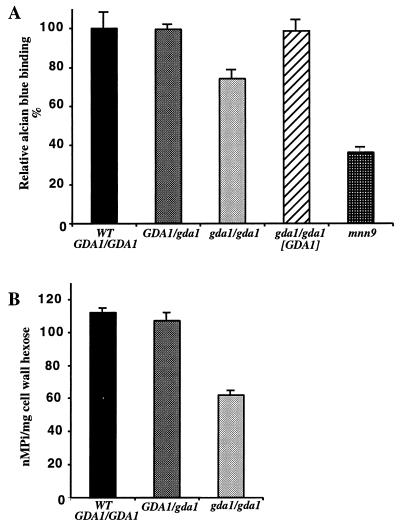
Cell surface hydrophobicity is considered to be an important virulence determinant of C. albicans, and it has been linked to the level of cell wall protein glycosylation (37). In S. cerevisiae, mannosylphosphate gives a net negative charge to cell surface mannoproteins which in turn allows binding, under acidic conditions, to the dye alcian blue (41). We therefore determined the level of alcian blue binding for wild-type, hemizygous, and mutant strains of C. albicans and also for the Camnn9 mutant, which is severely affected in glycosylation (50). Binding of the dye to gda1/gda1 cells was 70% of that of the parental strain, whereas for hemizygous and reconstituted strains, it was the same (Fig. 9A). To determine if these differences in binding corresponded to differences in phosphate content, cell walls were isolated and the level of phosphate was measured (Fig. 9B). The amount of phosphate in the cell wall of gda1/gda1 strains was nearly half of that of the wild-type cells, completely explaining the differential binding of alcian blue.
FIG. 9.
Cell surface charge in C. albicans strains. (A) Alcian blue binding assays. Relative dye binding was calculated as the percentage of dye bound compared with results for the parental strain (CAI4). (B) Cell wall phosphate content. Results are average of three independent determinations; bars indicate standard deviations. WT, wild type.
CaGda1p is not required for adhesion to epithelial cells.
Several studies have shown that mannoproteins are necessary for adhesion of C. albicans to the surfaces of host cells (38, 51). Because CaGDA1 mutants showed less cell surface charge and a severe defect in O-glycosylation, we measured their adherence to monolayers of HeLa cells. In these assays, yeast cells were placed on an epithelial monolayer for 45 min followed by removal of nonadhering cells by washing. The number of adhering cells was determined by growth in a YEPD agar overlay after washing. Adherence was determined as the percentage of fungal cells attached to monolayers of HeLa cells. No significant difference was found between the adherence of wild-type Candida cells (49% ± 8%) and gda1/gda1 mutant cells (47% ± 10%). Each experiment was done in triplicate, with two starting amounts of fungal cells.
CaGda1p is required for hyphal morphogenesis.
It has been reported that the C. albicans Capmt1 and Capmt6 mutants, both affected in O-glycosylation, are partially defective in hyphal development (55, 56). To determine whether CaGda1p exerts an effect on germ tube formation, experiments of hyphal induction in solid and liquid media were carried out. With the latter we also analyzed the effect of different stimuli on filamentous growth: pH, temperature, serum, and N-acetylglucosamine.
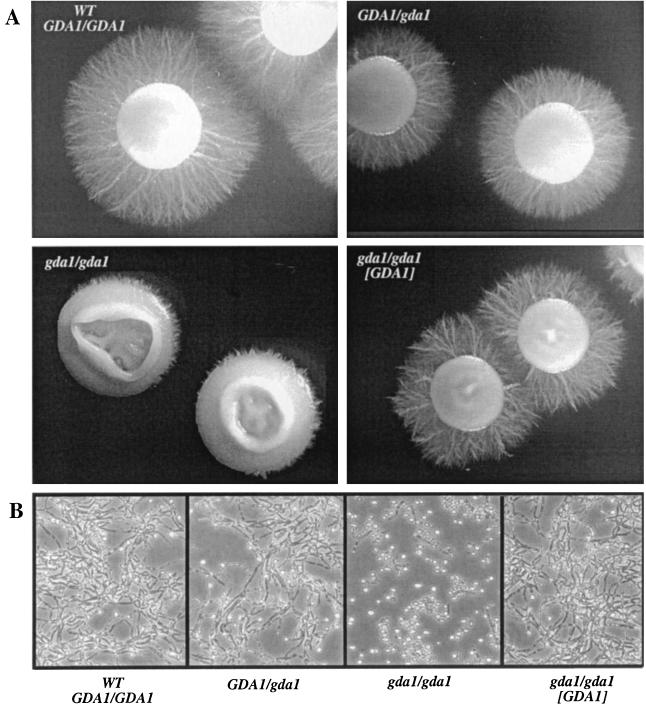
For the analysis of hyphal formation in solid medium, C. albicans strains were grown in yeast form in liquid Lee medium and then plated onto solid Lee medium with mannitol. The appearance of colonies after 6 days of growth at 37°C can be seen in Fig. 10A. As expected, the C. albicans wild-type strain CAI4 formed colonies with long hyphae. In contrast, the mutant BAB4 showed a clear defect in filamentation. The hemizygous and reconstituted strains BAB2 and BAB4-GDA displayed an intermediate phenotype (Fig. 10A). In liquid medium the gda1/gda1 mutants grow at the same rate as the wild type but show a partial block in hyphal formation following changes in temperature (not shown) and pH (Fig. 10B). The mutants formed normal hyphae in the presence of serum and N-acetylglucosamine (not shown).
FIG. 10.
Hypha formation of C. albicans strains. (A) Colonies grown for 6 days at 37°C in Lee solid medium; same magnification for all panels. (B) Hypha induction in liquid medium; cells were grown in Lee medium, pH 4.5, at 37°C and then incubated under conditions that promote hyphal growth in Lee medium at pH 6.8.
DISCUSSION
In this work we addressed the consequences of the elimination of the guanosine diphosphohydrolase of C. albicans, encoded by CaGDA1, on mannosylation, cell wall construction, and filamentation. Previous studies of the S. cerevisiae Golgi Gda1p provided strong evidence for the role of this enzyme in the nucleotide sugar transport/antiport cycle. After protein and lipid mannosylation, the reaction product GDP is converted by Gda1p to GMP, which can exit the lumen in an exchange, coupled to the entry of additional GDP-mannose from the cytosol (2). This cycle has a pivotal function in regulating posttranslational modifications that occur in the lumen of the Golgi apparatus of all eukaryotes (27).
CaGDA1 was cloned based on its similarity to the ScGDA1 gene and was shown to be its functional homologue. CaGda1p, like ScGda1p and KlGda1p, is localized to the Golgi apparatus and is also predicted to be a type II membrane protein with a short NH2-terminal cytosolic tail and the bulk of the catalytic domain facing the lumen of the organelle (1, 35). CaGda1p is different from the K. lactis and S. cerevisiae GDPases in that it contains no N-linked glycosylation sites. Gda1p is responsible for about 90% of the membrane-bound, calcium-dependent, in vitro GDPase activity of C. albicans yeast and hyphal forms as well as that of other yeasts, such as K. lactis and S. cerevisiae. Rather active manganese-dependent GDPases remain in the gda1/gda1 strains, as indicated by a 50% decrease in the GDP hydrolysis capabilities in hyphae and a 70% decrease in yeast forms of the homozygous mutant. CaGda1p appears to be an active UDPase in vitro, and manganese greatly stimulates the UDP hydrolysis activity of these and other enzyme(s) remaining in the Candida gda1/gda1 strains; again the phenomenon is more evident with hyphae, suggesting the existence of hypha-specific GDPases and/or UDPases not yet identified.
Only two nucleotide diphosphatases (GDA1 and YND1) have been found in the entire genome of S. cerevisiae, and both have their active sites facing the Golgi lumen (1, 60). The gda1Δ ynd1Δ double deletion has a synthetic effect on glycosylation, cell growth, and cell shape phenotype (21). Ynd1p appears to be regulated by the binding of its COOH-terminal cytoplasmic domain to an activator subunit of vacuolar H+ ATPase (61). The precise relationship between Ynd1p, Gda1p, and glycosylation in the Golgi is not understood. Our data suggest that either the CaYnd1p enzyme (we have identified the YND1 Candida gene, which shows 46% identity with the S. cerevisiae gene [unpublished results]) remaining in Cagda1/Cagda1 strains is more active in the hyphae or that there are other membrane bound nucleoside diphosphases in C. albicans unknown at present. Investigating both possibilities will further our understanding of the relationship between glycosylation, wall construction, and filamentation.
Loss of CaGDA1 function leads to cell wall-associated defects and to “medium-conditional” loss of hyphal development. Defects in the cell wall were not gene dosage dependent. We observed a significant reduction in binding of the positively charged dye alcian blue that was completely accounted for by a reduction of cell wall phosphate content with Cagda1 mutants. Although in C. albicans negatively charged sialylated glycoconjugates have been proposed to exist (3), we found no evidence for them. Cell walls of gda1/gda1 mutants contained twice as much chitin as those of the wild type, providing independent evidence for cell surface alteration. Recent work on S. cerevisiae (43, 31) shows that stress to the cell wall resulting from mutations or from environmental factors often leads to strengthening of the wall by intercalation of a substantial amount of chitin fibers. Our results indicate that mechanisms for induction of stress-related chitin synthesis are also present in C. albicans. While mutations in GDA1 have consequences in the structure and composition of the cell wall of the three yeast species studied so far, the alterations are not identical. C. albicans and K. lactis gda1 mutants become hypersensitive to attack by β-1,3-glucanase, while S. cerevisiae gda1 mutants become glucanase resistant. The mechanism underlying the above changes in GDA1 null mutants from the three yeast species are not yet understood, but the differences among them highlight the importance of direct studies with C. albicans. On the other hand, in Camnn9 and Capmt1 mutants, which are defective in N- and O-glycosylation of proteins, respectively, enhanced levels of chitin together with increased coupling of cell wall proteins through β-1,6-glucan to chitin were observed (31). It is likely that C. albicans and S. cerevisiae respond to cell wall weakening in similar but not identical fashions.
It has been shown that the ability of C. albicans to switch from yeast to hyphal forms is important for pathogenicity (34). There is rapid reshaping and expansion of the cell wall during hyphal formation; for this reason, mutations in genes involved in cell wall construction have filamentation defects. Some of them are as follows: KRE9 required for β-1,6-glucan synthesis (36); PHR1 and PHR2, a pair of pH-regulated β-1,3-glucanosyltransferases responsible for the elongation of β-1,3-glucan (19); SRB1 encoding GDP-mannose pyrophosphorylase (58); MNN9, required for N-linked outer chain glycosylation (50); and PMT1 and PMT6, responsible for the initiation of O-linked mannan chains (55, 56). We thus examined whether Cagda1 mutants could undergo a yeast-hypha transition. Loss of CaGDA1 function blocked pH- and temperature-induced hyphal formation but had no effect on filamentation induced by serum or N-acetylglucosamine as the sole carbon source. A clear gene dosage effect was seen for this partial block in hyphal formation.
The morphogenetic switch from budding yeast to hyphal growth occurs in response to a variety of stimuli and growth conditions. Recently it has been shown that environmentally induced filamentous growth can occur with C. albicans even in the absence of EGF1, CPH1, and TUP1, providing evidence for a fourth regulatory pathway (8). It appears that genes turned on during filamentous growth do not respond to a central regulator; rather, they respond individually to at least four pathways that regulate filamentous growth (52, 33, 7), strongly suggesting that a network of signaling pathways extends down to target genes. Thus, even morphologically similar phenotypes may be different at the molecular level, especially at the cell wall (8). Serum still stimulates hyphal formation not only in Cagda1 mutants but also in Capmt1 O-glycosylation mutants, as well as in mutants defective in elements of a conserved mitogen-activated protein kinase signaling pathway, all of which manifest a partial block in filamentation (55). For this reason it was suggested that not morphogenesis per se but rather sufficient levels of an unidentified O-glycosylated protein critical for the mitogen-activated protein kinase signaling pathway that is operative in some media could be defective in pmt1 mutants (55). Similar reasoning could be applied to Cagda1 mutants, which are also defective in O-glycosylation.
The O-glycosylation defects in gda1 mutants are not the result of a single missing mannosyltransferase. Shorter O-linked mannan chains are a consequence of the reduced availability of the substrate GDP-mannose in the lumen of the Golgi. In the absence of Gda1p there is insufficient generation of the antiporter molecule GMP to sustain the nucleotide sugar transport cycle. We found a large increase in single O-linked mannose residues in the Cagda1 mutants, indicating that the O-linked mannan chains were initiated normally in the ER but that elongation in the Golgi failed. This was expected, since it is well established that transfer of the first O-linked mannose to serine and threonine by protein-O-mannosyltransferases occurs in the ER with dolichol-P-mannose as the donor (24). GDP-mannose is the direct donor for the subsequent mannose residues, which are added stepwise to complete O-linked chains in the lumen of the Golgi (42). Analyses of bulk cell mannoproteins of Cagda1 mutants also showed a small decrease in chains with two mannose residues, a severe decrease in chains with three, and normal amounts of longer chains with four and five mannose residues. It is possible that the Km for GDP-mannose of some mannosyltransferases could be very low. Therefore, the reactions catalyzed by these enzymes would occur at maximum velocity even with a limited supply of substrate.
It was surprising to find that gda1 mutants exhibited apparently normal N-glycosylation together with a significant decrease in cell wall phosphate content. Although the amounts of N-glycans present in wild-type and gda1 mutant strains are about the same, alteration in the structure of N-linked oligosaccharides cannot be ruled out. It has been recently shown that both, N- and O-linked glycosylation are affected by the loss of function of the essential Golgi apparatus GDP-mannose transporter, CaVRG4 (40). It is not clear why phenotypic consequences when the transport/antiport cycle that supplies GDP-mannose to the Golgi lumen is affected by reduced expression of the transporter (CaVrg4p) are different from when the same cycle is disturbed by the absence of one of the antiporter generating enzymes (CaGda1p). One possibility could be a differential compartmentalization within the Candida Golgi of Gda1p and other enzymes able to convert GDP to GMP, which could in turn locally drive the transport/antiport cycle to some extent.
β-1,2-linked oligomannosides were first described by Shibata et al. (49) as associated with C. albicans cell wall phosphopeptidomannan. Our data strongly suggest that O-linked chains with 4 and 5 mannose units have β-linked mannose at the reducing end. Recently phospholipomannan, a family of cell surface glycolipids, was found to contain long linear chains of β-1,2-linked mannose residues (57). Several groups have investigated the recognition of β-1,2-oligomannosides by the immune system in relation to the pathogenic character of C. albicans. These molecules have been shown to elicit specific antibodies in humans (45). Moreover, β-1,2-oligomannosides have been shown to act as C. albicans adhesins for macrophages and stimulate the production of tumor necrosis factor alpha (29). A complete study of the structure and biosynthesis of wall glycans will help in understanding the immunological properties of the cell wall and its relation to the pathogenesis of C. albicans infections.
Acknowledgments
We thank Gretchen Carney for expert typing and P. Berninsone and P. Robbins for helpful discussions. We also thank Stuart M. Levitz and Michael Mansour for their help with the cell adhesion studies.
D.U. is a recipient of a fellowship from the Pasteur Institue-Cenci Bolognetti foundation. This work was supported by NIH grants GM59773 to C.A., GM 30365 to C.B.H., DGICYT grant PM 98-0317 to A.D., and by grant no. 99196 from the Fulbright Commission for Cultural, Educational and Scientific Exchange between the United States and Spain to A.D. and C.B.H.
REFERENCES
- 1.Abeijon, C., P. Orlean, P. W. Robbins, and C. B. Hirschberg. 1989. Topography of glycosylation in yeast: characterization of GDPmannose transport and lumenal guanosine diphosphatase activities in Golgi-like vesicles. Proc. Natl. Acad. Sci. USA 86:6935-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeijon, C., K. Yanagisawa, E. C. Mandon, A. Hausler, K. Moremen, C. B. Hirschberg, and P. W. Robbins. 1993. Guanosine diphosphatase is required for protein and sphingolipid glycosylation in the Golgi lumen of Saccharomyces cerevisiae. J. Cell Biol. 122:307-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alviano, C. S., L. R. Travassos, and R. Schauer. 1999. Sialic acids in fungi: a minireview. Glycoconj. J. 16:545-554. [DOI] [PubMed] [Google Scholar]
- 4.Berninsone, P., H. Y. Hwang, I. Zemtseva, H. R. Horvitz, and C. B. Hirschberg. 2001. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N-acetylgalactosamine, and UDP-galactose. Proc. Natl. Acad. Sci. USA 98:3738-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berninsone, P., Z. Y. Lin, E. Kempner, and C. B. Hirschberg. 1995. Regulation of yeast Golgi glycosylation. Guanosine diphosphatase functions as a homodimer in the membrane. J. Biol. Chem. 270:14564-14567. [DOI] [PubMed] [Google Scholar]
- 6.Berninsone, P. M., and C. B. Hirschberg. 2000. Nucleotide sugar transporters of the Golgi apparatus. Curr. Opin. Struct. Biol. 10:542-547. [DOI] [PubMed] [Google Scholar]
- 7.Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105-109. [DOI] [PubMed] [Google Scholar]
- 8.Braun, B. R., and A. D. Johnson. 2000. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulawa, C. E. 1992. CSD2, CSD3, and CSD4, genes required for chitin synthesis in Saccharomyces cerevisiae: the CSD2 gene product is related to chitin synthases and to developmentally regulated proteins in Rhizobium species and Xenopus laevis. Mol. Cell. Biol. 12:1764-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burda, P., and M. Aebi. 1999. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta 1426:239-257. [DOI] [PubMed] [Google Scholar]
- 11.Buurman, E. T., C. Westwater, B. Hube, A. J. Brown, F. C. Odds, and N. A. Gow. 1998. Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 95:7670-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderone, R. A., and P. C. Braun. 1991. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 55:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capasso, J. M., and C. B. Hirschberg. 1984. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc. Natl. Acad. Sci. USA 81:7051-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanova, M., J. L. Lopez-Ribot, C. Monteagudo, A. Llombart-Bosch, R. Sentandreu, and J. P. Martinez. 1992. Identification of a 58-kilodalton cell surface fibrinogen-binding mannoprotein from Candida albicans. Infect. Immun. 60:4221-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro, C., J. C. Ribas, M. H. Valdivieso, R. Varona, F. del Rey, and A. Duran. 1995. Papulacandin B resistance in budding and fission yeasts: isolation and characterization of a gene involved in (1,3)beta-d-glucan synthesis in Saccharomyces cerevisiae. J. Bacteriol. 177:5732-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187-218. [DOI] [PubMed] [Google Scholar]
- 17.Elorza, M. V., A. Marcilla, and R. Sentandreu. 1988. Wall mannoproteins of the yeast and mycelial cells of Candida albicans: nature of the glycosidic bonds and polydispersity of their mannan moieties. J. Gen. Microbiol. 134:2393-2403. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson, M. A., P. Murray, H. Rutherford, and M. J. McConville. 1993. A simple purification of procyclic acidic repetitive protein and demonstration of a sialylated glycosyl-phosphatidylinositol membrane anchor. Biochem. J. 291:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonzi, W. A. 1999. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of beta-1,3- and beta-1,6-glucans. J. Bacteriol. 181:7070-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, X. D., V. Kaigorodov, and Y. Jigami. 1999. YND1, a homologue of GDA1, encodes membrane-bound apyrase required for Golgi N- and O-glycosylation in Saccharomyces cerevisiae. J. Biol. Chem. 274:21450-21456. [DOI] [PubMed] [Google Scholar]
- 22.Gemmill, T. R., and R. B. Trimble. 1999. Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim. Biophys. Acta 1426:227-237. [DOI] [PubMed] [Google Scholar]
- 23.Goshorn, A. K., S. M. Grindle, and S. Scherer. 1992. Gene isolation by complementation in Candida albicans and applications to physical and genetic mapping. Infect. Immun. 60:876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haselbeck, A., and W. Tanner. 1983. O-glycosylation in Saccharomyces cerevisiae is initiated at the endoplasmic reticulum. FEBS Lett. 158:335-338. [DOI] [PubMed] [Google Scholar]
- 25.Hayette, M. P., G. Strecker, C. Faille, D. Dive, D. Camus, D. W. Mackenzie, and D. Poulain. 1992. Presence of human antibodies reacting with Candida albicans O-linked oligomannosides revealed by using an enzyme-linked immunosorbent assay and neoglycolipids. J. Clin. Microbiol. 30:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschberg, C. B. 2001. Golgi nucleotide sugar transport and leukocyte adhesion deficiency II. J. Clin. Investig. 108:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschberg, C. B., P. W. Robbins, and C. Abeijon. 1998. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 67:49-69. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim, A. S., F. Mirbod, S. G. Filler, Y. Banno, G. T. Cole, Y. Kitajima, J. E. Edwards, Jr., Y. Nozawa, and M. A. Ghannoum. 1995. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect. Immun. 63:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jouault, T., C. Fradin, P. A. Trinel, A. Bernigaud, and D. Poulain. 1998. Early signal transduction induced by Candida albicans in macrophages through shedding of a glycolipid. J. Infect. Dis. 178:792-802. [DOI] [PubMed] [Google Scholar]
- 30.Kandasamy, R., G. Vediyappan, and W. L. Chaffin. 2000. Evidence for the presence of pir-like proteins in Candida albicans. FEMS Microbiol. Lett. 186:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapteyn, J. C., L. L. Hoyer, J. E. Hecht, W. H. Muller, A. Andel, A. J. Verkleij, M. Makarow, H. Van Den Ende, and F. M. Klis. 2000. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol. Microbiol. 35:601-611. [DOI] [PubMed] [Google Scholar]
- 32.Kubota, S., Y. Yoshida, H. Kumaoka, and A. Furumichi. 1977. Studies on the microsomal electron-transport system of anaerobically grown yeast. V. Purification and characterization of NADPH-cytochrome c reductase. J. Biochem (Tokyo) 81:197-205. [DOI] [PubMed] [Google Scholar]
- 33.Liu, H., J. Kohler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723-1726. [DOI] [PubMed] [Google Scholar]
- 34.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous Candida albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Avalos, M. D., D. Uccelletti, C. Abeijon, and C. B. Hirschberg. 2001. The UDPase activity of the Kluyveromyces lactis Golgi GDPase has a role in uridine nucleotide sugar transport into Golgi vesicles. Glycobiology 11:413-422. [DOI] [PubMed] [Google Scholar]
- 36.Lussier, M., A. M. Sdicu, S. Shahinian, and H. Bussey. 1998. The Candida albicans KRE9 gene is required for cell wall beta-1, 6-glucan synthesis and is essential for growth on glucose. Proc. Natl. Acad. Sci. USA 95:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuoka, J., and K. C. Hazen. 1999. Differences in the acid-labile component of Candida albicans mannan from hydrophobic and hydrophilic yeast cells. Glycobiology 9:1281-1286. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 39.Mumberg, D., R. Muller, and M. Funk. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119-122. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa, A., J. B. Poster, Y. Jigami, and N. Dean. 2002. Molecular and phenotypic analysis of CaVRG4, encoding an essential Golgi apparatus GDP-mannose transporter. J. Bacteriol. 184:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odani, T., Y. Shimma, X. H. Wang, and Y. Jigami. 1997. Mannosylphosphate transfer to cell wall mannan is regulated by the transcriptional level of the MNN4 gene in Saccharomyces cerevisiae. FEBS Lett. 420:186-190. [DOI] [PubMed] [Google Scholar]
- 42.Orlean, P. 1997. Biogenesis of yeast wall and surface components, vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Osmond, B. C., C. A. Specht, and P. W. Robbins. 1999. Chitin synthase III: synthetic lethal mutants and “stress related” chitin synthesis that bypasses the CSD3/CHS6 localization pathway. Proc. Natl. Acad. Sci. USA 96:11206-11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parry, D. A. 1982. Coiled-coils in alpha-helix-containing proteins: analysis of the residue types within the heptad repeat and the use of these data in the prediction of coiled-coils in other proteins. Biosci. Rep. 2:1017-1024. [DOI] [PubMed] [Google Scholar]
- 45.Poulain, D., C. Faille, C. Delaunoy, P. M. Jacquinot, P. A. Trinel, and D. Camus. 1993. Probable presence of beta(1-2)-linked oligomannosides that act as human immunoglobulin G3 epitopes and are distributed over a Candida albicans 14- to 18-kilodalton antigen. Infect. Immun. 61:1164-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schonholzer, F., A. M. Schweingruber, H. Trachsel, and M. E. Schweingruber. 1985. Intracellular maturation and secretion of acid phosphatase of Saccharomyces cerevisiae. Eur. J. Biochem. 147:273-279. [DOI] [PubMed] [Google Scholar]
- 47.Schweingruber, A. M., F. Schoenholzer, L. Keller, R. Schwaninger, H. Trachsel, and M. E. Schweingruber. 1986. Glycosylation and secretion of acid phosphatase in Schizosaccharomyces pombe. Eur. J. Biochem. 158:133-140. [DOI] [PubMed] [Google Scholar]
- 48.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Shibata, N., T. Ichikawa, M. Tojo, M. Takahashi, N. Ito, Y. Okubo, and S. Suzuki. 1985. Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch. Biochem. Biophys. 243:338-348. [DOI] [PubMed] [Google Scholar]
- 50.Southard, S. B., C. A. Specht, C. Mishra, J. Chen-Weiner, and P. W. Robbins. 1999. Molecular analysis of the Candida albicans homolog of Saccharomyces cerevisiae MNN9, required for glycosylation of cell wall mannoproteins. J. Bacteriol. 181:7439-7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steele, C., J. Leigh, R. Swoboda, H. Ozenci, and P. L. Fidel, Jr. 2001. Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect. Immun. 69:7091-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strahl-Bolsinger, S., M. Gentzsch, and W. Tanner. 1999. Protein O-mannosylation. Biochim. Biophys. Acta 1426:297-307. [DOI] [PubMed] [Google Scholar]
- 54.Sundstrom, P. 1999. Adhesins in Candida albicans. Curr. Opin. Microbiol. 2:353-357. [DOI] [PubMed] [Google Scholar]
- 55.Timpel, C., S. Strahl-Bolsinger, K. Ziegelbauer, and J. F. Ernst. 1998. Multiple functions of Pmt1p-mediated protein O-mannosylation in the fungal pathogen Candida albicans. J. Biol. Chem. 273:20837-20846. [DOI] [PubMed] [Google Scholar]
- 56.Timpel, C., S. Zink, S. Strahl-Bolsinger, K. Schroppel, and J. Ernst. 2000. Morphogenesis, adhesive properties, and antifungal resistance depend on the Pmt6 protein mannosyltransferase in the fungal pathogen Candida albicans. J. Bacteriol. 182:3063-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trinel, P. A., Y. Plancke, P. Gerold, T. Jouault, F. Delplace, R. T. Schwarz, G. Strecker, and D. Poulain. 1999. The Candida albicans phospholipomannan is a family of glycolipids presenting phosphoinositolmannosides with long linear chains of beta-1,2-linked mannose residues. J. Biol. Chem. 274:30520-30526. [DOI] [PubMed] [Google Scholar]
- 58.Warit, S., N. Zhang, A. Short, R. M. Walmsley, S. G. Oliver, and L. I. Stateva. 2000. Glycosylation deficiency phenotypes resulting from depletion of GDP-mannose pyrophosphorylase in two yeast species. Mol. Microbiol. 36:1156-1166. [DOI] [PubMed] [Google Scholar]
- 59.Yanagisawa, K., D. Resnick, C. Abeijon, P. W. Robbins, and C. B. Hirschberg. 1990. A guanosine diphosphatase enriched in Golgi vesicles of Saccharomyces cerevisiae. Purification and characterization. J. Biol. Chem. 265:19351-19355. [PubMed] [Google Scholar]
- 60.Zhong, X., and G. Guidotti. 1999. A yeast Golgi E-type ATPase with an unusual membrane topology. J. Biol. Chem. 274:32704-32711. [DOI] [PubMed] [Google Scholar]
- 61.Zhong, X., R. Malhotra, and G. Guidotti. 2000. Regulation of yeast ectoapyrase ynd1p activity by activator subunit Vma13p of vacuolar H+-ATPase. J. Biol. Chem. 275:35592-35599. [DOI] [PubMed] [Google Scholar]