Abstract
Prader-Willi syndrome (PWS) and Angelman syndrome (AS) are neurogenetic disorders that are caused by the loss of function of imprinted genes in 15q11-q13. In a small group of patients, the disease is due to aberrant imprinting and gene silencing. Here, we describe the molecular analysis of 51 patients with PWS and 85 patients with AS who have such a defect. Seven patients with PWS (14%) and eight patients with AS (9%) were found to have an imprinting center (IC) deletion. Sequence analysis of 32 patients with PWS and no IC deletion and 66 patients with AS and no IC deletion did not reveal any point mutation in the critical IC elements. The presence of a faint methylated band in 27% of patients with AS and no IC deletion suggests that these patients are mosaic for an imprinting defect that occurred after fertilization. In patients with AS, the imprinting defect occurred on the chromosome that was inherited from either the maternal grandfather or grandmother; however, in all informative patients with PWS and no IC deletion, the imprinting defect occurred on the chromosome inherited from the paternal grandmother. These data suggest that this imprinting defect results from a failure to erase the maternal imprint during spermatogenesis.
Introduction
Prader-Willi syndrome (PWS [MIM 176270]) and Angelman syndrome (AS [MIM 105830]) are neurogenetic disorders caused by the loss of function of oppositely imprinted genes in the chromosomal region 15q11-q13 (for review, see Nicholls and Knepper 2001). Most of the patients with PWS and AS have a de novo deletion of 15q11-q13, uniparental disomy, or, in AS, a UBE3A mutation. In a few patients (1% in PWS and 2%–4% in AS), the disease is due to aberrant imprinting and gene silencing. In patients with PWS and an imprinting defect, the paternal chromosome carries a maternal imprint, whereas in patients with AS and an imprinting defect, the maternal chromosome carries a paternal imprint. In some of these patients, the incorrect imprint is caused by a microdeletion affecting a bipartite imprinting center (IC) (Buiting et al. 1995). Maternally inherited microdeletions affecting an 880-bp region 35 kb proximal to SNURF-SNRPN exon 1 impair the establishment of the maternal imprint and lead to AS. Paternally inherited microdeletions affecting a 4.3-kb region around exon 1 of SNURF-SNRPN impair the maintenance of the paternal imprint during early embryogenesis and lead to PWS (El-Maarri et al. 2001). The shortest regions of deletion overlap for each syndrome have been called “AS-SRO” and “PWS-SRO,” respectively.
We and others (Bürger et al. 1997; Buiting et al. 1998; Ohta et al. 1999a) have shown that IC deletions cannot account for all imprinting defects in patients with AS or PWS. Here, we describe the detailed analysis of >100 patients.
Material and Methods
Patients
AS or PWS was diagnosed in all patients after examination by experienced clinicians. Biparental inheritance of the PWS/AS region was shown by microsatellite analysis. Methylation analysis of the SNURF-SNRPN exon 1 region revealed that the patients with PWS have a maternal methylation pattern, and the patients with AS have a paternal methylation pattern. These findings classify the patients as having an imprinting defect. In the present study, a subset of the patients with AS and an imprinting defect were originally thought to have PWS. These patients may belong to the subgroup of patients with AS imprinting defect whose phenotype overlaps with PWS (Gillessen-Kaesbach et al. 1999).
DNA Methylation and Southern Blot Analysis
Genomic DNA was purified from whole blood, according to standard methods. Methylation at the SNURF-SNRPN locus was investigated by the methylation-specific (MS) SNURF-SNRPN PCR (Zeschnigk et al. 1997). To investigate the grandparental origin of the incorrectly imprinted chromosome, a combined RFLP/methylation Southern blot analysis was performed for the SNURF-SNRPN intron 1 region by use of probe 17 and DNA digested with BglII + MspI and BglII + HpaII (Buiting et al. 1998; Ohta et al. 1999b). Deletion screening of the PWS-SRO and AS-SRO was performed by quantitative Southern blot analysis with probes kb17 on BglII-digested DNA (Buiting et al. 1998) and IC3/RN285 on EcoRV-digested DNA (Schumacher et al. 1998), respectively. Aliquots of the DNA (2–3 μg) were digested with the appropriate restriction enzyme, resolved on 1% agarose gels, and analyzed by Southern blot hybridization. As internal standards, hybridization probes from the retinoblastoma locus on chromosome 13 and the tricho-rhino-phalangeal syndrome locus on chromosome 8 were used. Probes were labeled by random oligonucleotide priming and α-[32P] dCTP (NEN Dupont). Autoradiography was performed at −80°C with intensifying screens and Kodak XAR films.
Sequence Analysis of PWS-SRO (SNURF-SNRPN Exon 1/Intron 1 Region) and AS-SRO
A 1,185-bp PCR product for the AS-SRO was amplified using primers IC16 and MOP3. For the SNURF-SNRPN exon 1 region, a 344-bp PCR product spanning exon 1 was amplified using primers SNRPNe1 and DD40. For the SNURF-SNRPN intron 1 region, a 309-bp PCR product obtained with primers SNRPNi1 and SNRPNi2 was analyzed. PCR products were purified with Microcon-100 microconcentrators (Amicon). Sequencing reactions were performed using fluorescence-tagged dideoxynucleotides and the Taq cycle sequencing procedure (ABI). Sequences were analyzed on an ABI 377A or ABI 3100 DNA Sequencer.
Methylation Analysis in Sperm DNA Samples
For methylation analysis of the HpaII site at the PW71 locus, sperm DNA was digested with NciI, SpeI, and HpaII, resolved on a 1% agarose gel, and probed with u1A800 (Färber et al. 1999). Methylation analysis of the HpaII site at the AS-SRO was performed by an HpaII digest of two sperm DNA samples including a plasmid as a digest control. Completion of the digest was also proved by Southern blot methylation analysis at SNURF-SNRPN exon 1. Undigested, methylated DNA was amplified using primer pairs IC16 and MOP3, purified with Qiagen PCR purification kit, and cloned into a pGemT plasmid vector (Pharmacia). Clones were sequenced using vector-specific primers sp6 and T7.
Primer Sequences
Primer sequences for the ICs were as follows: IC16, 5′ GCT CAA GCC GTG TTT CAT TTT 3′; MOP3, 5′ TTG GCT TCC TTT ATA TGA AC 3′; SNRPNe1, 5′ TCT AGA GGC CCC CTC TCA TT 3′; DD40, 5′ GCT CCC CAG GCT GTC TCT TG 3′; SNRPNi1, 5′ GGT GCA GTG GTA AGG AGA GG 3′; and SNRPNi2, 5′ AAA GCA GTA GCC CAG TGC AG 3′.
Results and Discussion
Search for IC Deletions
During the past seven years we obtained blood or DNA samples from 136 unrelated patients with an imprinting defect. Fifty-one patients had PWS, and 85 patients had AS. We first searched for an IC deletion by quantitative Southern blot analysis of the AS-SRO in the patients with AS and the PWS-SRO in the patients with PWS. In families with PWS, the fathers were also included in the methylation analysis, since an aberrant methylation pattern would directly indicate a familial IC deletion. We detected an IC deletion on the paternal chromosome in seven (14%) patients with PWS and on the maternal chromosome in eight (9%) patients with AS (table 1). In most cases, the IC deletion was familial. In five PWS cases, the father carried the IC deletion on his maternal chromosome. In one PWS case, the father did not have the deletion in his blood cells, and thus it was either de novo or a consequence of a germline mosaicism. In another PWS case, parental DNA samples were not available. In five AS cases, the mother carried the IC deletion on her paternal chromosome. In two AS cases, the deletion was either de novo or the result of a germline mosaicism. In another family, the disease is due to an inversion, with one breakpoint inside the IC region (Buiting et al. 2000).
Table 1.
Distribution of Mutation Status among Patients with AS and PWS
|
No. (%) of Patients with |
||
| MutationStatus | AS | PWS |
| IC | 9a,b (10) | 7c (14) |
| No IC | 76d (80) |
44e (86) |
| Total | 85 | 51 |
Among patients with an IC deletion, three with PWS and three with AS have at least one affected sib. In contrast, none of the patients with a non-IC deletion has an affected sib. As reported elsewhere (Buiting et al. 1998), at least two patients with PWS and no IC deletion share the same paternal chromosome 15q11-q13 haplotype with an unaffected sib. Likewise, three patients with AS and no IC deletion reported elsewhere (Bürger et al. 1997; Buiting et al. 1998) and four heretofore unreported patients with AS share the same maternal chromosome 15 haplotype with an unaffected sib. These observations suggest that it is unlikely that the imprinting defect in families with PWS and AS and no IC deletion is due to a familial mutation.
Sequence Analysis of the AS-SRO
To search for very small deletions or point mutations, we performed sequence analysis of the AS-SRO plus flanking regions in 66 patients with AS and no IC deletion, including 20 patients from previous studies (Buiting et al. 1998; Gillessen-Kaesbach et al. 1999). Inside the 1,185-bp sequence, seven different sequence variations could be detected. One of these variations has been reported before to be an SNP (Ohta et al. 1999a) and leads to the presence or absence of a BstN1 restriction site. Five of the newly identified variants were also found in 29 control individuals, suggesting that they represent neutral polymorphisms (table 2). One T insertion (bp 6525–6526 [GenBank accession number AF148319]) found in patient ASID-28 was not present in any of the control samples. Sequence analysis of the parents' DNA revealed that this single-nucleotide insertion is also present in the patient's father and therefore is unlikely to represent a mutation. Except for these polymorphisms, no small deletions, point mutations, or structural rearrangements inside the AS-SRO could be detected. Of the 66 patients, 45 were found to be heterozygous for at least one of the polymorphisms; this excludes a deletion of the AS-SRO and confirms the results obtained by quantitative Southern blot analysis.
Table 2.
Allele Frequency of Polymorphisms inside the AS-SRO and SNURF-SNRPN Exon 1/Intron 1 Region in Unaffected Unrelated Individuals
| Regionand Position | Polymorphism | AlleleFrequency |
| AS-SRO: | ||
| bp 6152 | A/G | .84/.16a |
| bp 6219 | G/A | .81/.19a |
| bp 6284 | A/C | .98/.02a |
| bp 6311–6314 | ins/del TAT | .84/.16a |
| bp 6464 | C/G | .97/.03a |
| bp 6525–6526 | ins Tb | … |
| bp 6558 | G/T | .93/.07a |
| bp 6830 | C/T | .83/.17a |
| SNURF-SNRPN: | ||
| bp 15006 | G/C | .94/.06c |
| bp 15082–15085 | ins/del GGAG | .99/.01c |
| bp 15138 | G/C | .99/.01c |
| bp 15198 | G/Ad | … |
| bp 15233 | G/C | .99/.01c |
| SNURF-SNRPN: | ||
| bp 16726 | A/T | .42/.58e |
| bp 16792 | ins/del GTGGGGCGAGAC | .95/.05e |
| bp 16884 | A/T | .97/.03e |
| bp 16915 | G/Cf | … |
Frequency based on 58 chromosomes.
Present only in one patient with AS and his father.
Frequency based on 126 chromosomes.
Present only in one patient with PWS, his mother, and one patient with AS.
Frequency based on 38 chromosomes.
Present only in one patient with PWS and his mother.
Sequence Analysis of the PWS-SRO
In 32 patients with PWS and no IC deletion, including 9 patients reported elsewhere (Buiting et al. 1998), we sequenced two regions of the PWS-SRO. First, we analyzed a 344-bp PCR product containing the SNURF-SNRPN exon 1 and promoter. As shown by Schweizer et al. (1999), this region contains an HpaII site that is subject to nuclease hypersensitivity in a parent-of-origin–specific manner. In contrast to the AS-SRO, this region seems to be highly conserved, because only two rare single-nucleotide variations could be detected. In 7 of the 32 patients with PWS, a heterozygous C→G exchange was found at position −83 of exon 1 (bp 15006 3′–5′ orientation [GenBank accession number AC009696]). This variation was also found in 7 of 68 control samples in a heterozygous state, suggesting that this variation represents an SNP. In one patient (PWSID-04), a G→A exchange at position −4 of exon 1 (bp 15198 in AC009696 3′–5′ orientation) was found. This transition was not detected in any of the control subjects but was found in the patient's mother and in a patient with AS and no IC deletion (ASID-03). Thus, this single-nucleotide exchange also seems to be a rare neutral variant.
Second, we analyzed 309 bp spanning an HpaII site ∼2 kb distal to exon 1, which has previously been shown to be subject to nuclease hypersensitivity in a parent-of-origin–specific manner (Schweizer et al. 1999); 19 control individuals were included in this analysis. Again, we found no evidence of a structural or point mutation in any of the patients but observed heterozygosity in 22 patients for at least one of three newly identified SNPs, confirming that these patients have no deletion of the PWS-SRO. Only one patient (PWSID-04) showed a heterozygous G→C exchange (at position 16915 in AC009696, 3′–5′ orientation), which was not present in any other patient or the control group. Analysis of the parents' DNA revealed that this variation is also present in the patient's mother and therefore appears to be a rare variant.
Mosaic Methylation Defects in Patients with AS Imprinting Defect
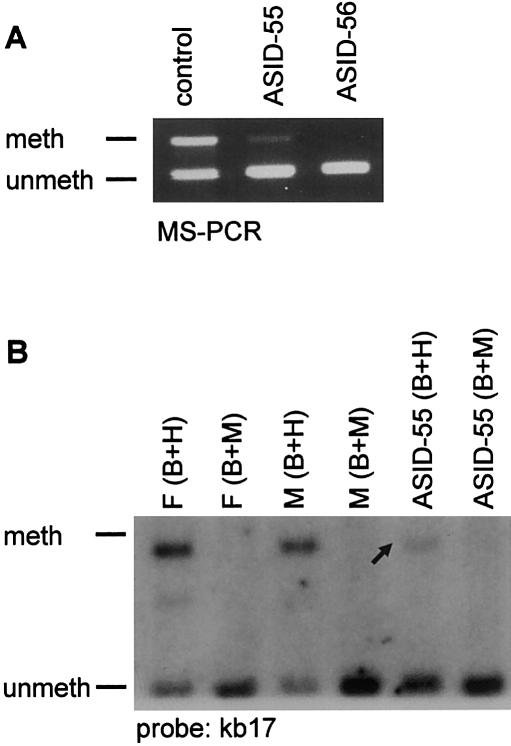
In five of the seven atypical non–IC-deletion patients with AS with an imprinting defect who were reported by Gillessen-Kaesbach et al. (1999), a faint maternal band, indicative of methylation mosaicism, had been observed by use of MS-PCR analysis for the SNURF-SNRPN exon 1 region. In the present study we found such a weak maternal band in 6 additional patients with atypical AS and no IC deletion but also in 10 patients with typical symptoms of AS (total, 27% of patients). In some of these patients, methylation analysis of an HpaII site inside intron 1 of SNURF-SNRPN by Southern blot hybridization showed the same result, thus excluding an MS-PCR–based artifact (fig. 1). We never observed a weak maternal band in patients with AS with a common large deletion, uniparental disomy, or IC deletion. Thus, this finding seems to be restricted to patients with an imprinting defect and no IC deletion. Methylation mosaicism is much rarer in patients with PWS and no IC deletion. In the present study, a faint paternal band was present in only 2 of 44 patients (PWSID-06 and PWSID-17). In one of these patients, we could also detect the band by Southern blot analysis (data not shown).
Figure 1.
Mosaic methylation defect. A, (MS)-PCR analysis of SNURF-SNRPN exon 1 in two patients with AS and an imprinting defect. Patient ASID-56 shows a typical AS methylation pattern, whereas patient ASID-55 has a faint methylated band. A faint methylated band (arrow) is also detected by MS Southern blot analysis. B, meth = methylated; unmeth = unmethylated; F = father; M = mother; B = BamHI; H = HpaII; M = MspI. In contrast to HpaII, MspI is not MS.
Grandparental Origin of the Chromosome Carrying the Imprinting Defect
To investigate the grandparental origin of the incorrectly imprinted chromosome, we performed microsatellite analysis or used a combined methylation/RFLP test for the SNURF-SNRPN locus (Buiting et al. 1998). In the patients with AS and no IC deletion, the maternal chromosome carrying an incorrect paternal imprint was inherited from the maternal grandfather in 11 patients and from the maternal grandmother in 7 patients. This finding suggests that the imprinting defect occurred after erasure of the parental imprints, possibly even only after fertilization, as discussed above. In contrast, in 19 informative patients with PWS and no IC deletion, the paternal chromosome carrying an incorrect maternal imprint was always derived from the paternal grandmother (table 3). This bias was highly significant (P=.000002). We can think of three explanations for this bias.
Table 3.
Grandparental Origin of the Chromosome Carrying the Imprinting Defect
| AS |
PWS |
|||
| Origin | ICMutation | No ICMutation | ICMutation | No ICMutation |
| Maternal: | ||||
| Grandfather | 5a | 7 | 0 | 0 |
| Grandmother | 1a | 11 | 0 | 0 |
| Paternal: | ||||
| Grandfather | 0 | 0 | 1b | 0 |
| Grandmother | 0 | 0 | 4a | 19c |
In one patient, the deletion is de novo or due to a germline mosaicism.
In one patient, the father is mosaic for the deletion.
Significantly different from equal grandpaternal and grandmaternal inheritance (P=.000002).
(1) The patients carry an unidentified IC mutation. If this were the case, we would expect to find affected sibs in this group of patients. As mentioned above, however, all patients with no IC deletion have sporadic disease, and some of them even share a paternal haplotype with an unaffected sib. Although the latter finding may be explained by germline mosaicism, we consider this possibility to be unlikely.
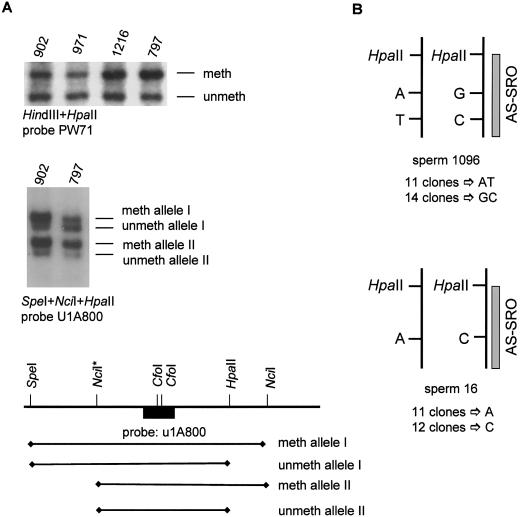
(2) The parental identity of the two homologues is maintained during male germ cell development. By analyzing the ontogeny of the H19 imprint in the mouse, Davis et al. (2000) have recently found that the reestablishment of the imprints on the parental alleles occurs at different times in male germ cell development. Whereas the paternal H19 allele becomes methylated during fetal stages, methylation of the maternal allele begins during perinatal stages and continues after birth through the onset of meiosis. We reasoned that certain CpG dinucleotides in human 15q11-q13 might be differentially methylated in spermatozoa and might occasionally become inadvertent nucleation sites for a maternal imprint. As shown elsewhere (e.g., El-Maarri et al. 2001), we found that most CpGs have a paternal methylation pattern in sperm. Interestingly, however, one HpaII site at the D15S63 (PW71) locus and one HpaII site just proximal of the AS-SRO showed ∼50% methylation (fig. 2A). The latter HpaII site, as well as other CpGs of the AS-SRO region, are heavily methylated in blood (Schumacher et al. 1998). To find out if methylation of the two HpaII sites is allele specific or random, we used two different approaches. For the D15S63 locus, we used a combined RFLP/methylation analysis. First, we screened for a sperm DNA sample informative for an NciI polymorphism (Dittrich et al. 1993) close to the HpaII site. Two heterozygous sperm samples (sample 902 and 797) were then used for Southern blot analysis. The DNA was digested with SpeI + HpaII + NciI (fig. 2A) and was probed with u1A800 (Färber et al. 1999). Because both parental alleles in sperm samples 902 and 797 are partially digested by HpaII, methylation is not allele specific.
Figure 2.
Methylation analysis of sperm DNA samples at D15S63 (PW71) and the AS-SRO A, Methylation analysis in different sperm samples with probe PW71 on DNA digested with HindIII + HpaII (top). In all sperm samples a methylated and an unmethylated band is present. Results are shown for methylation analysis in two sperm samples heterozygous for an NciI restriction site polymorphism (NciI*) at the D15S63 locus (middle). The different possible fragments for the methylated and unmethylated allele I and allele II are shown in the map below (not drawn to scale). The Southern blot (middle) shows the presence of a methylated and an unmethylated fragment for both alleles. B, Sequence analysis of two sperm DNA samples heterozygous for one or two SNPs inside the AS-SRO. Both sperm DNA samples were digested with HpaII and amplified with primers flanking the HpaII site. If methylation at this locus is allele specific, only the methylated allele should be amplified. By cloning the PCR products from sperm sample 1096, we obtained 11 clones from one allele and 14 from the other. For sperm sample 16, we obtained 11 clones from one allele and 12 clones from the other.
For the HpaII site at the AS-SRO, we used two sperm DNA samples that are heterozygous for one or two SNPs inside the AS-SRO, close to the HpaII site. We digested both sperm DNA samples with HpaII and performed PCR with primers flanking the HpaII site. If methylation at this locus were allele specific, only the methylated allele should be amplified. By cloning the PCR products, we obtained 23 clones for sperm sample 16 and 25 clones for sperm sample 1096. Given that both parental alleles were recovered (fig. 2B), methylation is not allele specific. A similar result was obtained for brain DNA (data not shown). Although we cannot exclude allele-specific DNA methylation at other loci (or parental imprints other than DNA methylation), there is no indication that the parental identity of the two homologues is maintained in mature spermatozoa.
(3) The incorrect maternal imprint in the patients results from a failure of the paternal germline to erase the grandmaternal imprint (epigenetic inheritance). There is increasing evidence to suggest that epigenetic marks at some mammalian alleles are not completely erased from one generation to the next. Morgan et al. (1999) and Kearns et al. (2000), for example, reported mouse lines carrying retrotransposons or transgenes that display incomplete penetrance and variable expressivity dependent on the parental origin. On the basis of detailed breeding and methylation studies, it was suggested that the imprint is not completely erased and reset when passed through the germline, resulting in unusual patterns of inheritance of gene expression. Although epigenetic inheritance of endogenous genes has not been reported in mammals, it is well known in other species. We propose that it occurs at a low frequency in 15q11-q13 and possibly at other human loci as well. Assuming that PWS occurs in 1/15,000 newborns and that 1% of patients have a non–IC-deletion imprinting defect, 1/1,500,000 spermatozoa should carry a maternal imprint that was not erased in the paternal germline. It will be a formidable task to detect such a cell.
Conclusions
In summary, by analyzing a very large series of patients with an imprinting defect and PWS or AS, we have determined that the vast majority of these defects are epimutations (aberrant epigenetic states) that occurred spontaneously in the absence of DNA sequence changes. The apparent absence of point mutations may indicate that the IC can tolerate small sequence changes or that it contains multiple, redundant elements. Epimutations of the maternal chromosome are often present in a mosaic form. This suggests that, in these patients, aberrant DNA methylation occurred after fertilization, when the maternal CpG methylation pattern is established (El-Maarri et al. 2001). Nevertheless, the underlying defect may be pre- or postzygotic. It is possible, for example, that the primary gametic imprint (which may be histone H3 lysine 9 methylation [Xin et al. 2001] or some other chromatin modification) is not always stable, that it is not always faithfully translated into CpG methylation, or that CpG methylation is not always faithfully replicated during early embryogenesis. It may be speculated that the frequency of these failures and the degree of mosaicism are influenced by the genetic or epigenetic background.
Methylation mosaicism makes it difficult to predict the phenotype. In fact, the clinical spectrum among these patients is very broad and ranges from typical AS, through mild AS, to atypical AS. All mosaic patients have a very low level of normal cells. This may be an ascertainment bias, because patients with a higher proportion of normal cells are likely to have a very mild phenotype and to escape clinical detection.
Epimutations of the paternal chromosome appear to occur during male germ cell development and to lead to epigenetic inheritance, that is, the inheritance of an epigenetic state from one generation to the next. The significance of epigenetic inheritance in humans is unclear. It is possible, however, that it is not only a curiosity in PWS but a contributor to genetic variation in humans (Rakyan et al. 2001).
Acknowledgments
We are very grateful to all colleagues who sent us blood or DNA samples from the patients. We are indebted to all the patients and their families for their participation in the study. We thank Stefan Böhringer for statistical analysis and two anonymous reviewers for helpful comments. Part of this work was supported by Deutsche Forschungsgemeinschaft grant BU907/1-2.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for PWS-SRO [accession number AC009696] and AS-SRO [accession number AF148319])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AS [MIM 105830] and PWS [MIM 176270])
References
- Buiting K, Barnicoat A, Lich C, Pembrey M, Malcolm S, Horsthemke B (2001) Disruption of the bipartite imprinting center in a family with Angelman syndrome. Am J Hum Genet 68:1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Dittrich B, Groß S, Lich C, Färber C, Buchholz T, Smith E, et al (1998) Sporadic imprinting defects in Prader-Willi syndrome and Angelman syndrome: implications for imprint-switch models, genetic counseling, and prenatal diagnosis. Am J Hum Genet 63:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Färber C, Kroisel P, Wagner K, Brueton L, Robertson ME, Lich C, Horsthemke B (2000) Imprinting centre deletions in two PWS families: implications for diagnostic testing and genetic counseling. Clin Genet 58:284–290 [DOI] [PubMed] [Google Scholar]
- Buiting K, Lich C, Cottrell S, Barnicoat A, Horsthemke B (1999) A 5-kb imprinting center deletion in a family with Angelman syndrome reduces the shortest region of deletion overlap to 880 bp. Hum Genet 105:665–666 [DOI] [PubMed] [Google Scholar]
- Buiting K, Saitoh S, Groß S, Dittrich B, Schwartz S, Nicholls R, Horsthemke B (1995) Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting center on human chromosome 15. Nat Genet 9:395–400 [DOI] [PubMed] [Google Scholar]
- Bürger J, Buiting K, Dittrich B, Groß S, Lich S, Sperling K, Horsthemke B, Reis A (1997) Different mechanisms and recurrence risks of imprinting defects in Angelman syndrome. Am J Hum Genet 61:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Yang GJ, McCarrey JR, Bartolomei MS (2000) The H19 methylation imprint is erased and re-established differentially on the paternal alleles during male germ cell development. Hum Mol Genet 9:2885–2894 [DOI] [PubMed] [Google Scholar]
- Dittrich B, Groß S, Buiting K, Horsthemke B (1993) An NciI RFLP at the D15S63 locus in the critical Prader-Willi syndrome region in 15q11-q13. Hum Mol Genet 2:1509 [DOI] [PubMed] [Google Scholar]
- El-Maarri O, Buiting K, Peery EG, Kroisel PM, Balaban B, Wagner K, Urman B, Heyd J, Lich C, Brannan CI, Walter J, Horsthemke B (2001) Maternal methylation imprints on human chromosome 15 are established during or after fertilization. Nat Genet 27:341–344 [DOI] [PubMed] [Google Scholar]
- Färber C, Dittrich B, Buiting K, Horsthemke B (1999) The chromosome 15 imprinting centre (IC) region has undergone multiple duplication events and contains an upstream exon of SNRPN that is deleted in all Angelman syndrome patients with an IC microdeletion. Hum Mol Genet 8:337–343 [DOI] [PubMed] [Google Scholar]
- Gillessen-Kaesbach G, Demuth S, Thiele H, Theile U, Lich C, Horsthemke B (1999) A previously unrecognised phenotype characterised by obesity, muscular hypotonia, and ability to speak in patients with Angelman syndrome caused by an imprinting defect. Eur J Hum Genet 7:638–644 [DOI] [PubMed] [Google Scholar]
- Kearns M, Preis J, McDonald M, Morris C, Whitelaw E (2000) Complex patterns of inheritance of an imprinted murine transgene suggest incomplete germ line erasure. Nucleic Acids Res 28:3301–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HD, Sutherland HG, Martin DL, Whitelaw E (1999) Epigenetic inheritance at the agouti locus in the mouse. Nat Genet 23:314–318 [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL (2001) Genome organization: function and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet 2:153–175 [DOI] [PubMed] [Google Scholar]
- Ohta T, Buiting K, Kokkonen H, McCandless S, Heeger S, Leisti H, Driscoll DJ, Cassidy SB, Horsthemke B, Nicholls RD (1999a) Molecular mechanism of Angelman syndrome in two large families involves an imprinting mutation. Am J Hum Genet 64:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Gray TA, Rogan PK, Buiting K, Gabriel JM, Saitoh S, Muralidhar, Bilienska B, Krajewska-Walasek M, Driscoll DJ, Horsthemke B, Butler MG, Nicholls RD (1999b) Imprinting-mutation mechanisms in Prader-Willi syndrome. Am J Hum Genet 64:397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan VK, Preis J, Morgan HD, Whitelaw E (2001) The marks, mechanisms and memory of epigenetic states in mammals. Biochem J 356:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Buiting K, Rogan PK, Buxton JL, Driscoll DJ, Arnemann J, König R, Malcolm S, Horsthemke B, Nicholls RD (1996) Minimal definition of the imprinting center and fixation of a chromosome 15q11-q13 epigenotype by imprinting mutations. Proc Natl Acad Sci USA 93:7811–7815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A, Buiting K, Zeschnigk M, Doerfler W, Horsthemke B (1998) Methylation analysis of the PWS/AS region does not support an enhancer-competition model. Nat Genet 19:324–325 [DOI] [PubMed] [Google Scholar]
- Schweizer J, Zynger D, Francke U (1999) In vivo nuclease hypersensitivity studies reveal multiple sites of parental origin–dependent differential chromatin conformation in the 150 kb SNRPN transcription unit. Hum Mol Genet 8:555–566 [DOI] [PubMed] [Google Scholar]
- Xin Z, Allis CD, Wagstaff J (2001) Parent-specific complementary patterns of histone H3 lysine 9 and H3 lysine 4 methylation at the Prader-Willi syndrome imprinting center. Am J Hum Genet 69:1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeschnigk M, Lich C, Buiting K, Doerfler W, Horsthemke B (1997) A single-tube PCR test for the diagnosis of Angelman and Prader-Willi syndrome based on allelic methylation differences at the SNRPN locus. Eur J Hum Genet 5:94–98 [PubMed] [Google Scholar]