Abstract
Models of disease susceptibility in multiple sclerosis (MS) often assume a dominant action for the HLA-DRB1*1501 allele and its associated haplotype (DRB1*1501-DQB1*0602 or DR2). A robust and phenotypically well-characterized MS data set was used to explore this model in more detail. A dose effect of HLA-DR2 haplotypes on MS susceptibility was revealed. This observation suggests that, in addition to the role of HLA-DR2 in MS, two copies of a susceptibility haplotype further increase disease risk. Second, we report that DR2 haplotypes modify disease expression. There is a paucity of benign MS and an increase of severe MS in individuals homozygous for DR2. Concepts of the molecular mechanisms that underlie linkage and association of the human leukocyte antigen (HLA) region to MS need to be revised to accommodate these data.
The association of multiple sclerosis (MS [MIM 126200]) with the human leukocyte antigen (HLA) region has been known for more than a quarter of a century (Bertrams and Kuwert 1972; Naito et al. 1972). A specific association with the DRB1*1501 molecule and its associated haplotype (DRB1*1501-DQB1*0602 or DR2 [MIM 142857 and MIM 604305, respectively]) is present in most populations with MS (Haines et al. 1998; Rubio et al. 2002); the only exceptions are an apparent association with DR3 and DR4 in patients with MS from Sardinia (Marrosu et al. 2002) and, perhaps, no DR2 association in some Asian populations who have a restricted form of MS, termed “neuromyelitis optica,” selectively affecting optic nerve and spinal cord myelin (Kira et al. 1996). Whole-genome mapping studies provide additional support for the presence of an MS susceptibility gene located within chromosome 6p21 (Haines et al. 1996, 1998). However, fine-mapping studies have not settled whether the effect is explained by the DRB1 gene itself; by variation at another closely linked gene within the class 2 HLA region in very high linkage disequilibrium (LD) with DRB1*1501, such as DQB1*0602; or by some other nearby gene in LD. Identification of the true predisposing gene or genes has been made more complex by extensive LD across the HLA region and by the presence of >240 genes within this superlocus, many of which have roles in immune function and are thus plausible MS candidates.
Linkage to the HLA region plus a specific association with the DR2 haplotype suggests that DRB1*1501-bearing haplotypes confer an effect on MS susceptibility that is distinct from that of other major histocompatibility (MHC) haplotypes. In support of this hypothesis, no evidence for linkage to 6p21 could be discerned in families who did not carry DR2 (Chataway et al. 1998; Haines et al. 1998; Barcellos et al. 2002). Locus heterogeneity may not be present in all populations, however, underscoring the complex genetic nature of this disease (Ligers et al. 2001).
To better delineate the role of the HLA locus in MS and, in particular, the risk and phenotype associated with the DR2 homozygous state, we analyzed a large and well-characterized family-based cohort of 549 families prone to MS (187 multicase and 362 single affected, or “singleton”) comprising 2,382 individuals, including 808 affected individuals and 1,574 unaffected family members. Diagnostic criteria, ascertainment protocols, and clinical and demographic characteristics of the multicase families are summarized elsewhere (Goodkin et al. 1991; Multiple Sclerosis Genetics Group [MSG Group] 1998; Haines et al. 1998; Barcellos et al. 2002). Singleton families were collected using identical ascertainment criteria; these families were required to have an affected proband with either two living parents or at least one unaffected sibling. The singleton family ascertainment was coordinated at the University of California San Francisco in collaboration with a network of specialized clinical sites throughout the United States.
All known ancestors were non-Hispanic whites of European descent. Complete clinical data were available for 96.0% of patients. For affected individuals, the female-to-male ratio was 3.0:1. The overall mean age at onset was 30.1±8.8 years, and mean disease duration was 14.6±10.2 years. Age at onset was defined as the first episode of neurological dysfunction suggestive of demyelinating disease (Doolittle et al. 1990; Barcellos et al. 2000). Disease course was recorded for patients at entry into study as either relapsing remitting (RR), secondary progressive (SP), primary progressive (PP), or relapsing progressive (RP). Disability was also assessed at entry with the Expanded Disability Status Scale (EDSS) (Kurtzke 1983). “Mild” (benign) and “severe” disease patient classifications were based on EDSS scores that were maintained over or achieved within designated time intervals were also used in the present study (see table 1 for details). The appropriate institutional review boards approved all studies, and informed consent was obtained from all participants.
Table 1.
Disease Course and Severity Phenotypes in Patients with MS[Note]
| Clinical Phenotype | N (%) |
| RR | 487 (62.8) |
| SP | 235 (30.3) |
| Othera | 53 (6.8) |
| Mild MS: EDSS <3 after ⩾10 yearsb | 71 (14.8) |
| Mild MS: EDSS <3 after ⩾15 yearsb | 40 (11.9) |
| Severe MS: EDSS >6 in ⩽10 yearsc | 35 (10.8) |
Note.— A total of 808 patients with clinically definite MS was studied. Complete clinical data were available for >95% of individuals.
PP and RP clinical subtypes.
Classifications of benign or mild MS. Patients in this group can walk normally or have mild gait disability only after ⩾10 or ⩾15 years from disease onset.
Patients in this group require bilateral assistance to walk or are wheelchair dependent in ⩽10 years from disease onset. The percentages shown for mild and severe phenotypes reflect the proportion of individuals in patient groups restricted by disease duration; for example, for mild MS, patients with disease duration of ⩾10 or ⩾15 years only are included (n=480 and n=335, respectively), and, for severe MS, only patients with disease duration of ⩽10 years are included (n=323).
HLA typing for DRB1 and DQB1 loci was performed using a nonradioactive PCR-based sequence-specific oligonucleotide probe reverse line-blot assay (PCR-SSOP) (Dynal). Generation of all genotypes was performed blind to pedigree structure and clinical status of the individual. PEDCHECK (O'Connell and Weeks 1998) was used to check for Mendelian consistency within all families with MS. Family-based association testing for alleles at the HLA-DR locus was performed for the combined family data set, using the “sum” option of the pedigree disequilibrium test (PDT v.3.11 [available at the Duke University Center for Human Genetics Web site]; Martin et al. 2000, 2001). The PDT can utilize genetic data from related nuclear families and discordant sibships within extended pedigrees. A strong association with HLA-DR overall (P<1.0×10-10) and with the DR2 haplotype specifically (P<1.0×10-11) was observed, as reported elsewhere, in a subset of this population (Barcellos et al. 2002). No evidence for excess transmission of other DR alleles to affected individuals was present.
All patients and unaffected family members were stratified by HLA-DR genotype (DR2/DR2, DR2/DRX, and DRX/DRX, where X denotes other DR alleles) to test for effects of HLA-DR2 dose on disease susceptibility. An effect of HLA-DR2 copy number on disease risk was suggested by the observed difference in proportion of affected individuals within each HLA-DR genotypic category (fig. 1). A total of 52.8% of DR2/DR2 individuals was affected, in contrast to 37.8% of DR2/DRX and 26.9% of DRX/DRX individuals. Conditional logistic regression modeling was then used to evaluate the effect of DR genotype on disease risk in the family data set. For these analyses, carriers of two (DR2/DR2) and one (DR2/DRX) DR2 haplotypes were compared with DRX/DRX individuals. Significant effects on disease risk were observed for both DR2/DR2 and DR2/DRX genotypes in all families (odds ratio [OR]=6.7, P<1.0×10-6 and OR=2.7, P<1.0×10-6), as well as multicase and singleton families considered separately (table 2). Significant results were also obtained when DR2/DR2 individuals were compared with individuals with DR2/DRX genotypes (table 2), indicating that two copies of DR2 confer a greater risk compared with one copy (OR=2.5, P<1.0×10-5, OR=2.4, P<1.0×10-3, and OR=2.7, P<.01 for all families, multicase, and singleton families, respectively). When analyses were restricted to sibships only, or just one single discordant sib pair selected from each family, the results were very similar (data not shown).
Figure 1.
Proportion of affected and unaffected individuals within 549 families with MS grouped by HLA-DR2 genotypes. The data set contained 2,382 individuals comprising 808 patients with MS and 1,574 unaffected family members. A total of 26.9% (n=319) DRX/DRX and 38.7% (n=395) DR2/DRX individuals were affected, in contrast to 52.8% (n=94) DR2/DR2 individuals, demonstrating a dose effect of HLA-DR2 on susceptibility.
Table 2.
OR for HLA-DR Genotypes in Patients with MS Compared with Unaffected Family Members[Note]
| OR (95% CI) |
||||
| Test Group | Referencea | All Families | Multicase Only | Singleton Only |
| DR2/DR2 | DRX/DRX | 6.7 (4.2–10.7)b | 6.1 (3.4–11.0)b | 7.9 (3.7–17.0)b |
| DR2/DRX | DRX/DRX | 2.7 (2.1–3.6)b | 2.6 (1.8–3.7)b | 3.0 (2.0–4.5)b |
| DR2/DR2 | DR2/DRX | 2.5 (1.7–3.7)c | 2.4 (1.4–3.9)d | 2.7 (1.4–5.1)e |
Note.— The analyses include all affected and unaffected individuals from families with multicase (n=187) and singleton (n=362) MS (total n=2,382 individuals; n=808 affected and 1,574 unaffected individuals).
The DRX group refers to all other non-DR2 (DRB1*1501–DQB1*0602) alleles. All analyses were performed using conditional logistic regression modeling and controlling for sex as implemented in PROC PHREG (SAS v. 8.2). All individuals were stratified by family for analyses. Unaffected family members included all parents and siblings of affected individuals.
P<10-6.
P<10-5.
P<10-3.
P<.01.
A second approach, a modification of the PDT, was also used to examine HLA-DR genotypic associations in the combined family data set. This test, the geno-PDT, is also applicable to nuclear family or extended pedigree data and can be used to test any specific genotype, or a global statistic can be computed to test all genotypes simultaneously. The PDT and geno-PDT both test the same null hypotheses but can have different powers, depending on the genetic model. Significant evidence for excess transmission of both DR2/DR2 and DR2/DRX genotypes was observed in the families with MS (P<.01 and P<1.0×10-5, respectively; data not shown), providing further support for both genotypes as disease risk factors.
Maximum-likelihood estimates of the relative penetrance values for MS-associated DR2/DR2 and DR2/DRX genotypes were determined using the ratio of the observed genotype frequency in patients over the frequency in controls (or P/C ratio), as described elsewhere (McWeeney and Thomson 2000). Control genotype frequency estimates were derived under the expectation of Hardy-Weinberg equilibrium, using nontransmitted (or non-MS) HLA-DR allele frequencies in the singleton families with MS (Thomson 1995). The penetrance values shown in table 3 have been normalized using a value of 1 for the reference DRX/DRX genotype. The results suggest that the relative penetrance for the DR2 homozygous genotype is at least twice as large as that for the DR2/DRX genotype in all families and in both multicase and singleton families considered separately.
Table 3.
Relative Penetrance Values for HLA-DR Genotypes[Note]
| Index Cases from | DR2/DR2 | DR2/DRX |
| All familiesa | 11.8 | 3.8 |
| Singleton families onlyb | 9.1 | 2.9 |
| Multicase families onlyc | 20.0 | 6.7 |
Note.— Control genotype frequencies for P/C ratios were derived using nontransmitted alleles from singleton families assuming Hardy Weinberg proportions (Thomson 1995) (DR2 or DRB1*1501-DQB1*0602: f=0.122). Nontransmitted allele frequencies derived using additional trios from multicase families (one per family) were similar (data not shown). All relative penetrance values were normalized to a value of 1 for the reference DRX/DRX genotype.
n=549.
n=362.
n=187.
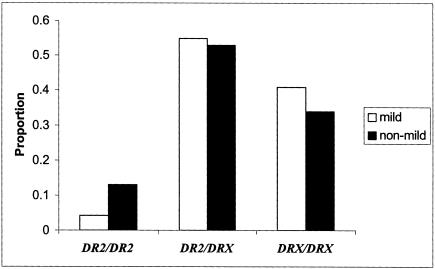
The effect of HLA-DR2 genotype on clinical phenotypes, including age at onset and disease course and severity, was tested in the patient data set, using linear and logistic models estimated by generalized estimating equations (Liang and Zeger 1986; Zeger and Liang 1992), which take into account any correlation between family members. The proportions shown in table 1 are derived from the total number of individuals with a particular clinical phenotype present within the data set. Although phenotypic categorization results in smaller sample sizes and a potential loss in statistical power, the increased clinical homogeneity in these subgroups may also increase the likelihood of detecting specific disease-modulating genetic effects. In the patient group with mild, or benign, MS, DR2 homozygotes were significantly less frequent compared with patients classified as having nonmild MS (5.4% vs. 13.0%), using the DRX/DRX genotype as a reference group (OR=0.3, 95% CI=0.1–0.9, P<.05; see fig. 2). Here, “mild MS” was defined as maintaining an EDSS score of <3 for at least 10 years. When a more stringent definition of “mild MS” was applied, in which disease duration of at least 15 years was imposed, no DR2 homozygotes were present in this subgroup. This result was also significant (Fisher’s exact test, P<.01; see fig. 2 legend for details). Both observations provide strong support for HLA-DR2 as a disease modifier. Furthermore, DR2 homozygotes were observed more frequently in patients with a severe disease course (those defined as reaching an EDSS score >6 in ⩽10 years) in contrast to patients classified as “nonsevere” (17.1% vs. 10.1%, OR=1.8, 95% CI=0.7–5.0; data not shown), though this observation did not reach statistical significance (P>.10).
Figure 2.
A comparison of HLA-DR genotypes for patient subgroups of mild and nonmild MS. Analyses were restricted to patients with disease duration of at least 10 years (n=480). P values are from PROC GENMOD (SAS version 8.2), using logistic regression with correction for familial correlations and adjustment for age at onset and sex. OR of mild MS for DR2 homozygotes (DR2/DR2)=0.3, 95% CI=0.1–0.9, P<.05. Reference group = DRX/DRX genotype. When DR2/DR2 individuals were compared with individuals with DR2/DRX genotype, OR=0.3, 95% CI=0.1–1.1, P<.10. There was no evidence for increased risk of mild MS phenotype in individuals carrying just one copy of HLA-DR2 haplotype, OR=0.8, 95% CI=0.5–1.4, P>.10. Because no DR2/DR2 individuals were present in analyses of mild MS ⩾15 years from onset, the statistical methods used above were not appropriate. Therefore, to determine statistical significance, unrelated mild MS cases were compared to other randomly selected unrelated cases using Fisher’s exact test, as implemented in PROC FREQ (version 8.2, SAS; P<.01; data not shown). Logistic regression modeling with correction for familial correlations and adjustments for age at onset and sex was also used for comparisons of HLA-DR genotypes in patient subgroups of severe and nonsevere MS (data not shown). See text for results.
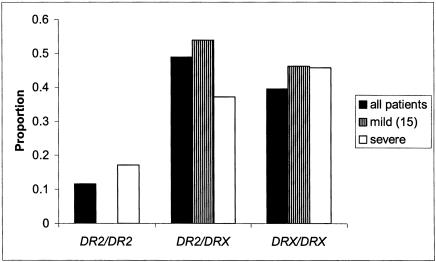
When HLA-DR genotypic proportions for individuals with either mild or severe disease classifications were compared with nonmild or nonsevere groups, respectively, the observed effect on disease expression was limited to those individuals carrying two copies of the HLA-DR2 haplotype. Using DR2/DRX as a reference group, trends for association were observed for both mild and severe phenotypes (OR=0.3, 95% CI=0.1–1.1, P<.10 and OR=2.3, 95% CI=0.8–6.4, P>.10), respectively. There was no evidence for effects of DR2/DRX, as compared with DRX/DRX, for either clinical phenotype (P>.40 for both mild and severe subgroups of MS, respectively; data not shown), suggesting that one copy of the haplotype may be insufficient for expression of these disease variants. The difference in DR2/DR2 genotype frequencies between the two most extreme clinical phenotypes, mild (disease duration of at least 15 years) and severe, was striking (Fisher’s exact test, P<.01; see fig. 3 for details), although the results must be interpreted cautiously because of small sample sizes. Significant effects were not present for DR2 dose on disease course (RR, SP, and PP), and no DR2-dependent difference in mean age at onset was detected.
Figure 3.
HLA-DR genotype frequencies for all patients (index cases only, n=549) and most extreme phenotypes, patients with mild (<3 for ⩾15 years) and severe MS. Because no DR2/DR2 individuals were present in the patient group with mild MS, unrelated mild MS cases (n=39) were compared with unrelated severe cases (n=33), using Fisher’s exact test, as implemented in PROC FREQ (version 8.2, SAS). The overall difference between genotype distributions for patients with mild and severe MS was significant (P<.01); 18.2% of patients with severe MS were homozygous for HLA-DR2, compared with 0% in patients with mild MS.
The use of a well-characterized data set of white MS-prone families permitted, for the first time in a large and prospectively ascertained population, an analysis of disease risk in individuals homozygous for the MS-associated HLA-DR2 haplotype. A dose effect on MS susceptibility was revealed. This finding is unexpected, although it is also supported by a recent meta-analysis of three small published data sets comprising a total of 35 DR2 homozygous individuals (Rasmussen et al. 2001). Although relative penetrance values for HLA-DR genotypes derived in the present study also support a DR2 dose effect for disease risk, the available data do not distinguish between additive and multiplicative models. In experimental models of autoimmune demyelination, a single copy of a disease-associated MHC haplotype, when present in the context of an appropriate genetic background, is generally sufficient for the induction of susceptibility. Dominantly acting MHC genes are thought to function via high-affinity binding to certain self-peptides, which are then efficiently presented to pathogenic T cells (Todd et al. 1987).
In the case of DRB1*1501, binding and structural data support a model of the peptide-binding region that is composed in part of a large hydrophobic pocket displaying high affinity for aromatic amino acids, including phenylalanine at aa 92 of an immunodominant peptide (aa 89–96) of the autoantigen myelin basic protein (MBP) (Valli et al. 1993; Smith et al. 1998). A direct role for autoimmunity against the aa 89–96 region of MBP in the pathogenesis of MS is suggested by the findings of immunodominance of the T-cell response to this peptide in DRB1*1501-positive individuals (Martin et al. 1990; Ota et al. 1990), specific activation of these T cells in the circulation of patients with MS (Allegretta et al. 1990; Scholz et al. 1998), and presence in MS lesions of T-cell receptors bearing antigen recognition CDR3 motifs likely to recognize this peptide (Oksenberg et al. 1993). The “tight binder” concept and immunodominant peptide model has been applied to a variety of other autoimmune diseases in which MHC alleles function as dominantly acting susceptibility genes (Svejgaard et al. 1983), including pemphigus vulgaris (Wucherpfennig et al. 1995).
One model to explain a dose effect of MHC genes on MS predicts that more than one gene within the MHC class 2 region, and perhaps beyond this region, contributes to disease risk. This model has been most fully developed for rheumatoid arthritis (RA) (Zanelli et al. 2000). Although disease-associated alleles differ between RA and MS, a dose effect of MHC genes on risk is common to the two conditions. In RA, DRB genes that encode a sequence containing a shared epitope—consisting of the motif Q(or R)K(or R)RRA in the third hypervariable region of the DRB protein contributing to the antigen binding properties of the molecule—influence susceptibility in a dominant manner (Gregersen et al. 1987). The adjacent DQ locus also influences risk but via a different mechanism, which may involve binding of the shared epitope itself to DQ, which results in the activation of protective regulatory T cells (van der Horst-Bruinsma et al. 1999).
In MS, the observed dose effects might be similarly explained by a dominantly acting susceptibility gene present on DRB1*1501 haplotypes plus the absence of a protective gene required for the maintenance of peripheral tolerance present on non-DRB1*1501 haplotypes. Loss of protection could conceivably result from a perturbation in the balance of Th1 and Th2 cytokines influenced by other class 2 MHC loci, such as DQ, or by other genes in the HLA region, such as tumor necrosis factor (TNF). Because of the tight LD between DRB1*1501 and DQB1*0602 in whites, it has not been possible to discern whether DQB1*0602 has any independent role in MS (Rubio et al. 2002). With respect to TNF, DRB1*1501 haplotypes are associated with a promoter polymorphism in TNF that modulates levels of expression of this proinflammatory Th1 cytokine (Garcia-Merino et al. 1996). T-cell clones triggered by antigen presented in the context of DRB1*1501 have also been reported to display a Th1-biased pattern of cytokine secretion (Zipp et al. 1995). Thus, homozygosity for DRB1*1501 could influence the outcome of interactions with MS autoantigenic peptides, by promoting an environment in which peripheral tolerance is not maintained. As an alternative explanation, current data cannot exclude the possibility that a dose effect of DRB1*1501 might result simply from higher levels of surface expression of DRB1*1501 on antigen-presenting cells in the homozygous state, increasing the likelihood that myelin autoantigens will be presented to encephalitogenic T cells.
The genotype-phenotype correlation revealed that patients with MS who were homozygous for HLA-DR2 were unlikely to have a benign course and to be at greater risk for a more severe disease outcome. In contrast to the observed HLA-DR2 effects on disease susceptibility, a single copy of the HLA-DR2 haplotype did not appear to influence mild or severe disease expression. The analyses of HLA-DR2 effects on disease behavior have been contradictory. Almost all studies have been based on phenotypic (presence or absence of DR2) rather than genotypic data and have shown associations with both favorable and unfavorable outcomes or no association at all (for review, see Kantarci et al. [2002]). A recent population-based case-control study showed no differences in DR2/DR2 genotype frequencies between PP MS and “bout onset” MS (Weinshenker et al. 1998). Although the numbers of individuals within the mild and severe phenotypic categories presented here are small, our results underscore the importance of including genotypic information in analyses of clinical data.
Our observation of a DR2 effect on disease outcome is also consistent with a model of protection mediated by DRB1*1501-negative haplotypes and the concept that a Th1 bias drives an aggressive disease course in experimental autoimmune demyelination (Powell et al. 1990) and in human MS (Beck et al. 1988; Tejada-Simon et al. 2001). Analogous to MS, in RA, a similar dose effect of MHC genes on indices of clinical severity, including erosive manifestations and rheumatoid factor production, is clearly present (Weyand et al. 1992; El-Gabalawy et al. 1999). The proposed model has several testable implications, including an expectation that myelin autoantigen-reactive T-cell clones derived from DRB1*1501 homozygous individuals should display a strong Th1 bias and that generation of regulatory T cells from such individuals might be impaired.
Acknowledgments
The Multiple Sclerosis Genetics Group also includes the following clinical investigators: D. Bourdette, M. Mass, B. Perry, J. Schafer, W. Au, R. Murray, L. Shaughnessy, R. Tillett, and D. Lockridge. We thank the patients with MS and their families for making this study possible. We also thank John Neuhaus and Glenys Thomson for very helpful comments. This work was funded by the National Multiple Sclerosis Society grants RG2542 (to S.L.H.) and RG2901 (to J.R.O.); National Institutes of Health grants NS26799 (to S.L.H. and J.R.O.), NS32830 (to J.L.H. and M.A.P.-V.) and AG-20135-01 (to E.R.M.); and the Nancy Davis Foundation.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Duke University Center for Human Genetics, http://wwwchg.mc.duke.edu/software/pdt.html (for Pedigree Disequilibrium Test computer program; a beta version of the geno-PDT program for genotype analysis is also available upon request: emartin@chg.mc.duke.edu)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MS [MIM 126200], DRB1 [MIM 142857], and DQB1 [MIM 604305])
References
- Allegretta M, Nicklas JA, Sriram S, Albertini RJ (1990) T cells responsive to myelin basic protein in patients with multiple sclerosis. Science 247:718–721 [DOI] [PubMed] [Google Scholar]
- Barcellos LF, Oksenberg JR, Green AJ, Bucher P, Rimmler JB, Schmidt S, Garcia ME, Lincoln RR, Pericak-Vance MA, Haines JL, Hauser SL (2002) Genetic basis for clinical expression in multiple sclerosis. Brain 125:150–158 [DOI] [PubMed] [Google Scholar]
- Barcellos LF, Schito AM, Rimmler JB, Vittinghoff E, Shih A, Lincoln R, Callier S, Elkins MK, Goodkin DE, Haines JL, Pericak-Vance MA, Hauser SL, Oksenberg JR (2000) CC-chemokine receptor 5 polymorphism and age of onset in familial multiple sclerosis. Multiple Sclerosis Genetics Group. Immunogenetics 51:281–288 [DOI] [PubMed] [Google Scholar]
- Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J (1988) Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand 78:318–323 [DOI] [PubMed] [Google Scholar]
- Bertrams J, Kuwert E (1972) HL-A antigen frequencies in multiple sclerosis: significant increase of HL-A3, HL-A10 and W5, and decrease of HL-A12. Eur Neurol 7:74–78 [DOI] [PubMed] [Google Scholar]
- Chataway J, Feakes R, Coraddu F, Gray J, Deans J, Fraser M, Robertson N, Broadley S, Jones H, Clayton D, Goodfellow P, Sawcer S, Compston A (1998) The genetics of multiple sclerosis: principles, background and updated results of the United Kingdom systematic genome screen. Brain 121:1869–1887 [DOI] [PubMed] [Google Scholar]
- Doolittle TH, Myers RH, Lehrich JR, Birnbaum G, Sheremata W, Franklin GM, Nelson LM, Hauser SL (1990) Multiple sclerosis sibling pairs: clustered onset and familial predisposition. Neurology 40:1546–1552 [DOI] [PubMed] [Google Scholar]
- El-Gabalawy HS, Goldbach-Mansky R, Smith D II, Arayssi T, Bale S, Gulko P, Yarboro C, Wilder RL, Klippel JH, Schumacher HR Jr (1999) Association of HLA alleles and clinical features in patients with synovitis of recent onset. Arthritis Rheum 42:1696–1705 [DOI] [PubMed] [Google Scholar]
- Garcia-Merino A, Alper CA, Usuku K, Marcus-Bagley D, Lincoln R, Awdeh Z, Yunis EJ, Eisenbarth GS, Brink SJ, Hauser SL (1996) Tumor necrosis factor (TNF) microsatellite haplotypes in relation to extended haplotypes, susceptibility to diseases associated with the major histocompatibility complex and TNF secretion. Hum Immunol 50:11–21 [DOI] [PubMed] [Google Scholar]
- Goodkin DE, Doolittle TH, Hauser SL, Ransohoff RM, Roses AD, Rudick RA (1991) Diagnostic criteria for MS research involving multiply affected families. Archives of Neurology 48:805–807 [DOI] [PubMed] [Google Scholar]
- Gregersen PK, Silver J, Winchester RJ (1987) The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30:1205–1213 [DOI] [PubMed] [Google Scholar]
- Haines JL, Ter-Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H, Pericak-Vance MA, et al (1996) A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. Nat Genet 13:469–471 [DOI] [PubMed] [Google Scholar]
- Haines JL, Terwedow HA, Burgess K, Pericak-Vance MA, Rimmler JB, Martin ER, Oksenberg JR, Lincoln R, Zhang DY, Banatao DR, Gatto N, Goodkin DE, Hauser SL (1998) Linkage of the MHC to familial multiple sclerosis suggests genetic heterogeneity. Hum Mol Genet 7:1229–1234 [DOI] [PubMed] [Google Scholar]
- Kantarci OH, de Andrade M, Weinshenker BG (2002) Identifying disease modifying genes in multiple sclerosis. J Neuroimmunol 123:144–159 [DOI] [PubMed] [Google Scholar]
- Kira J, Kanai T, Nishimura Y, Yamasaki K, Matsushita S, Kawano Y, Hasuo K, Tobimatsu S, Kobayashi T (1996) Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol 40:569–574 [DOI] [PubMed] [Google Scholar]
- Kurtzke JF (1983) Rating neurologic impairment in MS: an expanded disability status scale. Neurology 33:1444–1452 [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22 [Google Scholar]
- Ligers A, Dyment DA, Willer CJ, Sadovnick AD, Ebers G, Risch N, Hillert J and the Canadian Collaborative Study Groups (2001) Evidence of linkage with HLA-DR in DRB1*15-negative families with multiple sclerosis. Am J Hum Genet 69:900–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrosu MG, Cocco E, Lai M, Spinicci G, Pischedda MP, Contu P (2002) Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet 359:1461–1465 [DOI] [PubMed] [Google Scholar]
- Martin ER, Bass MP, Kaplan NL (2001) Correcting for a potential bias in the pedigree disequilibrium test. Am J Hum Genet 68:1065–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ER, Monks SA, Warren LL, Kaplan NL (2000) A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet 67:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Jaraquemada D, Flerlage M, Richert J, Whitaker J, Long EO, McFarlin DE, McFarland HF (1990) Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol 145:540–548 [PubMed] [Google Scholar]
- McWeeney S, Thomson G (2000) Meta analysis of relative penetrance rank order statistics with application to HLA DR-DQ genes and type 1 diabetes. A J Hum Genet 67:A214 [Google Scholar]
- MSG Group (1998) Clinical demographics of multiplex families with multiple sclerosis. Annals of Neurology 43:530–534 [DOI] [PubMed] [Google Scholar]
- Naito S, Namerow N, Mickey MR, Terasaki PI (1972) Multiple sclerosis: association with HL-A3. Tissue Antigens 2:1–4 [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg JR, Panzara MA, Begovich AB, Mitchell D, Erlich HA, Murray RS, Shimonkevitz R, Sherrit M, Rothbard J, Bernard CCA, Steinman L (1993) Selection for T-cell receptor V β-D β-J β gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature 362:68–70 [DOI] [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA (1990) T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 346:183–187 [DOI] [PubMed] [Google Scholar]
- Powell MB, Mitchell D, Lederman J, Buckmeier J, Zamvil SS, Graham M, Ruddle NH, Steinman L (1990) Lymphotoxin and tumor necrosis factor-α production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol 2:539–544 [DOI] [PubMed] [Google Scholar]
- Rasmussen HB, Kelly MA, Clausen J (2001) Additive effect of the HLA-DR15 haplotype on susceptibility to multiple sclerosis. Mult Scler 7:91–93 [DOI] [PubMed] [Google Scholar]
- Rubio JP, Bahlo M, Butzkueven H, van der Mei IA, Sale MM, Dickinson JL, Groom P, Johnson LJ, Simmons RD, Tait B, Varney M, Taylor B, Dwyer T, Williamson R, Gough NM, Kilpatrick TJ, Speed TP, Foote SJ (2002) Genetic dissection of the human leukocyte antigen region by use of haplotypes of Tasmanians with multiple sclerosis. Am J Hum Genet 70:1125–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz C, Patton KT, Anderson DE, Freeman GJ, Hafler DA (1998) Expansion of autoreactive T cells in multiple sclerosis is independent of exogenous B7 costimulation. J Immunol 160:1532–1538 [PubMed] [Google Scholar]
- Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW (1998) Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med 188:1511–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejgaard A, Platz P, Ryder LP (1983) HLA and disease 1982: a survey. Immunol Rev 70:193–218 [DOI] [PubMed] [Google Scholar]
- Tejada-Simon MV, Hong J, Rivera VM, Zhang JZ (2001) Reactivity pattern and cytokine profile of T cells primed by myelin peptides in multiple sclerosis and healthy individuals. Eur J Immunol 31:907–917 [DOI] [PubMed] [Google Scholar]
- Thomson G (1995) Mapping disease genes: family-based association studies. Am J Hum Genet 57:487–498 [PMC free article] [PubMed] [Google Scholar]
- Todd JA, Bell JI, McDevitt HO (1987) HLA-DQ β gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329:599–604 [DOI] [PubMed] [Google Scholar]
- Valli A, Sette A, Kappos L, Oseroff C, Sidney J, Miescher G, Hochberger M, Albert ED, Adorini L (1993) Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J Clin Invest 91:616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst-Bruinsma IE, Visser H, Hazes JM, Breedveld FC, Verduyn W, Schreuder GM, de Vries RR, Zanelli E (1999) HLA-DQ–associated predisposition to and dominant HLA-DR–associated protection against rheumatoid arthritis. Hum Immunol 60:152–158 [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Santrach P, Bissonet AS, McDonnell SK, Schaid D, Moore SB, Rodriguez M (1998) Major histocompatibility complex class II alleles and the course and outcome of MS: a population-based study. Neurology 51:742–747 [DOI] [PubMed] [Google Scholar]
- Weyand CM, Xie C, Goronzy JJ (1992) Homozygosity for the HLA-DRB1 allele selects for extraarticular manifestations in rheumatoid arthritis. J Clin Invest 89:2033–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Yu B, Bhol K, Monos DS, Argyris E, Karr RW, Ahmed AR, Strominger JL (1995) Structural basis for major histocompatibility complex (MHC)–linked susceptibility to autoimmunity: charged residues of a single MHC binding pocket confer selective presentation of self-peptides in pemphigus vulgaris. Proc Natl Acad Sci USA 92:11935–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli E, Breedveld FC, de Vries RR (2000) HLA association with autoimmune disease: a failure to protect? Rheumatology 39:1060–1066 [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY (1992) An overview of methods for the analysis of longitudinal data. Stat Med 11:1825–1839 [DOI] [PubMed] [Google Scholar]
- Zipp F, Weber F, Huber S, Sotgiu S, Czlonkowska A, Holler E, Albert E, Weiss EH, Wekerle H, Hohlfeld R (1995) Genetic control of multiple sclerosis: increased production of lymphotoxin and tumor necrosis factor-α by HLA-DR2+ T cells. Ann Neurol 38:723–730 [DOI] [PubMed] [Google Scholar]