Abstract
Charcot-Marie-Tooth type 2B (CMT2B) is clinically characterized by marked distal muscle weakness and wasting and a high frequency of foot ulcers, infections, and amputations of the toes because of recurrent infections. CMT2B maps to chromosome 3q13-q22. We refined the CMT2B locus to a 2.5-cM region and report two missense mutations (Leu129Phe and Val162Met) in the small GTP-ase late endosomal protein RAB7 which causes the CMT2B phenotype in three extended families and in three patients with a positive family history. The alignment of RAB7 orthologs shows that both missense mutations target highly conserved amino acid residues. RAB7 is ubiquitously expressed, and we found expression in sensory and motor neurons.
The inherited neuropathies of the peripheral nervous system show considerable clinical and genetical heterogeneity (De Jonghe et al. 2000). Some forms, the ulcero-mutilating neuropathies, are characterized by prominent sensory loss, often complicated by severe infections, arthropathy, and amputations (Auer-Grumbach et al., in press). So far, two loci and one gene have been reported for autosomal-dominant ulcero-mutilating neuropathies. However, molecular genetic studies have demonstrated that a third locus must exist (Auer-Grumbach et al. 2000b; Bellone et al. 2002). Hereditary sensory neuropathy type I (HSN I [MIM 162400]) maps to chromosome 9q22.1–q22.3 and is caused by mutations in the serine palmitoyltransferase, long chain base subunit-1 (SPTLC1) gene (Bejaoui et al. 2001; Dawkins et al. 2001). Charcot-Marie-Tooth type 2B (CMT2B [MIM 600882]) or hereditary motor and sensory neuropathy type IIB (HMSN IIB) was assigned to chromosome 3q13–q22 in an American family (Kwon et al. 1995). CMT2B is clinically characterized by marked distal muscle weakness and wasting and a high frequency of foot ulcers, infections, and amputations of the toes because of recurrent infections. CMT2B is mild to severe, with sensory loss and all modalities equally affected. Spontaneous pain is absent. Motor deficit is often the first and most prominent sign of the disease. The distal leg muscles are more affected than the hand muscles. Nerve-conduction–velocity studies indicate a primarily axonal neuropathy that allows clinical diagnosis in asymptomatic individuals (reviewed in Auer-Grumbach et al., in press).
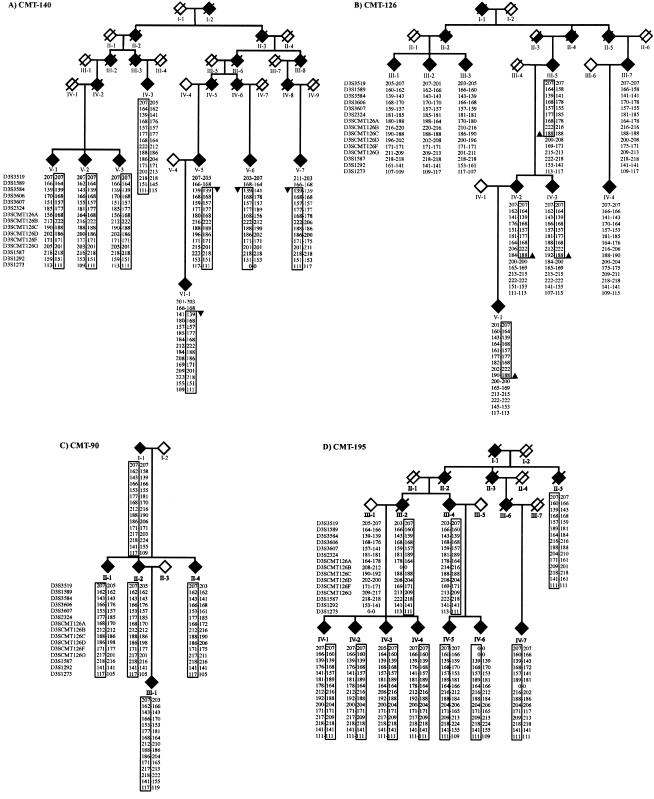
We performed a molecular genetic study of three families with an ulcero-mutilating phenotype who were previously linked to the CMT2B locus: an American family (CMT-195) (Kwon et al. 1995), a Scottish family (CMT-90) (De Jonghe et al. 1997), and an Austrian family (CMT-140) (Auer-Grumbach et al. 2000a). To determine whether these families share a common disease haplotype, we analyzed 15 STR markers in the CMT2B region. These markers included six new polymorphic STR markers (D3SCMT126A, D3SCMT126B, D3SCMT126C, D3SCMT126D, D3SCMT126F, and D3SCMT126G), which we isolated using sequence information from public databases. For each marker, alleles associated with the ulcero-mutilating phenotype were identified, and a disease haplotype was reconstructed in each family (fig. 1). No common disease haplotype was observed among families CMT-195, CMT-90, and CMT-140, suggesting the absence of a genetic relationship among these families with CMT2B. However, a common disease haplotype spanning seven STR markers, D3S3584–D3SCMT126C, was shared between the Austrian family CMT-140 and a small branch of another Austrian multigenerational pedigree, CMT-126 (patients III-5, IV-2, IV-3, and V-1), originally excluded from the CMT2B locus (Auer-Grumbach et al. 2000b; fig. 1). The five remaining patients (III-1, III-2, III-3, III-7, and IV-4) with a CMT phenotype in CMT-126 did not share this haplotype. The haplotype frequency for the shared disease haplotype in CMT-140 and part of CMT-126 is 4.6×10-6 and indicates that it is highly unlikely that both families share by chance the same alleles over a seven-marker interval. When examining the pedigree of CMT-126, it seems that the linked haplotype was married into the family by individual II-3. According to the family information, person II-3 was known to have a “strange gait,” but no clinical records were registered.
Figure 1.
Haplotype analysis of chromosome 3q13-22 STR markers in families with CMT2B. Pedigrees of four families with an ulcero-mutilating phenotype linked to the CMT2B locus: A, Austrian family CMT-140; B, Austrian family CMT-126; C, Scottish family CMT-90; and D, American family CMT-195. Symbols: open diamond = unaffected; filled diamond = affected; half-filled diamond = probably affected (i.e., CMT-126 II-3); slashed diamond = deceased; arrow = recombination; box = disease-associated haplotype. Pedigree structure and sex are disguised to preserve anonymity. The best genetic and physical order of STR markers is according to NCBI (GenBank/LocusLink). The six newly developed STR markers (D3SCMT126A, D3SCMT126B, D3SCMT126C, D3SCMT126D, D3SCMT126F, and D3SCMT126G) are localized between D3S2324 and D3S1587. Genotypes are represented by allele sizes in base pairs, and 0-0 = failed genotype. The RAB7 gene is located on the same contig (NT_005523) as markers D3SCMT126B and D3SCMT126C. For genotyping, fragment analysis was performed on an ABI Prism3700 DNA Analyzer and processed with the ABI GENESCAN 3.5 and GENOTYPER 3.6 software (Perkin Elmer Applied Biosystems).
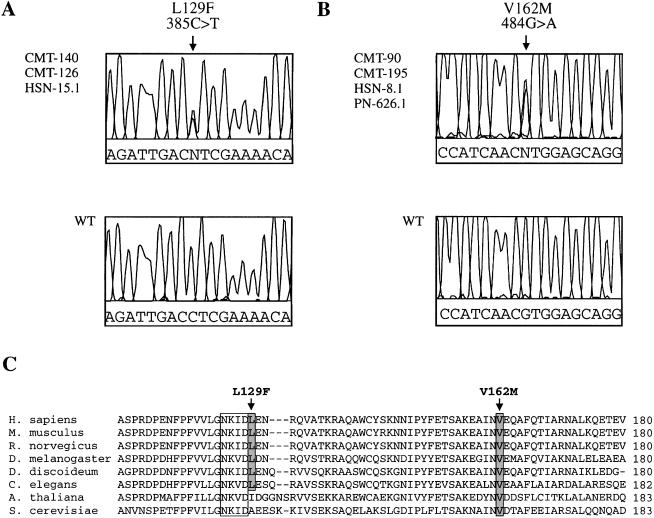
In family CMT-140, we observed a recombination in affected individuals V-5, V-6, V-7, and VI-1 between STR markers D3S1589 and D3S3584, mapping the CMT2B locus telomeric to D3S1589. The recombination in patients III-5, IV-2, IV-3, and V-1 of the CMT-126 family mapped the CMT2B locus centromeric to marker D3SCMT126D. These data refined the CMT2B region to 2.5 cM, between D3S1589 and D3SCMT126D (fig. 1). In the CMT2B locus, we selected three known positional candidate genes for mutation analysis in the families with CMT2B: ZNF9 (zinc finger protein 9), ABTB1 (ankyrin repeat and BTB domain containing 1) and RAB7 (small GTP-ase late endosomal protein RAB7) (NCBI Map viewer). For each gene, we determined the exon-intron boundaries of the candidate sequences by BLAST searches against the HTGS (intronic primers sequences available on request). We sequenced all known exons and intron-exon boundaries in the patients with CMT2B and detected no disease-causing mutations in ZNF9 or ABTB1. However, in exon 3 of RAB7, we observed a c.385C→T transition (Leu129Phe) in all patients of family CMT-140, which was also present in the patients belonging to the small linked branch of family CMT-126 (fig. 2A). A second c.484G→A transition (Val162Met) in exon 4 was observed in families CMT-195 and CMT-90 (fig. 2B). The missense mutations showed cosegregation with the CMT2B phenotype in all pedigrees and were absent from 200 control chromosomes.
Figure 2.
DNA and protein sequence analysis of RAB7. A, Electropherogram showing the c.385C→T sequence variation in part of exon 3, resulting in the Leu129Phe missense mutation in families CMT-140 and CMT-126 and patient HSN-15.1. B, Electropherogram of the c.484G→A sequence variation in part of exon 4, resulting in the Val162Met missense mutation in families CMT-90 and CMT-195 and patients HSN-8.1 and PN-626.1. The corresponding genomic sequence of a control person is shown below. DNA sequencing was performed using the DYEnamic ET Terminator Cycle Sequencing Kit (Amersham Pharmacia Biotech), and the sequencing reactions were loaded on the ABI Prism3700 DNA Analyzer (Perkin Elmer Applied Biosystems). The data were collected and analyzed via the ABI DATA COLLECTION version 1.1 and DNA SEQUENCING ANALYSIS version 3.6 software, respectively. C, ClustalW multiple protein alignment of the RAB7 orthologs of the region surrounding the Leu129Phe and Val162Met mutations. RAB7 orthologs: Human (Homo sapiens), mouse (Mus musculus), rat (Rattus norvegicus), fly (Drosophila melanogaster), slime mold (Dictyostelium discoideum), nematode (Caenorhabditis elegans), mouse-ear cress (Arabidopsis thaliana), and baker’s yeast (Saccharomyces cerevisiae). The highly conserved motif involved in guanine nucleotide binding is boxed. Both amino acid mutations are shaded and indicated by an arrow.
It is interesting that the remaining patients of family CMT-126 (III-1, III-2, III-3, III-7, and IV-4) did not have the Leu129Phe mutation in RAB7. The fact that individuals III-5, IV-2, IV-3, and V-1 of family CMT-126 shared the same disease haplotype (fig. 1) and the same c.385C→T (Leu129Phe) mutation as the patients in family CMT-140 (fig. 2A), indicated a close familial relationship between CMT-140 and part of CMT-126, who originated from the same Austrian province. In CMT-126, we found no obvious differences in neurological and electrophysiological findings between patients with the Leu129Phe mutation and those without the RAB7 mutation, except that the phenotype was more severe in the branch with the Leu129Phe mutation, including the occurrence of ulcers and amputations. Also, the ulcero-mutilating CMT phenotype of the remaining patients in family CMT-126 is likely to be caused by a mutation in another gene (since SPTLC1 was excluded; data not shown) and further supports the presence of a third locus for ulcero-mutilating neuropathies.
In addition to this study, we selected a set of 24 unrelated patients diagnosed with an HSN or ulcero-mutilating CMT phenotype for mutation analysis of RAB7. In three patients, two Austrian and one Belgian, we observed Leu129Phe (HSN-15.1) once and Val162Met twice (HSN-8.1 and PN-626.1). These three patients with RAB7 mutations have a family history of CMT and ulcero-mutilations, but the additional patients were not cooperative for genetic studies. Haplotype analysis confirmed that the Austrian patient HSN-15.1, with a family history of CMT2B, shared the linked haplotype with the Austrian families CMT-140 and part of CMT-126. These findings indicate a founder effect for the Leu129Phe mutation in families CMT-126, CMT-140, and HSN-15.1. However, the Austrian (HSN-8.1) and Belgian (PN-626.1) patients with the Val162Met mutation do not share a common disease haplotype and are not related to the Scottish CMT-90 and American CMT-195 families (data not shown).
The alignment of RAB7 orthologs shows that both missense mutations target highly conserved amino acid residues (fig. 2C). The Val162Met mutation affects a valine that is conserved among all species. The Leu129Phe mutation is located next to a conserved GTP-binding domain (NKID). The leucine residue at position 129 is not conserved in Arabidopsis and yeast.
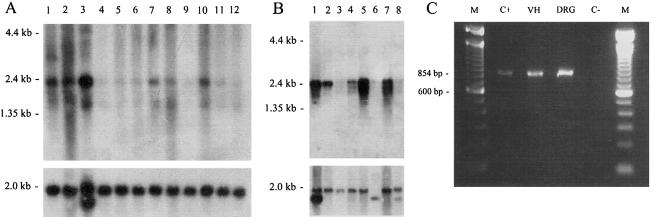
Vitelli and coworkers (1996) reported expression of two transcripts of 2.5 kb and 1.8 kb for the human RAB7 gene in different cell types. The expression information of human RAB7 and mouse Rab7 in the Unigene database suggests ubiquitous expression (Unigene Clusters: Hs.356386 and Mm.4268). We found expression in all tested tissues; in human, high expression was found in skeletal muscle (fig. 3A), and, in mouse, high expression was found in liver, heart, and kidney (fig. 3B). PCR analysis of cDNA from E13 mouse dorsal root ganglia and ventral horn showed expression of Rab7 in sensory and motor neurons, respectively (fig. 3C).
Figure 3.
Expression analysis of RAB7. A, Human 12-lane Multiple Tissue Northern blot (Clontech) containing poly A+ RNA from adult tissues: lane 1, brain; lane 2, heart; lane 3, skeletal muscle; lane 4, colon; lane 5, thymus; lane 6, spleen; lane 7, kidney; lane 8, liver; lane 9, small intestine; lane 10, placenta; lane 11, lung; and lane 12, peripheral blood leukocyte. The probe used for hybridization is a cDNA fragment of 800 bp, obtained from plasmid clones containing partial human RAB7 cDNA sequences (IMAGp956B0837, IMAGp956M0263, and IMAGp956M2246 [Resource Center of the German Human Genome Project]). B, Mouse multiple tissue northern blot containing poly A+ RNA from adult tissues (Clontech): lane 1, heart; lane 2, brain; lane 3, spleen; lane 4, lung; lane 5, liver; lane 6, skeletal muscle; lane 7, kidney; and lane 8, testis. The probe used for hybridization is a full-length mouse Rab7 cDNA cloned into the pCRII-TOPO vector (Invitrogen). The mouse Rab7 cDNA was generated via RT-PCR and SMART RACE cDNA Amplification (Clontech) from mouse brain RNA. Both northern blots (A and B) were also hybridized with a β-actin cDNA probe (Clontech) as a control for RNA loading (lower part of figure). C, PCR amplification of a mouse Rab7 cDNA fragment of 854 bp: M, 100-bp size marker; C+, Rab7 full-length cDNA cloned into pCRII-TOPO; VH, ventral horn cDNA; DRG, dorsal root ganglia cDNA; C-, blanco. VH and DRG were isolated from 13-d-old mouse embryos, total RNA was extracted using the Totally RNA Kit (Ambion), and RT-PCR was performed using the SMART PCR cDNA Synthesis Kit (Clontech). Mouse Rab7 cDNA primers (MRAB7-2F = 5′-CTGACCAAGGAGGTGATGGT-3′ and MRAB7-2R = 5′-GAACAGTTCTCTCACTCTCC-3′) were used to PCR amplify the Rab7 cDNA fragment of 854 bp in both neuron populations.
RAB7 belongs to the Rab family of Ras-related GTP-ases. These Rab proteins are essential for the regulation of intracellular membrane trafficking. The Rab proteins regulate vesicular transport through the recruitment of specific effector or motor proteins and may have a role in linking vesicles and target membranes to the cytoskeleton (Echard et al. 1998; Nielsen et al. 1999). RAB7 is involved in transport between late endosomes and lysosomes, and recent studies demonstrate that the Rab7-effector protein RILP (Rab interacting lysosomal protein) induces the recruitment of dynein-dynactin motors and regulates transport toward the minus-end of microtubules (Cantalupo et al. 2001; Jordens et al. 2001). Expression of Rab7 dominant-negative artificial mutants in mammalian cells inhibits lysosomal degradation and disperses lysosomes. One such mutant, rab7N125I, is localized in the GTP-binding domain of Rab7 (Press et al. 1998). In vitro studies, performed in hamster BHK kidney cells, demonstrated that this mutant rab7N125I protein exists preferentially in a nucleotide-free form and has been shown to have a dominant-negative effect on late endocytic transport (Press et al. 1998). In contrast, in human fibroblasts overexpressing Rab7, late endocytic vesicles accumulated in the perinuclear region, probably because of an increased motility in the minus-end direction of microtubules (Lebrand et al. 2002). Overexpression studies of wild-type Rab7 also demonstrated that the Rab7 protein is involved in the Golgi targeting of glycosphingolipids (Choudhury et al. 2002). It is interesting that the SPTLC1 gene, mutated in patients with ulcero-mutilating HSN type I, is involved in the biosynthesis of sphingolipids (Bejaoui et al. 2001; Dawkins et al. 2001).
To date, 60 human RAB genes have been identified, and the majority are likely to control highly specialized functions in many cell types. Mutations in RAB genes may, therefore, cause a wide range of inherited diseases. In addition, there is evidence for alteration in RAB function in the pathogenesis of acquired diseases, such as infection due to intracellular micro-organisms (Seabra et al. 2002). It is unclear how dysfunction of RAB7 causes sensory and motor neuropathy in patients with CMT2B. However, other Rab proteins are involved in trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons (Tang 2001). The RAB3A protein regulates neurotransmitter release and other forms of regulated secretion (Geppert et al. 1997). The Rab23 protein is implicated in neural patterning, as demonstrated in the mouse sonic hedgehog signaling pathway (Eggenschwiler et al. 2001).
In conclusion, we report two missense mutations in the RAB7 late endosomal protein as the cause for the ulcero-mutilating inherited peripheral neuropathy CMT2B. It will be a challenge to explain why mutations in such a universally expressed protein as RAB7 lead to an axonal pathology in CMT2B.
Acknowledgments
We gratefully acknowledge the cooperation and participation of all patients and their relatives in this study. We also thank Prof. Dr. Christine Van Broeckhoven for critical reading and helpful discussions. This research project was supported by the Fund for Scientific Research-Flanders (FWO); the University of Antwerp; the Geneeskundige Stichting Koningin Elisabeth; the Association Belge contre les Maladies Musculaires, Belgium; the Muscular Dystrophy Association, United States; and the Fonds zur Förderung der wissenschaftlichen Forschung, Austria (FWF P13563-BIO and FWF P15378). This research was also performed within the frame of the Interuniversity Attraction Poles programme P5/19 of the Federal Office for Scientific, Technical, and Cultural Affairs, Belgium. N.V. received a Ph.D. student fellowship from the Institute of Science and Technology, Belgium. K.V. is a postdoctoral fellow at the FWO.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- ClustalW, http://npsa-pbil.ibcp.fr/ (for multiple protein alignment)
- GenBank, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Protein (for LotusLink and protein sequences: RAB7_human [P51149]; Rab7_mouse [P51150]; Rab7_rat [P09527]; Rab-protein 7 Drosophila melanogaster [NP_524472]; Rab7_Dictyostelium discoideum [P36411]; Ras related protein_Caenorhabditis elegans [NP_496549]; Rab7_Arabidopsis thaliana [O04157]; and YPT7_YEAST [P32939])
- German Human Genome Project, http://www.rzpd.de/ (clones for partial human RAB7 cDNA sequences)
- NCBI Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/map_search.cgi?chr=hum_chr.inf&query (for finding known genes, ESTs, and putative novel genes in the CMT2B region)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CMT2B [MIM 600882] and HSN I [MIM 162400]).
References
- Auer-Grumbach M, De Jonghe P, Verhoeven K, Timmerman V, Wagner K, Hartung HP, Nicholson GA. Autosomal dominant inherited neuropathies with prominent sensory loss and mutilations: a review. Arch Neurol (in press) [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M, De Jonghe P, Wagner K, Verhoeven K, Hartung HP, Timmerman V (2000a) Phenotype-genotype correlations in a CMT2B family with refined 3q13-q22 locus. Neurology 55:1552–1557 [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M, Wagner K, Timmerman V, De Jonghe P, Hartung HP (2000b) Ulcero-mutilating neuropathy in an Austrian kinship without linkage to hereditary motor and sensory neuropathy IIB and hereditary sensory neuropathy I loci. Neurology 54:45–52 [DOI] [PubMed] [Google Scholar]
- Bejaoui K, Wu C, Scheffler MD, Haan G, Ashby P, Wu L, de Jong P, Brown RH Jr (2001) SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet 27:261–262 [DOI] [PubMed] [Google Scholar]
- Bellone E, Rodolico C, Toscano A, Di Maria E, Cassandrini D, Pizzuti A, Pigullo S, Mazzeo A, Macaione V, Girlanda P, Vita G, Ajmar F, Mandich P (2002) A family with autosomal dominant mutilating neuropathy not linked to either Charcot-Marie-Tooth disease type 2B (CMT2B) or hereditary sensory neuropathy type I (HSN I) loci. Neuromuscul Disord 12:286–291 [DOI] [PubMed] [Google Scholar]
- Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C (2001) Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J 20:683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Dominguez M, Puri V, Sharma DK, Narita K, Wheatley CL, Marks DL, Pagano RE (2002) Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest 109:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA (2001) Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet 27:309–312 [DOI] [PubMed] [Google Scholar]
- De Jonghe P, Timmerman V, FitzPatrick D, Spoelders P, Martin J-J, Van Broeckhoven C (1997) Mutilating neuropathic ulcerations in a chromosome 3q13-q22 linked Charcot-Marie-Tooth disease type 2B family. J Neurol Neurosurg Psychiatry 62:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe P, Timmerman V, Nelis E (2000) Hereditary peripheral neuropathies. In: Deymeer F (ed) Neuromuscular diseases: from basic mechanisms to clinical management. Karger, Basel [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B (1998) Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 279:580–585 [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Espinoza E, Anderson KV (2001) Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature 412:194–198 [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Sudhof TC (1997) The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 387:810–814 [DOI] [PubMed] [Google Scholar]
- Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol 11:1680–1685 [DOI] [PubMed] [Google Scholar]
- Kwon JM, Elliott JL, Yee WC, Ivanovich J, Scavarda NJ, Moolsintong PJ, Goodfellow PJ (1995) Assignment of a second Charcot-Marie-Tooth type II locus to chromosome 3q. Am J Hum Genet 57:853–858 [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Corti M, Goodson H, Cosson P, Cavalli V, Mayran N, Faure J, Gruenberg J (2002) Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J 21:1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M (1999) Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol 1:376–382 [DOI] [PubMed] [Google Scholar]
- Press B, Feng Y, Hoflack B, Wandinger-Ness A (1998) Mutant Rab7 causes the accumulation of cathepsin D and cation- independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol 140:1075–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra MC, Mules EH, Hume AN (2002) Rab GTPases, intracellular traffic and disease. Trends Mol Med 8:23–30 [DOI] [PubMed] [Google Scholar]
- Tang BL (2001) Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J Neurochem 79:923–930 [DOI] [PubMed] [Google Scholar]
- Vitelli R, Chiariello M, Lattero D, Bruni CB, Bucci C (1996) Molecular cloning and expression analysis of the human Rab7 GTP-ase complementary deoxyribonucleic acid. Biochem Biophys Res Commun 229:887–890 [DOI] [PubMed] [Google Scholar]