Abstract
The dimorphic fungus Histoplasma capsulatum is the etiologic agent of one of the most common systemic mycoses of humans, histoplasmosis. In the environment, H. capsulatum grows in a differentiated mold form and shifts to an undifferentiated yeast form after mold fragments or spores are inhaled. This mold-to-yeast shift is required for disease. Little is known about the molecular biology of dimorphism in Histoplasma, and most studies have been directed toward yeast-specific genes. While it is important to examine the role of genes upregulated in the yeast morphotype, genes which are silenced in the yeast (i.e., mold-specific genes) may also play a critical role in dimorphism. To begin to examine this hypothesis, we report here the first misexpression and knockout analysis of a mold-specific gene in Histoplasma. The strongly expressed MS8 gene encodes a predicted 21-kDa protein extremely rich in glycine and glutamine. Forced expression of MS8 driven by the TEF1 promoter in yeast did not alter the yeast morphology at 37°C or mold formation at 25°C. Yeast expressing MS8 did exhibit clumping in liquid medium and formed “sticky” colonies on agar plates. Allelic replacement of MS8 was accomplished by a positive-negative selection procedure. ms8 knockout mutants formed apparently normal yeast at 37°C but gave rise to aberrant mycelia at 25°C. The mold colonies of the knockouts were less than half as large as normal, had a granular surface, produced a dark-red pigment, and formed short hyphae which were 40% wider with a distinctive twisted “zig-zag” shape.
The dimorphic fungus Histoplasma capsulatum is the etiologic agent of one of the most common systemic mycoses of humans, histoplasmosis. Integral to pathogenesis is the mold-to-yeast (M-Y) dimorphic shift. The organism grows in soil as a differentiated multicellular mold. When mold fragments or spores are inhaled, the fungus shifts to an undifferentiated single-cell yeast growth form in the lungs of the host. If this M-Y shift is blocked, the disease cannot progress (11). In the laboratory, this reversible dimorphism can be observed by changing the incubation temperature to favor the desired form: 25°C for mold or 37°C for yeast.
The molecular basis of the shift from the differentiated multicellular form to the undifferentiated unicellular form is unknown. Investigators have searched for genes upregulated in the pathogenic yeast form to identify genes required for dimorphism and virulence, and several interesting genes have been identified in these studies, as summarized in the recent review by Retallack and Woods (13). Keath and Abidi (4), for example, reported that the yps-3 gene is specific to the yeast form and is expressed at higher levels in the most virulent strains. Sebghati et al. (16) demonstrated that the calcium binding protein gene CBP1 is required for survival within host phagocytic cells. Neither gene is required for dimorphism, however, since strains that do not express yps-3 and cbp1 knockout mutants still exhibit apparently normal dimorphism.
Although the search for yeast-specific genes as regulators of dimorphism is quite logical, it is possible that genes critical for the dimorphic shift are being overlooked due to the assumption that yeast-specific or yeast-upregulated genes are the most important regulators. It may be necessary, for example, to downregulate certain mold-specific genes in order to form the in vivo yeast morphotype. One can envision a scenario in which mold-specific (MS) genes would exert an overriding effect, i.e., expression of a mold-specific gene(s) would shift growth to the mold form and a shutdown of this gene(s) would be required to allow the shift to the undifferentiated yeast form. We have begun to examine this hypothesis by isolating a number of genes which are silent in the yeast morphotype (18). Here, we report the first misexpression and knockout analysis of a mold-specific gene, MS8, in H. capsulatum and show that ms8 knockout mutants form aberrant mycelia.
MATERIALS AND METHODS
Strains and growth conditions. (i) Yeast strains.
The H. capsulatum strains G186AS and G184AS (7) were used in these studies. Both strains represent restriction fragment length polymorphism class 3 isolates (5) and exhibit the same expression profile of MS8. A uracil auxotroph of G184AS, G184AS ura5-11, the kind gift of Jon Woods (University of Wisconsin, Madison), was used for knockout experiments. Routine cultures were grown in GYE medium (2% [wt/vol] glucose, 1% [wt/vol] yeast extract) at 37°C with 150-rpm gyratory shaking for yeast or at 25°C with 100-rpm gyratory shaking for mold. Cultures for transformation or knockout analysis were grown in HMM, a rich synthetic medium based on F-12 tissue culture medium supplemented with cysteine and glutamic acid and buffered to pH 7.5 with HEPES (22). HMM plates were made by adding 1% (wt/vol) agarose (A-5093; Sigma). To reduce the chance of bacterial contamination during the prolonged incubations, 50 μg of ampicillin/ml and 100 μg of streptomycin/ml were added to the HMM. The following additions to HMM were made when necessary: HMM/u, 50 μg of uracil/ml; HMM/h, 200 μg of hygromycin/ml; and HMM/f, 1 mg of 5-fluoroorotic acid/ml.
Yeast cultures for DNA or RNA extraction were grown to mid-log phase and harvested by centrifugation. Actively growing mold cultures for nucleic acid extraction were prepared as follows: 5 ml of a dense mold culture was inoculated into 100 ml of fresh medium, incubated at 25°C with gyratory shaking for 3 days, and then harvested by vacuum filtration.
(ii) Escherichia coli strains.
E. coli SURE-2 cells {e14 (McrA) Δ(mcrCB-hsdSMR-mrr)171 endA1 supE44 thi-1 gyrA96 relA1 lac recB recJ sbcC umuC::Tn5 (Kanr) uvrC [F′ proAB lacIqZ Δ(M15 Tn10) (Tetr) Amy Camr]; Stratagene}, which give better results with the H. capsulatum telomeric-repeat plasmids, were used for routine plasmid preparations. Telomere plasmids prepared in other strains occasionally exhibit sequence rearrangements, presumably because of the telomeric repeats.
Preparation of DNA.
Plasmids from E. coli were isolated by alkaline lysis and Celite chromatography with the Wizard kit (Promega, Madison, Wis.) according to the manufacturer's directions. Purification of DNA from agarose gels or after enzyme reactions was done by silica adsorption with the Zymoclean kit (Zymo Research, Orange, Calif.) according to the manufacturer's directions.
Large-scale genomic DNA preparations from Histoplasma were done as follows. Approximately 35 ml of mid-log-phase yeast was harvested by centrifugation at 2,000 × g for 5 min, resuspended in 35 ml of ice-cold water, and collected by centrifugation for 5 min. The cell pellet was resuspended in 0.6 ml of DNA extraction buffer (100 mM Tris [pH 8.0], 100 mM EDTA, 250 mM NaCl). Phenol-chloroform (5:1; pH 7.9) (0.6 ml) and 0.8 ml of 0.45-mm-diameter glass beads were added, and the tubes were vortexed on a Vortex Genie 2 (Fisher Scientific) at maximum speed for 1 min. The tubes were cooled on ice for 1 min, followed by vortexing for 1 min. The cycles of vortexing and cooling were repeated for a total vortex time of 5 min. The cell debris and beads were pelleted by centrifugation at 11,000 × g for 10 min. The supernatant was removed to a new tube, and 30 μl of a 5-mg/ml RNase A solution was added. The tubes were incubated at 37°C for 60 min to digest the RNA. The mixture was extracted with an equal volume of phenol-chloroform (5:1; pH 7.9), and the aqueous phase was removed to a new tube. DNA was precipitated from the aqueous phase by the addition of 2 volumes of ethanol followed by centrifugation at 11,000 × g for 10 min. The DNA pellet was dissolved in 10 mM Tris (pH 7.8)-1 mM EDTA, and the concentration was determined by absorbance at 260 nm. The DNA typically migrated as a single large band slightly larger than a 23-kb lambda HindIII marker when analyzed by agarose gel electrophoresis.
DNA miniprep for PCR testing of putative knockout colonies was as follows. A single colony was scraped from an HMM plate with a sterile toothpick and resuspended in 50 μl of H2O in a 500-μl microcentrifuge tube. DNA extraction buffer (100 μl), 0.45-mm-diameter glass beads (50 μl), and 100 μl of phenol-chloroform (5:1; pH 8.0) were added, and the tube was vortexed for 3 min. The tube was centrifuged at 10,000 × g for 5 min, and the aqueous phase was recovered. RNase A was added to the aqueous phase to a final concentration of 100 μg/ml, and the tube was incubated at room temperature for 10 min. The DNA was then ethanol precipitated as described above and dissolved in sterile water.
Preparation of RNA.
RNA was isolated by extraction with acidic phenol, which partitions RNA in the aqueous phase and DNA and protein in the lower phases (8). Mid-log-phase yeast cells were collected by centrifugation at 400 × g for 5 min. Actively growing mold was vacuum filtered onto 0.45-μm-pore-size nitrocellulose membranes and washed with ice-cold sterile water. The mycelium was scraped from the filter with a plastic spatula. Yeast or mold cells were resuspended in 0.4 ml of ice-cold RNA extraction buffer (0.1 M sodium acetate [pH 5.0], 0.2 M NaCl, 0.2% [wt/vol] sodium dodecyl sulfate [SDS]) in a 1.5-ml screw cap microcentrifuge tube. An equal volume of acidic phenol-chloroform (5:1; equilibrated with RNA extraction buffer) and 300 μl of 0.45-mm-diameter glass beads were added. The tubes were vortexed at maximum speed for 10 min at room temperature and centrifuged for 3 min at 10,000 × g, and the aqueous phase was recovered to a new tube. RNA was precipitated from the aqueous phase by the addition of 2 volumes of ethanol followed by centrifugation at 10,000 × g for 10 min. The RNA pellet was washed once with 75% ethanol and dissolved in 10 mM Tris (pH 7.5)-1 mM EDTA-0.2% (wt/vol) SDS by heating the solution at 65°C for 10 min. Any insoluble material was removed by centrifugation at 10,000 × g for 10 min, and the RNA in the supernatant was quantitated by absorbance at 260 nm.
Southern and Northern blotting.
For Southern blotting, approximately 1 μg of H. capsulatum genomic DNA was digested with restriction enzymes (New England Biolabs) and electrophoresed on a 0.7% (wt/vol) agarose gel. The DNA was transferred onto charged nylon membranes (Hybond N+; Amersham Corp.) by downward blotting with 0.4 N NaOH using a Turboblotter (Schleicher and Schuell, Keene, N.H.). The membrane was neutralized with several washes of 2× SSC (1× SSC is 0.15 M NaCl-0.015 M sodium citrate, pH 7.0) and allowed to air dry. The membranes were prehybridized in 0.5 M sodium phosphate (pH 7.0)-5% (wt/vol) SDS at 65°C for 1 h. Denatured radiolabeled probe was added and allowed to hybridize at 65°C for 12 to 16 h. The membranes were washed once at 65°C with 2× SSC-1% (wt/vol) SDS and two times with 0.1× SSC-0.1% (wt/vol) SDS. Radiolabeled probes were prepared with [α-32P]dATP with the Strip-EZ kit (Ambion). The blots were later stripped according to the manufacturer's directions.
Northern blots were prepared by electrophoresis of approximately 10 μg of total RNA per lane on a 2% denaturing formaldehyde agarose gel according to standard methods (9). RNA was transferred onto charged nylon membranes (Hybond N+) with 20× SSC by using a Turboblotter. After the transfer, the membrane was soaked in 50 mM NaOH for 4 min and neutralized with 2× SSC. RNA was cross-linked to the membrane by exposure to 254-nm UV light for a dosage of 120 mJ/cm2. Probe labeling, prehybridization, hybridization, and washes were done as described for Southern blots.
MS8 genomic walking and sequencing.
The MS8 gene and flanking sequence were isolated by genomic-walking PCR (3). Forward and reverse primers specific for the previously cloned MS8 cDNA (GenBank accession no. AF292398) were used to isolate approximately 2 kb of upstream and 1.6 kb of downstream sequence. Primers were then designed to isolate the entire genomic DNA for MS8 via PCR. DNA was sequenced by the dideoxy chain termination method. To reduce sequence errors from PCR, a proofreading polymerase (Advantage 2; Clontech) was used and three independent PCR products were sequenced.
Plasmid construction.
An H. capsulatum telomere vector was made based on the successful plasmid constructs of Woods and Goldman (20). A DNA construct containing (in 5′-3′ order) a NotI site, 12 H. capsulatum telomeric repeats (GGGTTA), a PmeI site, a 1.3-kb HindIII-StyI tetracycline resistance marker from pBR322, a PmeI site, 12 H. capsulatum telomeric repeats (TAACCC), and a NotI site was cloned into the NotI site of pGem11 Zf+ (Promega) to generate pG11TelTet. A 1.7-kb EcoRI fragment containing the Pa Ura5 gene from pWU55 (21) was cloned into the EcoRI site of pG11TelTet to generate pXTU1. A 1.0-kb fragment of H. capsulatum genomic DNA (representing approximately 950 nucleotides [nt] of 5′ untranscribed flanking sequence and 50 nt of the 5′ untranslated region of the H. capsulatum translation elongation factor 1 [TEF1] gene) containing the strong constitutive H. capsulatum TEF1 promoter was cloned into the XhoI site of pXTU1 to generate pXTU2. ApaI linkers were ligated to the MS8 cDNA (GenBank accession no. AF292398), which was then inserted into the ApaI site of pXTU2 immediately downstream of the TEF1 promoter to generate pXTU3. Several clones were isolated and analyzed by restriction cutting to select the sense orientation relative the TEF1 promoter.
An MS8 knockout vector was constructed as follows. A 4.3-kb H. capsulatum genomic DNA PCR product containing the MS8 gene and 2 kb of 5′ flanking sequence plus 1.5 kb of 3′ flanking sequence was cloned into pGemTeasy (Promega) to generate pMS8g. A 760-nt BclI/BstBI fragment in pMS8g representing most of the MS8 coding sequence was replaced with a 2.9-kb BamHI/ClaI hygromycin resistance marker from pMAD93 (21) to generate pMS8g::hph. pXTU3 was cut with SmaI and ApaI to remove the TEF1 promoter and MS8 cDNA. The remaining vector was blunted with Klenow and ligated to NotI adapters. A 6.4-kb NotI fragment containing the knockout construct was isolated from pMS8g::hph and ligated to the resulting vector to form the knockout vector pXTU4Δ. The vector pXTU3g was constructed for ms8 knockout complementation experiments as follows. pXTU3 was cut with SmaI and ApaI to remove the TEF1 promoter and MS8 cDNA. The remaining vector was blunted with Klenow and ligated to NotI adaptors. The 4.3-kb MS8 genomic fragment was cut from pMS8g with NotI and ligated to the remaining pXTU3 vector to generate pXTU3g.
Histoplasma transformation.
Plasmids were digested with PmeI to remove the Tetr marker and expose the telomeric repeats. The resulting linear vector was purified by agarose gel electrophoresis and recovered from the gel using a silica gel column (Zymoclean). H. capsulatum yeast was transformed by electroporation by a modification of the method of Woods et al. (21). Briefly, 5 ml of mid-log-phase yeast cells from an HMM broth culture was centrifuged at room temperature at 300 × g for 5 min to harvest the cells. The cells were washed in 5 ml of 10% (wt/vol) mannitol and collected by centrifugation. The cell pellet was resuspended in 350 μl of 10% (wt/vol) mannitol, and 0.5 μg of linearized vector (contained in less than 10 μl of sterile water) was added and mixed. The cell-DNA mixture was transferred to a 0.2-cm electroporation cuvette (Bio-Rad). Electroporation was done in an ElectroPorator (Invitrogen) at the following settings: 750V; 71 μF; 150Ω (which yields a pulse of 3,750 V/cm for 10.6 ms). For uracil selection, the cells were immediately plated on HMM plates. For hygromycin selection, the cells were mixed with 0.8 ml of HMM and incubated on a roller drum at 37°C for 12 h to allow expression of the hph marker, followed by spreading the mixture on HMM/h plates. The plates were incubated in a humidified chamber at 37°C. Small colonies were typically visible in about 7 days.
Construction of knockout mutants.
Genomic replacement of the native MS8 locus with the hph-disrupted MS8 construct was accomplished by a modification of the positive-negative selection method of Sebghati et al. (16). G184AS ura5-11 yeast was transformed with linearized pXTU4Δ. The cells were spread on HMM and incubated for 7 to 10 days at 37°C. Ten colonies were picked and inoculated into 1 ml of HMM broth in 10 disposable 15-ml plastic screw cap centrifuge tubes. A small hole was made in the cap of each tube with a 26-gauge needle, and the tubes were incubated on a roller drum at 37°C for 1 week to allow time for the double-crossover event to occur. Positive-negative selection was imposed by spreading 200 μl from each tube onto HMM/uhf plates to select for the hygromycin marker and against the Ura5 marker. The plates were incubated at 37°C for 7 to 10 days, and 10 colonies were selected for miniprep DNA extraction and analysis by PCR to identify knockout mutants.
RESULTS
Sequence analysis of MS8.
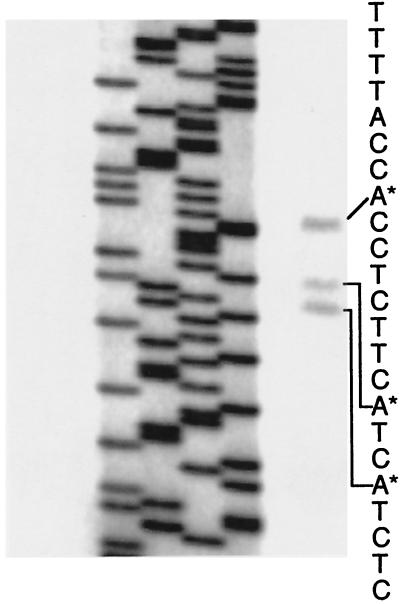
The complete nucleotide sequence of the MS8 gene is shown in Fig. 1. The gene is interrupted by a single intron of 95 nt with canonical GT/AG splice junction sequences and a putative splice box (GCTAAC), which matches the filamentous-fungus consensus sequence RCTRAC (2, 19), starting 27 nt upstream of the 3′ terminus of the intron. The predicted protein is 203 residues long with a mass of 21,332 Da and an estimated pI of 6.76. Sequencing of several cDNA clones identified two poly(A) sites: one site at nt 1360, 20 nt downstream of a putative poly(A) signal (AATAAA), and a second poly(A) site 42 nt upstream of the first site. Primer extension experiments (Fig 2) identified three transcriptional start points, each starting at the A in a CAY motif, located at +1, +9, and +12. A TATA box (TATATAA) was found starting 44 nt upstream of the first transcriptional start site.
FIG. 1.
Sequence of the MS8 gene (GenBank accession no. AY049031). Nucleotide numbers are shown on the right, and amino acid numbers are shown in parentheses. The first transcriptional start site is designated +1 and underlined. Two additional start sites were found at +9 and +12. A putative TATA box (underlined) was found 44 nt upstream of the +1 site. The single intron is shown in lowercase letters and includes a putative splice box (underlined). Two poly(A) sites were found at nt 1318 (single underline) and 1360 (double underline). A putative poly(A) signal was found starting at nt 1335 (double underline). Asterisk, stop codon.
FIG. 2.
Primer extension analysis of the MS8 gene. The four lanes on the left show DNA sequencing reactions used as size markers. The rightmost lane shows a primer extension reaction, with the corresponding sequence of the MS8 gene.
Expression of MS8 during the Y-M shift.
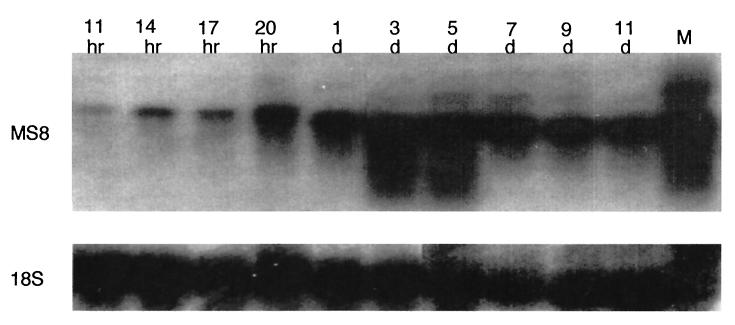
The MS8 transcript is very abundant in the mold morphotype but is not detectable on Northern blots with RNA from the yeast growth form of H. capsulatum (18). To determine the time course of induction, yeast cells growing at 37°C were downshifted to 25°C, which results in a switch to the mold growth form. As shown in Fig. 3, weak expression was first detected 11 h after the temperature shift, corresponding to the first emergence of germ tubes. Expression then increased dramatically, particularly at day 3, corresponding to branch formation in the growing germ tubes. MS8 transcript levels remained high in stable mold cultures. In several lanes, some signal was present in a slightly higher molecular weight band, which we believe represents a longer transcript from the secondary poly(A) site.
FIG. 3.
Expression analysis of MS8 during the Y-M shift. Total RNA was extracted from cells shifted from 37 to 25°C from 11 h (hr) to 11 days (d) as indicated and electrophoresed on a denaturing agarose gel (20 μg/lane). The blot was probed with MS8 cDNA (top), stripped, and probed with a Histoplasma 18S ribosomal DNA probe (bottom) as a control. The rightmost lane (M) shows RNA extracted from fully developed mold.
MS8 expression in yeast results in clumping and altered colony texture.
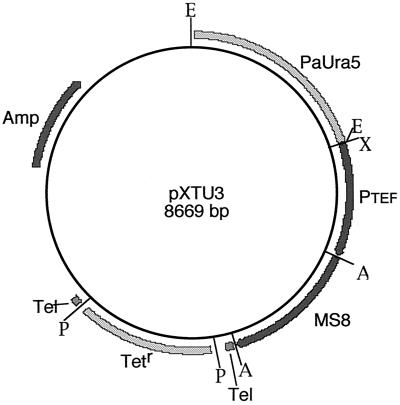
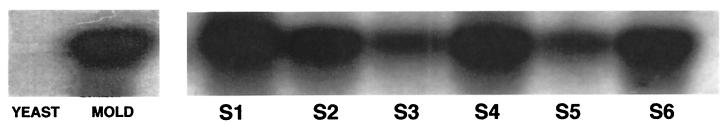
To test the hypothesis that expression of MS8 in yeast might shift the morphology to become more mold-like at 37°C, we forced yeast cells to express the MS8 transcript at levels similar to that seen in mold cultures. The H. capsulatum telomere vector pXTU3 (Fig. 4) was constructed with the MS8 cDNA (GenBank accession no. AF292398), including a 30-nt poly(A) tail, fused to the strong constitutive H. capsulatum TEF1 promoter in the sense orientation. G184AS ura5-11 cells were transformed with the linearized plasmid, and transformants were identified by uracil selection on HMM plates. Six transformants were selected at random, and the expression level of MS8 was determined by Northern blotting, as shown in Fig. 5. Transformants S1, S4, and S6 had levels similar to that seen in mold cells, while S2, S3, and S5 showed less expression than in mold. All six transformants, maintained by uracil selection, exhibited normal yeast morphology as determined by microscopic examination. In HMM broth culture, however, yeast expressing MS8, but not yeast transformed with the vector lacking MS8 sequences, exhibited pronounced clumping. Yeast colonies expressing MS8 on HMM plates had no obvious alteration in size or color but were quite sticky, i.e., when touched with an inoculating loop, the cells tended to stick to the loop like very thick honey. These transformants formed apparently normal mold after the growth temperature was shifted to 25°C. No detectable change in the Y-M transformation or in the microscopic morphology of the mold was noted.
FIG. 4.
Plasmid for forced expression of MS8 in yeast. MS8 cDNA was fused to the strong constitutive Histoplasma TEF1 promoter. The plasmid was cut with PmeI (to remove the Tet marker and expose the telomeric repeats), and cells were transformed with the linear vector. A, ApaI; E, EcoRI; P, PmeI; X, XhoI; Tel, telomeric repeats.
FIG. 5.
Forced expression of MS8 in yeast. Yeast was transformed with the linearized pXTU3 vector and selected on HMM plates. Total RNA was isolated and analyzed by Northern blotting. Each lane contained 20 μg of RNA. The image on the left shows RNA from normal yeast and mold. The image on the right shows RNA from six independent transformants (S1 to S6). The blot was probed with radiolabeled MS8 cDNA.
Positive-negative selection of knockout mutants.
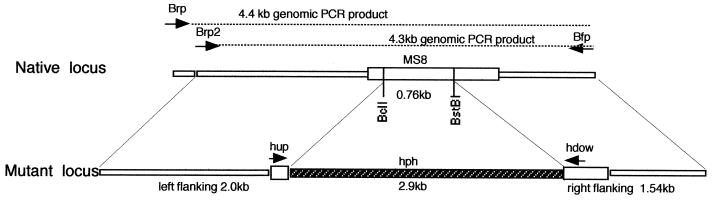
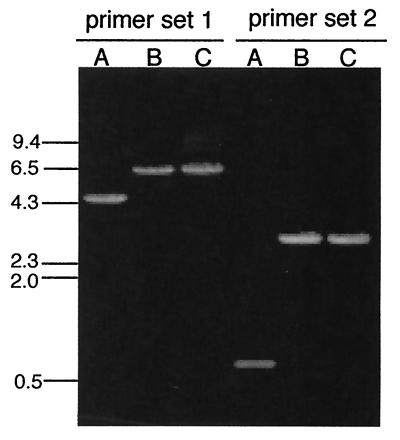
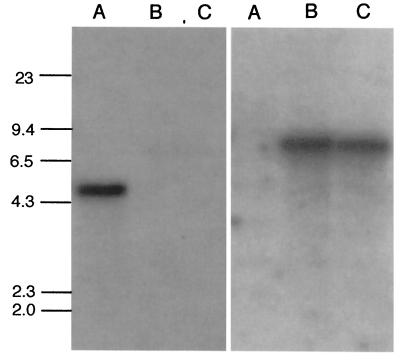
To test the effect of MS8 loss of function on H. capsulatum growth and dimorphism, knockout mutants were constructed by a modification of the positive-negative selection method of Sebghati et al. (16). H. capsulatum G184AS ura5-11 yeast (which shows the same MS8 expression pattern as G186AS [data not shown]) was transformed with a construct containing the MS8 gene with a large section of the coding sequence replaced by a hygromycin marker (hph), as shown in Fig. 6. The knockout construct contained 2 kb of 5′ flanking sequence and 1.5 kb of 3′ flanking sequence relative to the open reading frame. Approximately 0.8 kb of the MS8 coding region was replaced with the 2.9-kb hph marker. After 1 week of uracil selection followed by 1 week of positive-negative selection on 5-fluoroorotic acid-hygromycin plates, 10 colonies were randomly selected and examined for MS8 allelic replacement. Analysis by PCR and Southern blotting showed that 3 of the 10 colonies were ms8 knockout mutants. Figure 7 shows PCR analysis of two knockout mutants. Analysis with two sets of primers (set 1, Brp plus Bfp; set 2, Brp2 plus Bfp) showed that the knockout mutants yielded a band 2.1 kb larger than that of the parental strain, consistent with replacement of the single-copy MS8 gene with the knockout construct (i.e., replacement of 0.8 kb of MS8 sequence with the 2.9-kb hph marker). To confirm the loss of the native locus, DNA from the parental strain and two knockout mutants was digested with BamHI and examined by Southern blotting, as shown in Fig. 8. A radiolabeled probe representing the 0.8 kb of MS8 sequence deleted from the knockout construct hybridized to a 5.4-kb band only in the parental strain. In contrast, an hph probe hybridized to a 7.6-kb band only in the knockout mutants, as predicted for allelic replacement of MS8.
FIG. 6.
Map of MS8 knockout vector. A map of the native locus (The MS8 open reading frame is indicated by the large open box) is shown at the top, and the knockout construct is at the bottom. PCR primer sites are indicated by arrows. Approximately 0.8 kb of MS8 coding sequence was replaced with the 2.9-kb hygromycin marker (hph). Approximately 2 kb of 5′ flanking sequence and 1.5 kb of 3′ flanking sequence were included. This linear construct was used to replace the TEF1-MS8 sequence (XhoI to ApaI) in pXTU3 to form the knockout vector pXTU4Δ.
FIG. 7.
PCR analysis of knockout mutants. Lanes A, DNA from parental strain G184AS ura5-11; lanes B and C, DNA from putative knockout mutants. Primer set 1, Brp and Bfp; primer set 2, Brp2 and Bfp. Replacement of the native locus should yield a band 2.1 kb larger than that in the parental strain.
FIG. 8.
Southern blot analysis of knockout mutants. DNA was extracted from the G184AS ura5-11 parental strain (lanes A) and two putative knockout mutants (lanes B and C), cut with BamHI, separated on a 0.7% gel, and blotted onto a nylon membrane. The left-hand image shows the blot hybridized with a radiolabeled probe representing the 0.8 kb of MS8 sequence removed in the knockout vector. The right-hand image shows the same blot stripped and probed with a radiolabeled hph marker.
ms8 knockout mutants form aberrant mycelia.
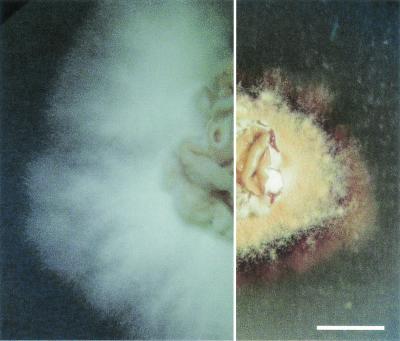
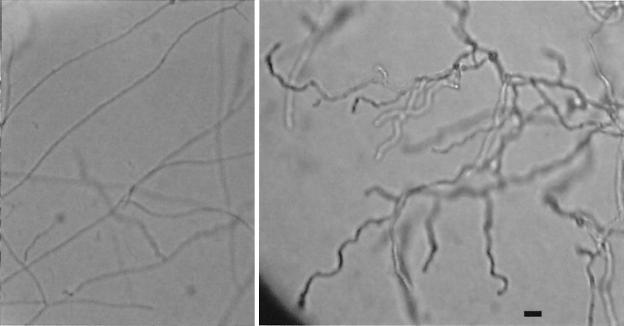
ms8 knockout mutants shifted to 25°C showed no significant phenotypic changes early in the Y-M transformation. Germ tube emergence and elongation occurred in both the parental strain and knockout mutants at approximately the same time and rate. After several days of growth on HMM/u plates, however, the mycelia of the ms8 knockouts were substantially different from those of the parental strain, as shown in Fig. 9. Colonies of the knockout mutants were less than half the diameter of normal colonies, had a brownish granular appearance, and produced a deep-red pigment particularly remarkable upon viewing the back of the agar plate. Microscopic examination showed that the hyphae of knockout mutants were nearly 40% wider (0.32 versus 0.23 μm) than normal, as shown in Fig. 10. Whereas hyphae in the parental strain generally gave rise to long, straight filaments, those in the knockout mutants gave rise to much shorter branches, with a dramatic “zig-zag” twisted appearance.
FIG. 9.
Colony morphology of G184AS ura5-11 parental strain (left) and ms8 knockout mutant (right). The colonies are shown on HMM/u agar after 6 weeks of growth at 25°C. The photographs were taken from the same plate and with the same lighting conditions and magnification. Bar, 5 mm.
FIG. 10.
Microscopic morphology of G184AS ura5-11 parental strain (left) and ms8 knockout (right) stained with lactophenol cotton blue and photographed at the same magnification. Bar, 2 μm.
To ensure that the aberrant mycelial morphology of the ms8 knockouts was the result of loss of function of MS8, we transformed the knockout mutants with pXTU3 (MS8 cDNA driven by the TEF1 promoter) and pXTU3g (an MS8 genomic fragment with the native promoter) to complement the ms8 mutation. Knockout mutants were transformed with pXTU3 and pXTU3g and plated on HMM plates to maintain the plasmids by uracil selection. Colonies grown at 25°C demonstrated normal colony and microscopic morphologies, as shown for the parental strain in the left-hand images of Fig. 9 and 10. In contrast, ms8 knockout mutants transformed with pXTU1 (which has the Ura5 marker but no MS8 sequence) still exhibited the aberrant morphology, as shown for the ms8 knockouts in the right-hand images of Fig. 9 and 10. Thus, the aberrant phenotype was not incidental to the experimental protocol but due to MS8 loss of function.
DISCUSSION
The most obvious environmental cue for the M-Y shift after spores or mold fragments are inhaled is the upshift to 37°C. Laboratory cultures shifted from 25 to 37°C mimic this process in vitro, and nearly all studies of H. capsulatum dimorphism in the literature have used a simple temperature shift to favor mold (25°C) or yeast (37°C). Although the temperature shift is clearly an important dimorphism signal in H. capsulatum, it is not necessary or sufficient for the morphological transition. Under certain conditions, yeast can be grown at the “nonpermissive” temperature of 25°C (10) and mold can be grown at 37°C (10, 14, 15). Presumably, the temperature shift is the natural trigger for induction of a gene regulation cascade which results in the dramatic differentiation process from a multicellular form to a unicellular form or vice versa. Clearly, the dimorphic shift is associated with dramatic changes in gene regulation, as demonstrated by the numerous differentially regulated genes found in subtracted-library and differential-display experiments in our laboratory and others (6, 13, 18).
The MS8 gene was interrupted by a single intron with typical GT-5′ and AG-3′ termini (Fig. 1). A putative splice box matching the filamentous-fungus consensus RCTRAC (GCTAAC) was found starting 27 nt upstream of the 3′ end of the intron. Comparison of several cDNA clones revealed that MS8 had two poly(A) sites, as do many of the H. capsulatum genes we have isolated. The most distal poly(A) site was preceded by a canonical poly(A) signal sequence, AATAAA, starting 26 nt upstream. The second poly(A) site results in a transcript 42 nt shorter and is not preceded by an obvious poly(A) signal sequence. The sequence surrounding the putative translational start site (boldface and underlined), TCATCATGTC, showed high similarity to the Kozak sequence [TCA(C/A)(A/C)ATG(G/T)C] seen in filamentous fungi (1). Primer extension experiments showed three closely spaced transcriptional start sites of similar strengths 228, 220, and 217 nt upstream of the translational start site. Each transcriptional start was the A in a CAY motif. A consensus TATA box (TATATAA) was located starting 44 nt upstream of the first transcriptional start site. The predicted MS8 protein was 203 amino acids long with an expected mass of 21.3 kDa and a pI of 6.77. Particularly striking was the abundance of two amino acids in the putative MS8 protein. Over 40% of MS8p was composed of only two amino acids, glycine (22%) and glutamine (19%). GenBank similarity searches revealed only one significant match in the database: a glycine-rich protein from the plant-pathogenic fungus Colletotrichum gloeosporioides (accession no. U94186; BLAST similarity, 10−21). The C. gloeosporioides protein is similar to MS8p in mass (22 kDa), G and Q content (24 and 19%, respectively), and cysteine content (0%) but is significantly more basic (pI, 9.18) than MS8p. Unfortunately, the function of this protein in Colletotrichum is also unknown.
Expression of MS8 is not detected on Northern blots of RNA from the yeast morphotype but is quite abundant in mold cells (18). During the 25°C Y-M transition, expression was first detected at 11 h (Fig. 3), corresponding to the emergence of germ tubes from the yeast. As the germ tubes elongated, the level of the MS8 transcript increased dramatically, particularly on day 3, which corresponded to hyphal branch formation in most of the cells. MS8 transcript levels remained high in actively growing mold cultures.
H. capsulatum has inefficient homologous recombination, and only very recently has allelic replacement become feasible in Histoplasma. Our knockout experiments with a slight modification of the positive-negative selection methodology of Sebghati et al. (16) showed that nearly one-third of the colonies on the final selection plates were ms8 knockouts. This is similar to the frequency reported for their knockout of the calcium binding protein gene (CBP1). This positive-negative selection method now makes it possible to isolate H. capsulatum knockout mutants in less than a month.
If MS8 was essential for formation of mold cells, we would expect ms8 knockout mutants to be unable to form mycelia. Clearly, MS8 is not an absolute requirement for mold formation, since the knockout mutants did form hyphae (albeit of aberrant shape and size). The presence of a functional MS8 gene is also not required for the shift back to the yeast morphotype, since ms8 knockout mutants growing as mold can shift to apparently normal yeast at 37°C (not shown). The identity of the red pigment produced by the ms8 mutants is not known. Red-pigmented variants of H. capsulatum have been reported, one isolated from a canebrake (12) and one from biopsy material from a human immunodeficiency virus-positive patient (17). The identity of the pigment produced by these variants is also not known. Although it is possible that the pigments produced by the variants and the ms8 knockout mutants are related, we have no data at this time to allow a conclusion.
Clearly, it is not essential that MS8 be silent to maintain the yeast morphotype, since yeast forced to express high levels of the MS8 transcript (Fig. 5) still grew as yeast at 37°C. MS8 is required, however, for normal hypha formation. ms8 knockout mutants formed aberrant short, twisted hyphae and gave rise to small, pigmented, slow-growing mold colonies (Fig. 9). The function of MS8p within the cell is unknown. Examination of the putative MS8p with various proteomics structural analysis programs (http://www.expasy.ch/tools) revealed primarily that the protein is expected to be quite flexible and hydrophilic, with two short regions of alpha helix in the C terminus. The lack of diagnostic structural features and the BLAST similarity match to a single protein whose function is also unknown make it impossible to postulate a particular function for this protein. It is quite possible that MS8p is a structural protein used in hyphal wall formation. This hypothesis is consistent with the MS8 expression experiments with yeast (Fig. 5). The clumping of yeast expressing MS8 and the sticky texture of the yeast on plates indicate that the cell surface is altered in some fashion, perhaps because MS8p is localized in or on the cell wall. In future work, we plan to examine the cellular localization of MS8p with GFP-MS8 fusions to help elucidate the role of this protein in mold formation.
Acknowledgments
This work was supported in part by grant AI49357 from the National Institutes of Health.
REFERENCES
- 1.Ballance, D. J. 1986. Sequences important for gene expression in filamentous fungi. Yeast 2:229-236. [DOI] [PubMed] [Google Scholar]
- 2.Gurr, S. J., S. E. Unkles, and J. R. Kinghorn. 1987. The structure and organization of nuclear genes of filamentous fungi, p. 93-139. In J. R. Kinghorn (ed.), Gene structure in eukaryotic microbes. IRL Press, Oxford, United Kingdom.
- 3.Jones, D. H., and S. C. Winistorfer. 1993. Genome walking with 2- to 4-kb steps using panhandle PCR. PCR Methods Appl. 2:197-203. [DOI] [PubMed] [Google Scholar]
- 4.Keath, E. J., and F. E. Abidi. 1994. Molecular cloning and sequence analysis of yps-3, a yeast-phase-specific gene in the dimorphic fungal pathogen Histoplasma capsulatum. Microbiology 140:759-767. [DOI] [PubMed] [Google Scholar]
- 5.Keath, E. J., G. S. Kobayashi, and G. Medoff. 1992. Typing of Histoplasma capsulatum by restriction fragment length polymorphisms in a nuclear gene. J. Clin. Microbiol. 30:2104-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keath, E. J., A. A. Painter, G. S. Kobayashi, and G. Medoff. 1989. Variable expression of a yeast-phase-specific gene in Histoplasma capsulatum strains differing in thermotolerance and virulence. Infect. Immun. 57:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klimpel, K. R., and W. E. Goldman. 1987. Isolation and characterization of spontaneous avirulent variants of Histoplasma capsulatum. Infect. Immun. 55:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar, D., Y. J. Avissar, and J. H. Wyche. 1991. Simultaneous and rapid isolation of bacterial and eukaryotic DNA and RNA: a new approach for isolating DNA. BioTechniques 11:94-101. [PubMed] [Google Scholar]
- 9.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Maresca, B., G. Medoff, D. Schlessinger, and G. S. Kobayashi. 1977. Regulation of dimorphism in the pathogenic fungus Histoplasma capsulatum. Nature 266:447-448. [DOI] [PubMed] [Google Scholar]
- 11.Medoff, G., M. Sacco, B. Maresca, D. Schlessinger, A. Painter, G. S. Kobayashi, and L. Carratu. 1986. Irreversible block of the mycelial-to-yeast phase transition of Histoplasma capsulatum. Science 231:476-479. [DOI] [PubMed] [Google Scholar]
- 12.Morris, P. R., A. A. Terreni, and A. F. DiSalvo. 1986. Red-pigmented Histoplasma capsulatum—an unusual variant. J. Med. Vet. Mycol. 24:231-233. [PubMed] [Google Scholar]
- 13.Retallack, D. M., and J. P. Woods. 1999. Molecular epidemiology, pathogenesis, and genetics of the dimorphic fungus Histoplasma capsulatum. Microbes Infect. 1:817-825. [DOI] [PubMed] [Google Scholar]
- 14.Rippon, J. W. 1968. Monitored environment system to control cell growth, morphology, and metabolic rate in fungi by oxidation-reduction potentials. Appl. Microbiol. 16:114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacco, M., B. Maresca, B. V. Kumar, G. S. Kobayashi, and G. Medoff. 1981. Temperature- and cyclic nucleotide-induced phase transitions of Histoplasma capsulatum. J. Bacteriol. 146:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebghati, T. S., J. T. Engle, and W. E. Goldman. 2000. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science 290:1368-1372. [DOI] [PubMed] [Google Scholar]
- 17.Staib, F., and G. Grosse. 1996. Brown-red pigment formation by the mycelial phase of a clinical isolate of Histoplasma capsulatum on Staib agar. A preliminary report. Zentbl. Bakteriol. 283:515-521. [DOI] [PubMed] [Google Scholar]
- 18.Tian, X., and G. Shearer. 2001. Cloning and analysis of mold-specific genes in the dimorphic fungus Histoplasma capsulatum. Gene 275:107-114. [DOI] [PubMed] [Google Scholar]
- 19.Unkles, S. E. 1992. Gene organization in industrial filamentous fungi, p. 29-53. In J. R. Kinghorn and G. Turner (ed.), Applied molecular genetics of filamentous fungi. Blackie, Glasgow, United Kingdom.
- 20.Woods, J. P., and W. E. Goldman. 1993. Autonomous replication of foreign DNA in Histoplasma capsulatum: role of native telomeric sequences. J. Bacteriol. 175:636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods, J. P., E. L. Heinecke, and W. E. Goldman. 1998. Electrotransformation and expression of bacterial genes encoding hygromycin phosphotransferase and beta-galactosidase in the pathogenic fungus Histoplasma capsulatum. Infect. Immun. 66:1697-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worsham, P. L., and W. E. Goldman. 1988. Selection and characterization of ura5 mutants of Histoplasma capsulatum. Mol. Gen. Genet. 214:348-352. [DOI] [PubMed] [Google Scholar]