Abstract
The human microbiome exerts profound influence over various biological processes within the body. Unlike many host determinants, it represents a readily accessible target for manipulation to promote health benefits. However, existing commercial microbiome-directed products often exhibit low efficacy. Advancements in technology are paving the way for the development of novel microbiome therapeutics, across a wide range of indications. In this narrative review, we provide an overview of state-of-the-art technologies in late-stage development, examining their advantages and limitations. By covering a spectrum, from fecal-derived products to live biotherapeutics, phage therapy, and synthetic biology, we illuminate the path toward the future of microbiome therapeutics.
Key Points
| The human gut microbiome is an accessible target for manipulation to promote health. |
| An overview of state-of-the-art microbiome therapeutics and technologies for a range of indications, their advantages and limitations are summarized in this review article. |
Introduction
The advent of sequencing technologies has revolutionized our understanding of the human microbiome, a diverse and intricate community of microorganisms that resides within us. Comprising predominantly bacteria but also encompassing viruses, fungi, archaea, and protists, this ecosystem thrives throughout the body, with the intestinal tract serving as its primary habitat, displaying escalating density and complexity from the esophagus to the large intestine. Despite the existence of a relatively stable core of microbial members, the variability between individuals is influenced by lifestyle choices, health conditions, and medication usage [1]. Notably, taxonomic classification often fails to capture functional diversity, as there exists considerable overlap in metabolic capabilities among taxa. Indeed, the metabolic functions of the microbiome exhibit more similarity across individuals than across their taxonomic compositions [2]. The presence and function of these microorganisms exert profound effects on the host, impacting various aspects of health and disease. Recognizing the significance of microbe–host interactions has spurred endeavors to modulate the gut microbiome to promote beneficial health outcomes. From dietary interventions to fecal microbiota transplantation (FMT), the field of microbiome therapeutics is rapidly evolving, driven by advancements in technology and a growing focus on precision medicine. In this narrative review, we provide a concise overview of the current state-of-the-art in microbiome therapeutics and highlight recent breakthroughs with translational potential. From untargeted interventions to more targeted interventions (Fig. 1), we describe the available tools and provide a constructed summary of the current and future methods (Table 1). We also address the challenges and prospects associated with each therapeutic approach. Although microbial manipulation also holds promise for disease prevention, this topic will not be discussed in this review as it involves many aspects beyond its scope.
Fig. 1.
Schematic distinction of targeted and untargeted approaches of microbial therapeutics. Microbial consortia are considered both targeted and untargeted. All microbial therapeutic modalities are in close relationship with the gut microbiome and the intestinal mucosa of the host. LBT live biotherapeutic product, NLBT nonlive biotherapeutic product. The figure was created using BioRender.com
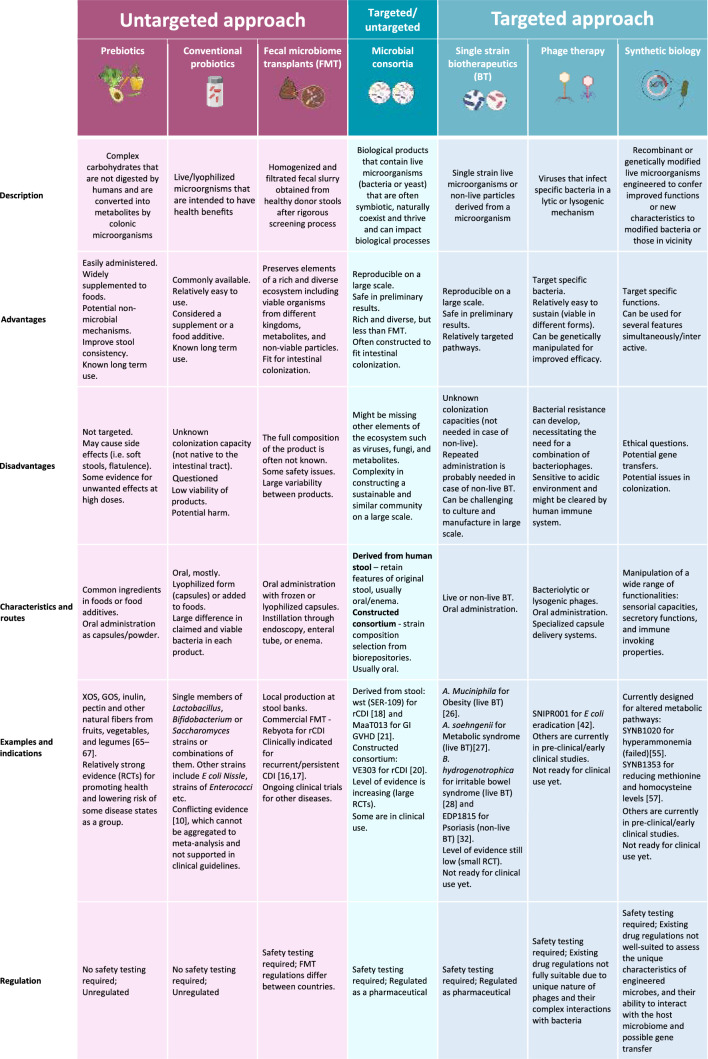
Table 1.
Current microbiome therapeutic modalities
Recommendations for most of the compounds in this table are aimed for the prevention, treatment, or cure of a human disease, or condition. Clinical evidence is scarce for certain modalities, and more studies are needed to determine effectiveness in particular indications
XOS xylo-oligosaccharides, GOS galacto-oligosaccharides, RCT randomize clinical trial, CDI Clostridioides difficile infection, GVHD graft-versus-host-disease, FMT fecal microbiome transplants, BT biotherapeutics
The Microbiome and the Host
The human gastrointestinal (GI) tract stands as a pivotal player in human physiology, recognized as one of the largest neurologic, endocrine, and immune organs. Recent evidence indicates that the gut orchestrates homeostasis not only within its immediate vicinity, but also extends its influence to distant organs, such as the liver, lungs, central nervous system, and muscle [3–6]. Central to this intricate interplay is the intestinal microbiome, which interacts extensively with the host through multifaceted pathways. Perturbations in the microbial community can disrupt the integrity of the intestinal mucus layer and are associated with poor barrier function (e.g., “leaky gut”) [7]. Our microbial community is a metabolic factory that can metabolize most types of nutrients and host products, as well as perform metabolic functions that humans do not possess (i.e., degradation of complex carbohydrate). These metabolites can deliver local and distant signals. With ongoing research, an expanding repertoire of receptors for microbial metabolites is being identified, spanning various cell types, including immune cells. Notably, the microbiome exerts regulatory effects on the immune system. For example, it is now thought that early-life exposure to microbial antigens augments the host’s immune system and predisposes individuals to diseases later in life [8]. Furthermore, “aging” of the microbiome is associated with many age-related diseases, possibly through some level of inflammation. Given the profound impact on the host, microbial interventions emerge as compelling strategies for promoting health and combating disease.
Current Microbial Interventions
Microbiome therapeutics, intended to improve health and treat disease, are ultimately mediated by one or more of the above-mentioned mechanisms i.e., through direct or indirect enhancement of microbes’ beneficial functions. Dietary modifications or supplements, such as prebiotics, mostly include dietary fibers that reach the colon undigested, and are utilized there by microorganisms to produce metabolites which affect the host. Another therapeutic option are conventional probiotics, which constitute “live microorganisms (mostly bacteria but also yeasts) that, when administered in adequate amounts, confer a health benefit on the host” [9]. The current use of untargeted probiotics is (largely) unsubstantiated, backed only by low, real-world efficacy and poor clinical evidence for most indications [10]. Although widely used, strong evidence exists for only a few clinical indications [11, 12]. This is likely owing to the flawed concept that organisms, which are non-native to the adult intestinal tract, will colonize for the long term, together with uncertain quality control of the products and loose regulations by the authorities [probiotics are considered foods or dietary supplements by the Food and Drug Administration (FDA)]. FMT is an untargeted, but ecological therapeutic modality that involves the transfer of fecal material from a healthy donor to a recipient in order to achieve a new balance of gut microbiota and enhance its functionalities and diversity. The high success rate of FMT for the treatment of recurrent Clostridioides difficile infection (rCDI) (above 80% with a single administration, and > 90% with repeating doses) [13], has given hope that this therapeutic modality may be successful for other non-infectious diseases associated with skewed microbial composition. The FDA established that the clinical use of FMT for the treatment of rCDI no longer needs an investigational new drug application (IND) [14]. However, this exemption does not apply to other therapeutic applications of FMT. Overall, FMT is considered a potent and holistic approach which has been extremely successful for rCDI; however, while benefits for some other indications have been shown, for many indications the benefits are still uncertain and FMT treatment is currently not recommended.
Microbial Consortia
Given the efficacy and feasibility of FMT as a comprehensive ecological intervention, alongside the inherent risks associated with the transmission of undetected or emerging pathogens, there is a compelling rationale for exploring alternative combinations of microbial communities that are disease specific. A microbial consortium is a group of microorganisms that are symbiotic (e.g., a mutual host-microorganism bidirectional and beneficial relationship), naturally coexist and thrive, and can collectively impact biological processes. Unlike mixtures of bacteria in current probiotic formulations, which are single strains mixed at the processing step, microbial consortia are designed to work together in a specific, synergistic way, focusing on recreating a more diverse and stable microbial community similar to natural microbiomes. Initial efforts in this area focused on standardizing the manufacturing of an FMT-derived product, in the form of Rebyota (RBX2660), which is an enema-delivered product approved by the FDA for rCDI. Although superior to placebo for reducing rCDI (n = 180, RBX2660; n = 87, placebo; success rate 70.6 versus 57.5%, respectively), the results are not comparable with prior FMT studies, while the incidence of adverse events was similar among interventions [13, 15–17]. Subsequently, fecal material processing was implemented to preserve some level of diversity while increasing precision and safety. Vowst (SER-109), an FDA-approved, oral fecal-derived purification of Firmicutes spores has shown superiority to placebo in reducing rCDI (n = 89, SER-109; n = 93, placebo; relative risk 0.32) [18]. The inactivation and purification of stool to remove vegetative forms of microorganisms [19], support the positive safety results [18]. As opposed to this “top-down” approach (FMT derivatives), a different approach that constructs a rationally selected microbial community (“bottom-up”) is also being attempted. One example is VE303, a bacterial consortium constructed from eight strains of Clostridia originating from stools of healthy individuals, obtained from bacterial biorepositories. VE303 has shown superiority over placebo in reducing rCDI (n = 29, high-dose VE303; n = 22, placebo; 13.8 versus 45.5%, respectively) with favorable safety outcomes [20]. The current strategies of microbial consortia are conservative and mostly designed for rCDI treatment, although other indications are being explored. MaaT013 for example, a compound of pooled fecal matter from 3–8 donors, was investigated for the treatment of refractory gastrointestinal graft-versus-host-disease with satisfactory safety outcomes in this highly immunocompromised patient population [21]. This oral product is enriched in butyrate-producing bacteria, which promotes epithelial integrity and restores immune homeostasis. Other formulations have been tested in animal models and are currently being explored in human pilot studies for different indications. Preliminary results are promising and pave the way to safe and effective alternatives to FMT. However, constructing a de novo, sustainable and symbiotic microbial consortium that will efficiently engraft is challenging. Microbial consortia lack other aspects of the microbiome ecosystem, such as the variety of substrate resources and the trans-kingdom interactions with viruses and fungi that are naturally found in the intestines [22, 23]. This might explain the lower efficacy of these interventions compared with the complex but primitive FMT.
Single Strain Biotherapeutics
Considering the challenges in the manufacture of microbial consortia, exploring single strain biotherapeutics as another form of microbial intervention has become an attractive strategy. Live biotherapeutic products (LBPs) are a class of biological, medicinal products that contain live microorganisms to prevent, treat, or cure diseases or medical conditions. Unlike traditional probiotics, LBPs are classified as pharmaceuticals and not dietary supplements, and the FDA and the European Medicines Agency are involved in their regulation to ensure safety and efficacy [24]. An example of single strain (ss) LBPs is Akkermansia muciniphila, a mucin-degrading commensal bacterium that has been associated with a good safety profile and contributes to the maintenance of an impermeable gut barrier, thereby regulating microbial host interactions and immunity. In animal models, this LBP has been associated with improving biomarkers of obesity, type 1 and type 2 diabetes mellitus, hepatic steatosis, intestinal inflammation, and several cancers [25]. Oral administration of A. muciniphila has been associated with a beneficial metabolic status and improved metabolic and clinical outcomes in individuals with obesity [26]. Another potential candidate for ssLBP is Anaerobutyricum soehngenii (formerly Eubacterium halli), a small, intestine-derived butyrate-producing bacterium that affects glucose metabolism and insulin resistance. Oral and enteral administration of A. soehngenii is safe and shows positive results on peripheral insulin resistance in patients with metabolic syndrome [27]. Blautia hydrogenotrophica (Blautix™) is another short-chain fatty acid producer (mainly acetate), shown to be safe and potentially beneficial in patients who suffer from irritable bowel syndrome [28]. Other ssLBPs that target specific pathophysiological aspects of disease are being studied in pre-clinical phases. Some of these bacteria have mutualistic properties and can be considered as cross-feeders [29], emphasizing the need for further research on combination of single strains and prebiotics to test the added value of co-supplementation. Non-live biotherapeutics (NLBPs) are medicinal products containing microorganisms that have been killed, inactivated, or stabilized. EDP1815 is an example of a NLBP derived from Prevotella histicola cultures that possesses broad and potent anti-inflammatory effects, targeting Th1, Th2, and Th17 inflammatory pathways, and which lead to reduced skin inflammation and tissue cytokines in mouse models [30, 31]. Clinical studies have demonstrated that EDP1815 is well tolerated, has a favorable safety profile and shows clinical efficacy in patients with mild to moderate psoriasis [32]. Disappointing results led to the discontinuation of EDP1815 in atopic dermatitis [33], further highlighting the specificity of these interventions. Taken together, LBPs have gained great attention in microbiome therapeutics as promising alternatives. Still, for sustainable long-term impact on the intestinal ecosystem, single or scarce organisms may not be sufficient. Furthermore, since NLBPs are not expected to colonize, frequent and ongoing administration will be needed to exert their beneficial effects.
Phage Therapy
The gut virome vastly interacts with other members of the microbiome, and can serve as another potential modality for gut microbiome modulation through, for example, fecal virome transplantation (e.g., administration of filtered and processed fecal material that contain particles below a certain size) [34, 35]. A more specific application of the gut virome is the use of bacteriophages, which are viruses that infect specific bacteria and can be used as antimicrobial agents. Unlike antibiotics, phages exhibit high specificity, often to the strain level, sparing unwanted off-target effects on the microbiota [36]. After the phage’s DNA is injected into the bacterium, one of two modes of action are initiated: lytic or lysogenic. Bacteriolytic phages utilize bacterial machinery to manufacture their own proteins leading to bacterial lysis and release of newly formed phage copies to the environment. In lysogenic replication, the phage’s DNA is integrated into the bacterial genome, replicates and passes on without killing the host (bacteria) [37]. Both phage modalities can serve as methods for intestinal microbiome manipulation. Federici et al. identified lytic phages that target Klebsiella pneumoniae clades, common in patients with inflammatory bowel disease [38]. These bacteriolytic phages were able to improve colitis in mice, and were found to be safe in an artificial gut model and phase I trial. Clustered Regularly Interspaced Short Palindromic Repeats-associated protein 9 (CRISPR-Cas9) is a DNA nuclease that serves as a genomic-editing tool [39]. This machinery can be delivered by lysogenic phages to target specific bacteria and specific bacterial functions [40, 41]. SNIPR001 (by SNIPR BIOME) is an example of a CRISPR-armed, phage delivery therapeutic with broad activity against 429 Escherichia coli strains. The goal is to promote the degradation of specific segments of bacterial DNA and by that, kill antibiotic resistant species [42]. Rubin et al. reported a versatile editing system that selects for specific strains within a microbial community, without the need for strain isolation as required by the above-mentioned technologies. This system enabled the introduction of a loss-of-function mutation to a growth inhibitory gene or the introduction of antibiotic resistance transposons to Klebsiella michiganensis and Pseudomonas simiae. By inserting those properties, bacteria were granted the ability to dominate the community under these specific stresses [43]. Combination of these technologies can enhance our capacity to personalize microbial interventions. While phage therapy pharmacodynamics and pharmacokinetics are yet to be fully investigated, preliminary findings (as reviewed elsewhere) suggest their safety, with minor and anecdotal adverse events reported in human and animal studies [44, 45]. However, resistance [46], immunogenicity [47], efficient delivery methods, and optimal dosing remain significant hurdles that need to be properly addressed. To tackle the acidity and proteases in the upper GI tract that bacteriophages in-particular face, methods are being designed for distal delivery, allowing for minimally disruptive in situ modification of microbes in the gut [48]. This approach paves the way for future research, which will be crucial in achieving the full potential of phage therapy.
Synthetic Biology
Synthetic biology is another powerful tool that involves genetic manipulation, in this case through the integration of selected traits into specific bacteria. Transcriptional regulators and genes encoding proteins of interest can be assembled into a plasmid (a circular DNA fragment that can be utilized as an accessory genome) that can be inserted into the bacterial cell or integrated into its genome [49, 50]. Microbial candidates for synthetic biology are selected according to specific features such as the ability to engraft the intestine, ease of manipulability, and previous use as a probiotic strain [51, 52]. Notably, the commercially available probiotic Escherichia coli Nissle 1917 (EcN) is frequently used as a vehicle strain due to the relative ease with which it is genetically manipulated. These manipulations encompass a wide range of functionalities, such as sensorial capacities, secretory functions, and immune-invoking properties. Koh et al. engineered EcN to express bile salt hydrolases under the control of a sialic acid-responsive promoter. This situation of altered bile acid metabolism is seen after antibiotic treatment and is often associated with the occurrence of CDI. Koh’s engineered EcN improved infection severity and survival in an antibiotic-exposed mouse model of CDI [53]. Metabolic pathway disorders are another appealing condition to target using synthetic biology. One of these is hyperammonemia, that can be seen in primary urea cycle disorders and hepatic encephalopathy. Kurtz et al. manipulated the EcN genome to generate the strain SYNB1020 that, through oral administration, converts ammonia to l-arginine in the gut to mitigate disease symptoms [54]. SYNB1020 (by Synlogic) lowered systemic hyperammonemia in preclinical trials, and was well tolerated by healthy volunteers and patients with cirrhosis and increased urinary nitrate. However, SYNB1020 was not able to reduce ammonia blood levels in a double-blind randomized placebo-controlled study in subjects with cirrhosis and hyperammonemia [55]. Another candidate (SYNB1353) was designed to reduce methionine levels in patients with classical homocystinuria, a condition usually managed with dietary restriction of the amino acid. In mice experiments and in healthy volunteers, SYNB1353 showed a reduction in plasma methionine and homocysteine following oral methionine intake [56]. The drug, granted Orphan Drug Designation by the FDA, is now advancing to a phase-2 trial [57]. Microbial engineering also takes advantage of microbial–host interactions to target inflammation. Lynch et al. designed a bacterial platform (PROT3EcT) that utilizes the Shigella type III secretion apparatus in EcN to allow active secretion of proteins directly into the intestinal environment that targets proinflammatory factors. A variant of PROT3EcT, with the ability to secrete an anti-TNF-α nanobody, showed improvement in a murine model of inflammatory bowel disease and was as effective as systemic anti-TNF-α treatment [58]. The latest development in this field is the intelligent responsive bacteria for diagnosis and therapy (iROBOT) system that can sense local inflammatory signals from the host, produce molecules that influence the environment/host and noninvasively record the level of inflammation in the target tissue [59]. Overall synthetic biology is a versatile tool that holds great promise for a targeted and personalized approach to many medical conditions. However, bacterial gene transfer may undesirably alter other metabolic pathways, impact environmental microbes [60], and potentially lead to off-target effects on bacterial members of the human microbiome. Of note, this gene transfer system can also be beneficial and leveraged for in-situ microbiome manipulation, as suggested by Ronda et al. [61]. They utilize EcN as a vehicle to horizontally transfer genetic elements to members of the microbiome community in vivo. However, concerns are raised from the previous use of genetically manipulated organisms (GMOs) and potentially unwanted effects of these interventions [62]. In an effort to overcome these unwanted traits, future probiotics should be engineered with an understanding of metabolic cooperation and competition within microbial communities using complex metabolic models [63].
Looking Forward
Advancements in our understanding of microbiome-associated disease mechanisms, coupled with the progress in molecular technology, have equipped us with the means to precisely target the host–microbiome interaction in a multitude of diseases. The extensive spectrum of conditions linked to microbiome alterations, some with established causal relationships, present numerous avenues for exploration. Efforts to harness the microbiome for therapeutic purposes extend across various medical disciplines, underscoring its vast therapeutic potential. However, significant challenges remain, including enhancing efficacy, establishing long-term safety, scaling up production, and ensuring product quality. An important fact is that while biotherapeutics become more precise, they currently overlook individual variations in microbiome composition. Future approaches should involve analyzing the pre-intervention microbiome structure to tailor therapy on a personalized basis to optimize efficacy. Although advances in molecular characterization and analytical platforms have improved our ability to characterize an individual’s microbiome, daily fluctuations and difficulties in sampling various gastrointestinal tract locations pose limitations. Recent progress in smart capsule technology for sampling along the intestine [64], together with advanced bioinformatic tools, offers promise in addressing these challenges. Additionally, combining these modalities and integrating lifestyle and dietary interventions can expand therapeutic options and enhance effectiveness and sustainability. The widespread use of probiotics and prebiotics indicate acceptance of microbiome therapeutics, often perceived as “natural” interventions, though concerns surrounding GMO foods highlight the necessity for open scientific dialogue to improve patient and physician acceptance. Now, more than ever, the future of microbiome therapeutics looks bright.
Declarations
Funding
Open access funding provided by Technion - Israel Institute of Technology.
Conflict of interest
M.P., I.K., D.S., D.B.H., and H.B.-Y. have no conflicts to declare.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Data availability
Not applicable.
Author contributions
M.P., I.K., D.S., D.B.H., and H.B.-Y. all contributed to the review. All authors approved the final version of the article, including the authorship list.
References
- 1.Zhou X, Shen X, Johnson JS, Spakowicz DJ, Agnello M, Zhou W, et al. Longitudinal profiling of the microbiome at four body sites reveals core stability and individualized dynamics during health and disease. bioRxiv: the preprint server for biology. United States; 2024. p. 2024.02.01.577565. [DOI] [PMC free article] [PubMed]
- 2.Visconti A, Le Roy CI, Rosa F, Rossi N, Martin TC, Mohney RP, et al. Interplay between the human gut microbiome and host metabolism. Nat Commun. 2019;10(1):4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albillos A, De Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–77. [DOI] [PubMed] [Google Scholar]
- 4.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol. 2017;15(1):55–63. [DOI] [PubMed] [Google Scholar]
- 5.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Cheng J, Hu Y. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev. 2022;81:101739. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber F, Balas I, Robinson MJ, Bakdash G. Border control: the role of the microbiome in regulating epithelial barrier function. Cells. 2024;13(6). [DOI] [PMC free article] [PubMed]
- 8.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014. 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 10.Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159(2):697–705. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Acute Respiratory Infections Group, editor. Cochrane Database Syst Rev. 2022. 10.1002/14651858.CD006895.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AlFaleh K, Anabrees J, Bassler D, Al-Kharfi T. Cochrane review: probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid-Based Child Health. 2012;7(6):1807–54. [DOI] [PubMed] [Google Scholar]
- 13.Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration (FDA). Fecal microbiota products [Internet]. 2023. Available from: https://www.fda.gov/vaccines-blood-biologics/fecal-microbiota-products.
- 15.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing clostridium difficile infection. JAMA. 2014;312(17):1772–8. [DOI] [PubMed] [Google Scholar]
- 16.Dubberke ER, Lee C, Orenstein R, Khanna S, Hecht G, Fraiz J. Efficacy and safety of RBX2660 for the prevention of recurrent Clostridium difficile infection: results of the PUNCH CD 2 trial. Open Forum Infect Dis. 2016;3(suppl_1):1341. [Google Scholar]
- 17.Khanna S, Assi M, Lee C, Yoho D, Louie T, Knapple W, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med. 2022;386(3):220–9. [DOI] [PubMed] [Google Scholar]
- 19.Khanna S, Sims M, Louie TJ, Fischer M, LaPlante K, Allegretti J, et al. SER-109: an oral investigational microbiome therapeutic for patients with recurrent Clostridioides difficile infection (rCDI). Antibiotics. 2022;11(9):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie T, Golan Y, Khanna S, Bobilev D, Erpelding N, Fratazzi C, et al. VE303, a defined bacterial consortium, for prevention of recurrent Clostridioides difficile infection: a randomized clinical trial. JAMA. 2023;329(16):1356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malard F, Loschi M, Huynh A, Cluzeau T, Guenounou S, Legrand F, et al. Pooled allogeneic faecal microbiota MaaT013 for steroid-resistant gastrointestinal acute graft-versus-host disease: a single-arm, multicentre phase 2 trial. EClinicalMedicine. 2023;62: 102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu NN, Jiao N, Tan JC, Wang Z, Wu D, Wang AJ, et al. Multi-kingdom microbiota analyses identify bacterial–fungal interactions and biomarkers of colorectal cancer across cohorts. Nat Microbiol. 2022;7(2):238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemian N, Ramezankhani M, Sehgal A, Khalid FM, Kalkhoran AHZ, Narayan A, et al. The trans-kingdom battle between donor and recipient gut microbiome influences fecal microbiota transplantation outcome. Sci Rep. 2020;10(1):18349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2(5):1–6. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19(10):625–37. [DOI] [PubMed] [Google Scholar]
- 26.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wortelboer K, Koopen AM, Herrema H, de Vos WM, Nieuwdorp M, Kemper EM. From fecal microbiota transplantation toward next-generation beneficial microbes: the case of Anaerobutyricum soehngenii. Front Med (Lausanne). 2022;9:1077275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Requena T, Solo de Zaldívar B, Peláez C, Martínez-Cuesta MC. Chapter 4—dietary influence on human microbiome. In: Goel G, Requena T, Bansal S, editors. Human-gut microbiome [Internet]. Academic Press; 2022. p. 59–80. Available from: https://www.sciencedirect.com/science/article/pii/B9780323913133000076.
- 29.Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B(12) production by intestinal symbionts. mBio. 2017;8(5). [DOI] [PMC free article] [PubMed]
- 30.Shahi SK, Freedman SN, Murra AC, Zarei K, Sompallae R, Gibson-Corley KN, et al. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front Immunol. 2019. 10.3389/fimmu.2019.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan B, Luckey D, Bodhke R, Chen J, Marietta E, Jeraldo P, et al. Prevotella histicola protects from arthritis by expansion of allobaculum and augmenting butyrate production in humanized mice. Front Immunol. 2021. 10.3389/fimmu.2021.609644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itano A, Maslin D, Ramani K, Mehraei G, Carpenter N, Cormack T, et al. Clinical translation of anti-inflammatory effects of Prevotella histicola in Th1, Th2, and Th17 inflammation. Front Med. 2023. 10.3389/fmed.2023.1070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evelo Biosciences, Inc. A long-term extension trial in participants with atopic dermatitis who participated in previous EDP1815 trials [Internet]. Bethesda: National Library of Medicine (US); 2023. Report No.: NCT05439941. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05439941.
- 34.Shkoporov AN, Turkington CJ, Hill C. Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat Rev Microbiol. 2022;20(12):737–49. [DOI] [PubMed] [Google Scholar]
- 35.Raeisi H, Noori M, Azimirad M, Mohebbi SR, Asadzadeh Aghdaei H, Yadegar A, et al. Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: challenges and perspectives. Gut Pathog. 2023;15(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mousavinasab F, Karimi R, Taheri S, Ahmadvand F, Sanaaee S, Najafi S, et al. Microbiome modulation in inflammatory diseases: progress to microbiome genetic engineering. Cancer Cell Int. 2023;23(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasman LM, Porter LD. Bacteriophages. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK493185/.
- 38.Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185(16):2879-2898.e24. [DOI] [PubMed] [Google Scholar]
- 39.Hryhorowicz M, Lipiński D, Zeyland J, Słomski R. CRISPR/Cas9 immune system as a tool for genome engineering. Arch Immunol Ther Exp (Warsz). 2017;65(3):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014;32(11):1146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam KN, Spanogiannopoulos P, Soto-Perez P, Alexander M, Nalley MJ, Bisanz JE, et al. Phage-delivered CRISPR-Cas9 for strain-specific depletion and genomic deletions in the gut microbiome. Cell Rep. 2021;37(5):109930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gencay YE, Jasinskytė D, Robert C, Semsey S, Martínez V, Petersen AØ, et al. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat Biotechnol. 2023;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin BE, Diamond S, Cress BF, Crits-Christoph A, Lou YC, Borges AL, et al. Species- and site-specific genome editing in complex bacterial communities. Nat Microbiol. 2022;7(1):34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danis-Wlodarczyk K, Dąbrowska K, Abedon ST. Phage therapy: the pharmacology of antibacterial viruses. Curr Issues Mol Biol. 2021;40:81–164. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Van Belleghem JD, de Vries CR, Burgener E, Chen Q, Manasherob R, et al. The safety and toxicity of phage therapy: a review of animal and clinical studies. Viruses. 2021;13(7):1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oechslin F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses. 2018;10(7):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majewska J, Kaźmierczak Z, Lahutta K, Lecion D, Szymczak A, Miernikiewicz P, et al. Induction of phage-specific antibodies by two therapeutic staphylococcal bacteriophages administered per OS. Front Immunol. 2019;10:2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu BB, Plant IN, Lyon L, Anastassacos FM, Way JC, Silver PA. In situ reprogramming of gut bacteria by oral delivery. Nat Commun. 2020;11(1):5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Froger A, Hall JE. Transformation of plasmid DNA into E. coli using the heat shock method. JoVE (J Vis Exp). 2007;(6):e253. [DOI] [PMC free article] [PubMed]
- 50.Prather KJ, Sagar S, Murphy J, Chartrain M. Industrial scale production of plasmid DNA for vaccine and gene therapy: plasmid design, production, and purification. Enzyme Microb Technol. 2003;33(7):865–83. [Google Scholar]
- 51.Mays ZJ, Nair NU. Synthetic biology in probiotic lactic acid bacteria: at the frontier of living therapeutics. Curr Opin Biotechnol. 2018;53:224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephens K, Bentley WE. Synthetic biology for manipulating quorum sensing in microbial consortia. Trends Microbiol. 2020;28(8):633–43. [DOI] [PubMed] [Google Scholar]
- 53.Koh E, Hwang IY, Lee HL, De Sotto R, Lee JWJ, Lee YS, et al. Engineering probiotics to inhibit Clostridioides difficile infection by dynamic regulation of intestinal metabolism. Nat Commun. 2022;13(1):3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, et al. An engineered E coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med. 2019;11(475):e7975. [DOI] [PubMed] [Google Scholar]
- 55.Safety T. Pharmacodynamics of SYNB1020. NCT03447730.
- 56.Perreault M, Means J, Gerson E, James M, Cotton S, Bergeron CG, et al. The live biotherapeutic SYNB1353 decreases plasma methionine via directed degradation in animal models and healthy volunteers. Cell Host Microbe. 2024;32(3):382-395.e10. [DOI] [PubMed] [Google Scholar]
- 57.Synlogic. Transforming medicine through synthetic biology [Internet]. 2024. Available from: https://investor.synlogictx.com/static-files/40aabe52-b953-4af4-a0de-13309cce7e23.
- 58.Lynch JP, González-Prieto C, Reeves AZ, Bae S, Powale U, Godbole NP, et al. Engineered Escherichia coli for the in situ secretion of therapeutic nanobodies in the gut. Cell Host Microbe. 2023;31(4):634-649 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou ZP, Du Y, Fang TT, Zhou Y, Ye BC. Biomarker-responsive engineered probiotic diagnoses, records, and ameliorates inflammatory bowel disease in mice. Cell Host Microbe. 2023;31(2):199-212.e5. [DOI] [PubMed] [Google Scholar]
- 60.Lerner A, Benzvi C, Vojdani A. The potential harmful effects of genetically engineered microorganisms (GEMs) on the intestinal microbiome and public health. Microorganisms. 2024;12(2):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ronda C, Chen SP, Cabral V, Yaung SJ, Wang HH. Metagenomic engineering of the mammalian gut microbiome in situ. Nat Methods. 2019;16(2):167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C, Wohlhueter R, Zhang H. Genetically modified foods: a critical review of their promise and problems. Food Sci Hum Wellness. 2016;5(3):116–23. [Google Scholar]
- 63.van den Berg NI, Machado D, Santos S, Rocha I, Chacón J, Harcombe W, et al. Ecological modelling approaches for predicting emergent properties in microbial communities. Nat Ecol Evol. 2022;6(7):855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rehan M, Al-Bahadly I, Thomas DG, Young W, Cheng LK, Avci E. Smart capsules for sensing and sampling the gut: status, challenges and prospects. Gut. 2024;73(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian R, Yu L, Tian F, Zhao J, Chen W, Zhai Q. Effect of inulin, galacto-oligosaccharides, and polyphenols on the gut microbiota, with a focus on Akkermansia muciniphila. Food Funct. 2024;15(9):4763–72. [DOI] [PubMed] [Google Scholar]
- 66.Juhász AE, Greff D, Teutsch B, Gede N, Hegyi P, Horváth EM, et al. Galactomannans are the most effective soluble dietary fibers in type 2 diabetes: a systematic review and network meta-analysis. Am J Clin Nutr. 2023;117(2):266–77. [DOI] [PubMed] [Google Scholar]
- 67.Micheletti C, Medori C, Bonetti G. Effects of carob extract on the intestinal microbiome and glucose metabolism: a systematic review and meta-analysis. La Clinica Terapeutica. 2023;(SUPPL 2(6)):169–72. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.