Abstract
We previously identified a functional variant of KLOTHO (termed “KL-VS”), which harbors two amino acid substitutions in complete linkage disequilibrium and is associated with reduced human longevity when in homozygosity. Klotho-deficient mice display extensive arteriosclerosis when fed a normal diet, suggesting a potent genetic predisposition. To determine whether klotho influences atherosclerotic risk in humans, we performed cross-sectional studies to assess the association between the KL-VS allele and occult coronary artery disease (CAD) in two independent samples of apparently healthy siblings of individuals with early-onset (age <60 years) CAD (SIBS-I [N=520] and SIBS-II [N=436]). Occult CAD was defined as the occurrence of a reversible perfusion defect during exercise thallium scintigraphy and/or as an abnormal result of an exercise electrocardiogram (SIBS-I, n=97; SIBS-II, n=56). In SIBS-I, the KL-VS allele conferred a relative odds of 1.90 (95% confidence interval 1.21–2.98) for occult CAD, after adjusting for familial intraclass correlations (P<.005). Logistic regression modeling, incorporating known CAD risk factors, demonstrated that the KL-VS allele is an independent risk factor (P<.019) and that the imposed risk of KL-VS allele status is influenced by modifiable risk factors. Hypertension (P<.022) and increasing high-density lipoprotein cholesterol (HDL-C) levels (P<.022) mask or reduce the risk conferred by the KL-VS allele, respectively, whereas current smoking (P<.004) increases the risk. Remarkably concordant effects of the KL-VS allele and modifying factors on the risk of occult CAD were seen in SIBS-II. These results demonstrate that the KL-VS allele is an independent risk factor for occult CAD in two independent high-risk samples. Modifiable risk factors, including hypertension, smoking status, and HDL-C level, appear to influence the risk imposed by this allele.
Introduction
We previously identified an allele of the gene KLOTHO (MIM 604824), termed “KL-VS,” that is prevalent in the general population (frequency 0.157) and, in homozygosity, is associated with reduced human longevity (Arking et al. 2002). This allele is characterized by six SNPs that occur within an 800-bp region spanning exon 2 and flanking sequence. Of the three mutations in exon 2, one is silent, and two code for amino acid substitutions, F352V and C370S, that influence klotho metabolism. Klotho-deficient mice display extensive and accelerated arteriosclerosis in association with medial calcification of the aorta and both medial calcification and intimal thickening of medium-sized muscular arteries (Kuro-o et al. 1997). In addition, they exhibit impaired endothelium-dependent vasodilation, decreased levels of nitric oxide metabolites (NO2 and NO3) in urine, and impaired angiogenesis, suggesting that klotho protein may protect the cardiovascular system through endothelium-derived nitric oxide production (Saito et al. 1998, 2000; Nagai et al. 2000; Fukino et al. 2002). Remarkably, in a rat model with multiple atherogenic risk factors (including hypertension, diabetes, obesity, and hyperlipidemia), in vivo klotho gene delivery can ameliorate vascular endothelial dysfunction and prevent medial hypertrophy and perivascular fibrosis (Shiraki-Iida et al. 2000).
Klotho is a member of the family 1 glycosidases and is composed of two internal repeats, each of which exhibits 20%–40% sequence identity to β-glucosidases across phylogeny, as well as mammalian lactase phlorhizin hydrolase. However, an enzymatic substrate for klotho has not been identified. Alternative RNA splicing yields both membrane-bound and secreted forms of klotho. In addition, klotho mRNA expression was not detectable in many organs in which severe pathology occurs in klotho-deficient mice. These observations and parabiosis experiments have led to the hypothesis that klotho acts as a humoral factor.
To determine whether KL-VS allele status influences risk of atherosclerotic coronary artery disease (CAD) in humans and is related to known modifiable risk factors, we employed cross-sectional association studies in two independent samples of apparently healthy siblings of hospitalized index cases with documented early-onset (age <60 years) CAD. Subjects who appeared to be healthy were chosen in order to enrich for factors that predispose to atherosclerosis rather than for factors that may trigger cardiac events.
Subjects and Methods
Study Population
The Johns Hopkins Sibling Study is an investigation of risk factors and their relationship to occult and incident CAD events in asymptomatic, apparently healthy 30–59-year-old siblings of individuals hospitalized with documented CAD at <60 years of age. Siblings were asked to participate in a comprehensive cardiovascular screening protocol and were eligible if they were <60 years of age and had experienced no clinically manifest CAD. Two separate samples were recruited using the same methods and were screened identically. The first sample (SIBS-I) was recruited in 1983–1996 from three Baltimore hospitals. The second sample (SIBS-II) was composed exclusively of African Americans and was recruited in 1998–2001 from 10 Baltimore hospitals. Recruitment methods were described in detail in a prior publication (Becker et al. 1998). The study was approved by the Johns Hopkins institutional review board, and all participating siblings gave informed consent.
Risk Factor Assessment
A cardiovascular history was taken, physical examination was performed, and blood was obtained for measurement of fasting levels of plasma total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) in the Johns Hopkins chemistry laboratory, which is standardized to the requirements of the Centers for Disease Control. Low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald formula for persons with TG levels <400 mg/dl (Friedewald et al. 1972). For adjusted analysis (see below), individuals with TG levels >400 mg/dl were assigned an average LDL-C for their race and sex (n=29). Blood pressure was measured at standard intervals, according to American Heart Association guidelines (1990), during an 8-h screening day. Three readings were averaged to characterize blood pressure for all subjects. Hypertension was defined as an average blood pressure of 140/90 mm Hg or the current use of antihypertensive medication. Current smoking status was defined by self-reported smoking of any cigarettes within the past month and was validated by measurement of expired carbon monoxide levels (>8 ppm) (Morabia et al. 2001). BMI was calculated as weight in kilograms divided by the square of the height in meters.
Exercise Thallium Scintigraphy
All siblings underwent a maximal symptom-limited graded exercise treadmill test (ETT), using a modified Bruce protocol (Blumenthal et al. 1996). In men, a positive stress test was defined as horizontal or downsloping ST-segment depression of ⩾1 mm over baseline at 0.06 s after the J-point in ⩾3 consecutive beats during the stress test or during the first 3 min of recovery. In women an abnormal response was defined as ⩾2 mm flat or downsloping ST-segment depression over baseline in leads II, III, or AVF or as ⩾1.5-mm ST-segment depression in any other lead.
Scintigraphy with thallium 201 was performed in conjunction with the ETT, as described elsewhere (Blumenthal et al. 1996). In brief, 1 min before the end of exercise, 3–4 mCi thallium 201 was injected intravenously, and tomographic imaging was begun 5–10 min later. After 4 h, delayed imaging was performed. Images were reconstructed by filtered back-projection, using a ramp filter after prefiltering of the projection images with a two-dimensional Fourier (Weiner) filter and correction for translational motion. Images were interpreted visually by an experienced nuclear cardiologist (L.C.B.), who had no knowledge of the subject’s identity, genotype, or risk factors.
Occult CAD was defined as an abnormal thallium tomography test represented by a segmental perfusion defect on the immediate postexercise images in more than two contiguous tomographic slices and two image orientations, accompanied by definite improvement or normalization on the delayed images. When receiver-operating-curve analysis is used, the visual interpretation using these criteria provides a 95% sensitivity for detection and a false-positive rate <10% (Fintel et al. 1989). The presence of perfusion defects is strongly associated with subsequent occurrence of premature CAD in the SIB-I population (Blumenthal et al. 1996).
KL-VS Screening
After DNA was extracted from whole blood buffy coat preparations or Guthrie cards, according to standard protocols, sample DNA was amplified using PCR (sense primer 5′-AGGCTCATGCCAAAGTCTGG-3′; antisense primer 5′-GTTTCCATGATGAACTTTTTGAGG-3′) with AmpliTaq Gold (Perkin Elmer) and supplied buffer under the following conditions: 95°C for 10 min, followed by 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, followed by a 10-min 72°C final extension. PCR products were then digested with MaeIII (Roche) at 55°C for 16 h and were electrophoretically separated on a 1.6% agarose gel. The KL-VS allele is characterized by diagnostic MaeIII restriction fragments of 265 and 185 bp.
Statistical Analysis
Descriptive statistics for each risk variable included means ± SD (continuous variables) or frequencies (qualitative variables) calculated separately for siblings found to have occult CAD and those without CAD. Unadjusted univariate comparisons between groups were performed via Shapiro-Wilk tests or the χ2 statistic in SAS, version 8.2 (SAS Institute). Adjusted analyses for KL-VS allele status and interactive effects were performed via general linear modeling using PROC GENMOD in SAS (employing a log-link function to reflect logistic regression) and using generalized estimating equations (GEE) (Liang and Zeger 1993) to account for the familial intraclass correlations between siblings when obtaining parameter estimates and variances. Because of the limited number of KL-VS homozygotes, adjusted analyses that include more than one interaction term assumed a dominant genetic model in comparisons of KL-VS carriers with noncarriers. The influence of modifiable risk factors on the risk conferred by KL-VS allele status was evaluated via the inclusion of interaction terms, and the significance was assessed via likelihood ratio testing. Odds ratios and CIs were obtained via GEE methods.
Results
The characteristics of the study population shown in table 1 are stratified by the independent samples (SIBS-I and SIBS-II) and by occult CAD status. SIBS-I (n=520) was predominantly white, and SIBS-II (n=436) was exclusively African American. The study population was middle-aged, and individuals with occult CAD were slightly but significantly older in both SIBS samples. Overall, the risk factors were generally more prevalent in persons with occult CAD, and the differences in levels and prevalence that were observed between SIBS-I and SIBS-II likely reflect racial composition and socioeconomic factors (Becker et al. 1998; Clark 1999).
Table 1.
Risk Factors and Demographic Characteristics Stratified by Sample and the Presence or Absence of Occult CAD
|
Occult CAD in SIBS-I |
Occult CAD in SIBS-II |
|||||
| Variable | Absent(n = 423) | Present(n = 97) | Pa | Absent(n = 380) | Present(n = 56) | Pa |
| Continuous (mean ± SD): | ||||||
| Age (years) | 45.3 ± 7.1 | 48.7 ± 7.0 | .0001 | 47.0 ± 6.7 | 49.0 ± 6.3 | .0367 |
| HDL-C (mg/dl) | 51.4 ± 16.0 | 46.4 ± 14.9 | .0050 | 54.4 ± 16.9 | 53.8 ± 15.9 | .7939 |
| LDL-C (mg/dl) | 147.4 ± 42.7 | 162.3 ± 44.7 | .0021 | 129.1 ± 39.2 | 136.2 ± 39.4 | .2021 |
| TG (mg/dl) | 149.1 ± 123.8 | 194.3 ± 208.7 | .0426 | 123.5 ± 84.7 | 109.5 ± 57.9 | .1184 |
| Systolic BP (mm Hg) | 130.6 ± 14.2 | 138.8 ± 14.5 | .0001 | 135.5 ± 16.6 | 139.8 ± 12.6 | .0236 |
| Diastolic BP (mm Hg) | 83.3 ± 9.4 | 87.7 ± 9.0 | .0001 | 86.5 ± 10.5 | 88.0 ± 8.3 | .2347 |
| BMI | 27.6 ± 5.8 | 28.5 ± 3.8 | .0478 | 31.9 ± 6.9 | 29.7 ± 5.4 | .0064 |
| Qualitative (%): | ||||||
| Diabetes | 6.4 | 3.1 | .2116 | 15.0 | 14.3 | .8886 |
| Hypertension | 39.6 | 54.6 | .0069 | 58.9 | 67.9 | .2037 |
| Current Smokers | 31.1 | 28.9 | .6625 | 31.6 | 32.1 | .9325 |
| African American | 16.0 | 13.4 | .5181 | 100 | 100 | … |
| Male | 46.5 | 76.3 | .0001 | 30.3 | 64.3 | .0001 |
Analysis using t tests or χ2 tests comparing occult CAD status groups within each sample.
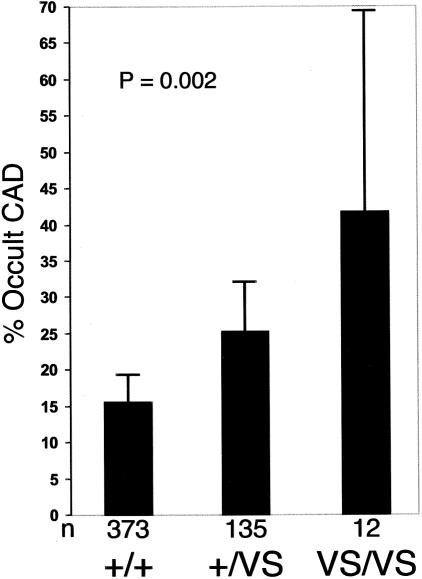
Having previously identified a functional variant of klotho (termed “KL-VS”) involved in human survival (Arking et al. 2002), we wanted to determine whether this allele also influences the risk of early-onset occult CAD. Both SIBS-I and SIBS-II have KL-VS allele frequencies (0.153 and 0.164, respectively) similar to that seen in previous studies (Arking et al. 2002). Figure 1 shows the frequency of occult CAD in SIBS-I stratified by KL-VS genotype. Univariate logistic regression analysis, assuming an additive genetic model (on a logit scale) and incorporating a GEE adjustment for familial intraclass correlations, indicates a relative odds of 1.90 (95% CI 1.21–2.98) for occult CAD conferred by the KL-VS allele (P<.005). To test whether KL-VS allele status is an independent risk factor, we constructed a logistic regression model (LRM) incorporating known CAD risk factors. Addition of KL-VS allele status significantly improved the model after adjustment for age, sex, race, BMI, hypertension, HDL-C level, LDL-C level, TG level, diabetes, and current smoking status (P<.019), indicating that KL-VS allele status is a novel risk factor for occult atherosclerosis in this sample.
Figure 1.
Frequency of occult CAD in the SIBS-I sample, stratified by KLOTHO genotype. P=.002 for trend (unadjusted). Error bars indicate 95% CIs.
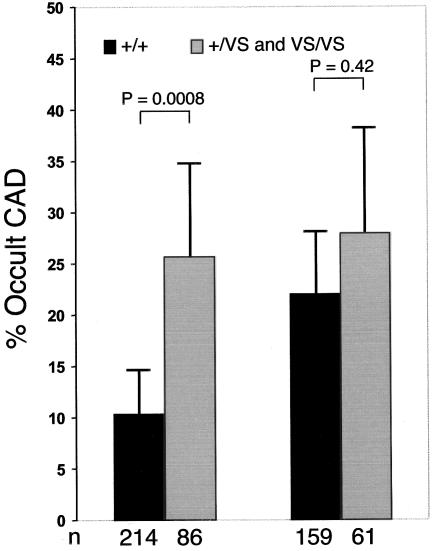
Experimental data in rats have demonstrated that klotho mRNA and protein levels are down-regulated under sustained circulatory stress (Aizawa et al. 1998; Nagai et al. 2000). It is therefore plausible that hypertensive conditions in humans could also lead to global down-regulation of klotho. In this view, the presence of concomitant hypertension could modify the risk imposed by the KL-VS allele, which is believed to manifest relative loss of functional klotho. Incorporation of an interaction term (klotho*hypertension) into the LRM to test whether hypertension modifies the risk associated with the KL-VS allele significantly improved the model (P<.022) and indicates that the detrimental effect of the KL-VS allele is more pronounced in normotensive individuals (fig. 2 and table 2).
Figure 2.
Frequency of occult CAD in the SIBS-I sample, stratified by hypertension and KLOTHO genotype. Left, Normotensive individuals. Right, Hypertensive individuals. Heterozygous and homozygous KL-VS allele carriers were combined because of the small numbers in the latter group after stratification for hypertension. Error bars indicate 95% CIs.
Table 2.
Multiple Logistic Regression Predicting Occult CAD for SIBS-I and SIBS-II Samples[Note]
| SIBS-I |
SIBS-II |
|||||
| Risk Factora | βb | SE | P | βb | SE | P |
| Intercept | −9.2071 | 1.7987 | .0001 | −3.0780 | 1.9622 | .12 |
| Age | .0971 | .0238 | .0001 | .0512 | .0302 | .09 |
| Sex | −1.4290 | .3709 | .0001 | −1.2623 | .3292 | .0001 |
| Race | −.0690 | .3985 | .86 | … | … | … |
| LDL-C | .0062 | .0034 | .30 | .0077 | .0046 | .10 |
| HDL-C | .0190 | .0121 | .12 | .0040 | .0153 | .79 |
| TG | .0010 | .0007 | .17 | −.0046 | .0030 | .13 |
| Diabetes | −1.0038 | .6767 | .14 | .5087 | .5145 | .32 |
| Hypertension | .5211 | .3404 | .13 | .4938 | .4703 | .29 |
| BMI | .0478 | .0278 | .09 | −.0585 | .0351 | .10 |
| Smoking | −.4201 | .4039 | .30 | .0885 | .4470 | .84 |
| Klotho | 2.4170 |
1.0447 | .021 | 2.6908 |
1.3959 | .05 |
| Klotho*hypertension | −.9834 |
.5851 | .09 | −1.2558 |
.7344 | .09 |
| Klotho*smoking | 1.4019 | .6477 | .030 | −.0048 | .7282 | .99 |
| Klotho*HDL-C | −.0383 |
.0210 | .07 | −.0341 |
.0212 | .11 |
| Overall klotho effectc | .0002 | .06 | ||||
Note.— Analyses were GEE adjusted.
Klotho is incorporated under the assumption of a dominant model.
Underlined values show dramatic concordance between the SIBS-I and SIBS-II populations.
Likelihood ratio tests comparing models that included klotho and significant modifier terms with models that did not include klotho terms.
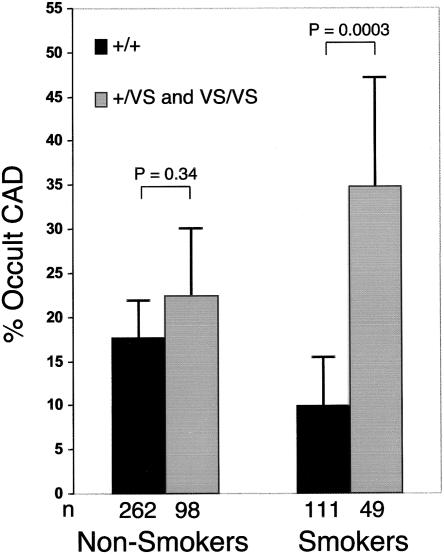
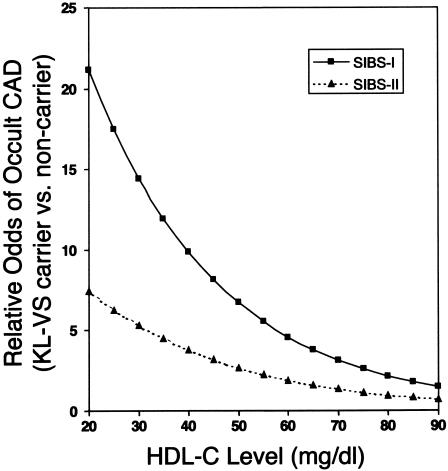
The possibility that additional modifiable risk factors influence the association between KL-VS allele status and occult atherosclerosis was analyzed by sequentially incorporating the risk factor interaction term that most significantly improved the LRM (P<.05). The klotho term is incorporated under the assumption of a dominant model, because of an insufficient number of homozygotes for analysis under the assumption of an additive model. Following this strategy, we found significant modifications to the risk associated with KL-VS allele status for current smoking status (P<.004) (fig. 3 and table 2) and HDL-C levels (P<.022) (table 2). The addition of KL-VS allele status, including the modifications of risks due to hypertension, smoking, and HDL-C levels, resulted in a highly significant increase in the overall fit of the LRM (P<.0002) and indicate a relative odds ⩾9.8 (95% CI 3.27–29.6) for occult CAD among normotensive smokers with HDL-C levels ⩽40 mg/dl who carry the KL-VS allele versus noncarriers (fig. 4).
Figure 3.
Frequency of occult CAD in the SIBS-I sample, stratified by KLOTHO genotype and current smoking status. Heterozygous and homozygous KL-VS allele carriers were combined because of the small numbers in the latter group after stratification for current smoking status. Error bars represent 95% CIs.
Figure 4.
Relative odds of occult CAD conferred by the KL-VS allele with increasing HDL-C Levels. Blackened squares represent SIBS-I normotensive smokers. Closed triangles represent all SIBS-II normotensive individuals.
We analyzed an additional sample (SIBS-II), in which enrollment was limited to African Americans recruited under the same criteria as the SIBS-I sample (table 1). Univariate logistic regression analysis did not indicate an increased risk of occult CAD for carriers of the KL-VS allele (data not shown). This result was somewhat predictable, given the higher frequency of hypertension and the higher HDL levels in SIBS-II. However, inclusion of KL-VS allele status in an LRM constructed to incorporate known risk factors and risk modifications due to hypertension and HDL-C level significantly improved the ability to predict individuals with occult CAD in SIBS-II (P<.06), as was observed in SIBS-I (P<.0002) (table 2). These results are particularly striking when one takes into account the remarkable similarity in the magnitude of effect attributable to the KL-VS allele and significant modifying risk factors in the two groups (table 2). Current smoking status had no effect on the risk of occult CAD conferred by the KL-VS allele in the SIBS-II population (P>.99). In SIBS-II, the relative odds for occult CAD is ⩾3.8 (95% CI 1.29–10.9) among normotensive individuals with HDL-C levels ⩽40 mg/dl who carry the KL-VS allele versus noncarriers (fig. 4). These results confirm that KL-VS allele status is an important predictor of occult CAD in family members of individuals with early-onset, clinically manifest CAD. However, modification of the risk conferred by the KL-VS allele by known CAD risk factors may not be identical in all populations.
Discussion
Family history of premature CAD in a first-degree relative is a major predictor of CAD, even after adjustment for known risk factors, suggesting the presence of as yet unidentified genetic contributors to this complex disease (Shea et al. 1984; Schildkraut et al. 1989; Yuhanna et al. 2001). In a study reported elsewhere (Arking et al. 2002), we identified the KL-VS allele of the KLOTHO gene as a functional variant that influences longevity, and we demonstrated that the derived protein likely has reduced activity. Individuals who are homozygous for this allele exhibit a 2.6-fold reduction in survival to age ⩾65 years. Partly on the basis of observations in klotho-deficient mice, a role for klotho in protecting the cardiovascular system has been proposed (Saito et al. 1998; Nagai et al. 2000). We have now demonstrated that the KL-VS allele of KLOTHO confers risk of occult atherosclerosis in a high-risk sample composed of siblings of individuals with premature CAD. The effect of the KL-VS allele is evident after adjustment for known risk factors (including sex), which indicates that it is a novel risk factor for both men and women. Modifiable risk factors, including hypertension, current smoking, and HDL-C level, significantly modulated the risk associated with the KL-VS allele. To confirm these results, we analyzed an additional independent sample. We did not see a univariate klotho effect in this sample, owing to the increased levels of HDL-C and prevalence of hypertension in its members. These factors mitigate the contribution of the KL-VS allele to increased risk of occult CAD. However, in an LRM, we did observe striking similarities in klotho and klotho*HDL/hypertension modifier effects between the two samples.
The identification of modifiable factors that are known to influence the risk conferred by the KL-VS allele is of particular interest, because these factors may shed light on the mechanism by which klotho influences atherogenic CAD risk and may suggest productive strategies for therapeutic intervention. Hypertension and HDL-C levels significantly modified the risk associated with KL-VS allele status in both samples. Normotensive KL-VS carriers demonstrated a greater increase in risk of occult atherosclerosis than did hypertensive carriers. Experimental data generated in rat models demonstrate that klotho levels are down-regulated under hypertensive conditions (Aizawa et al. 1998; Nagai et al. 2000) and by angiotensin II (Mitani et al. 2002). Thus, the levels of klotho are likely to be reduced in hypertensive individuals, which could mask the detrimental effect of the KL-VS allele. This hypothesis also raises the intriguing question of whether the status of elevated angiotensin II as an independent risk factor for cardiovascular disease (Brunner 2001; Gavras and Gavras 2002) may be due, at least in part, to down-regulation of klotho levels. Because a system for measuring serum levels of klotho has not yet been developed, this hypothesis remains to be tested. Alternatively, the modification, as a result of hypertension, of the risk conferred by the KL-VS allele could arise if hypertensive individuals who carry the KL-VS allele exhibit greatly increased frequency of coronary events, because only individuals who are apparently unaffected were included in the present study. Similarly, a differential response, depending on KL-VS genotype, to antihypertensive medication could contribute to this result. Prospective studies are required to address these hypotheses.
Klotho has been proposed to protect the cardiovascular system through endothelium-derived nitric oxide production (Saito et al. 1998, 2000; Nagai et al. 2000; Fukino et al. 2002), suggesting that the KL-VS allele may increase the risk of occult CAD, at least in part, because of a decrease in nitric oxide production. Thus, in light of recent evidence that HDL-C activates endothelial nitric oxide synthase and increases nitric oxide expression (Yuhanna et al. 2001; Kuvin et al. 2002), it is particularly intriguing that increased HDL-C levels specifically protect against the detrimental effect associated with the KL-VS allele. These data are consistent with a model that invokes opposing influences of klotho deficiency and elevated HDL-C levels on nitric oxide production and ultimate risk of CAD. This result raises the possibility that the use of drugs such as statins or niacin to increase HDL-C levels may prove to be particularly effective in preventing CAD in KL-VS carriers and that such an intervention might be beneficial even in individuals with normal HDL-C levels.
CAD has been proposed to be subject to interaction between environmental factors and genetic predispositions. Several studies have shown a relationship between genetic factors and lifestyle choices such as diet (McCombs et al. 1994; Ordovas 1999; Denke et al. 2000) and alcohol consumption (Hines et al. 2001). Recently, Talmud et al. (2000) identified an allele of lipoprotein lipase that augments risk of ischemic heart disease, specifically in male smokers. We have now demonstrated that KL-VS carriers who smoke exhibit increased risk of occult CAD in the SIBS-I sample, indicative of a strong gene-environment interaction. This result was not seen in the SIBS-II sample, which is restricted to African Americans. It is possible that the marked increase in other risk factors in the SIBS-II sample (including diabetes, high BMI, and hypertension) may mask any influence of smoking on the risk conferred by the KL-VS allele. In addition, smoking habits (including starting age and number of cigarettes per day) and the stability of metabolites in the bloodstream in African Americans are distinct from those in white Americans (Ahijevych 1999; Woods et al. 2001). Further studies are required to determine whether smoking modifies the risk conferred by KL-VS allele status in other populations and whether racial differences and/or smoking habits modify this effect.
We deliberately studied apparently healthy siblings of individuals with premature incident CAD, to enrich for subjects with a genetic predisposition to CAD. Although this study design was successful and may serve as a model for identifying genetic contributions to CAD, the extent to which the KL-VS allele contributes to occult atherosclerotic CAD in the general population remains to be elucidated.
In conclusion, we have identified a novel genetic risk factor for early-onset CAD. The KL-VS allele of the KLOTHO gene displays a strong gene-environment interaction, and the risk associated with this allele is modulated by modifiable risk factors. Importantly, ∼25% of individuals are carriers of this allele. These results suggest that genotyping for the KL-VS allele will identify individuals who are at higher relative odds of CAD and who could benefit from a genetically tailored therapeutic strategy.
Acknowledgments
This research was supported by the Howard Hughes Medical Institute (H.C.D.), the Smilow Family Foundation (H.C.D.), The Broccoli Foundation (H.C.D.), and National Institutes of Health grants AR41135 (to H.C.D. and D.E.A.), NR0224 and HL49762 (to D.M.B, L.R.Y., T.F.M., and L.C.B.), and M01 RR00052 (to Johns Hopkins University School of Medicine General Clinical Research Center, supporting D.M.B, L.R.Y., T.F.M., and L.C.B).
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for KLOTHO) [PubMed]
References
- Ahijevych K (1999) Nicotine metabolism variability and nicotine addiction. Nicotine Tob Res Suppl 1:S59–S62 [DOI] [PubMed] [Google Scholar]
- Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R (1998) Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun 249:865–871 [DOI] [PubMed] [Google Scholar]
- American Heart Association (1990) Human blood pressure determination by sphygmomanometry. American Heart Association, Dallas, pp 2460–2467 [Google Scholar]
- Arking DE, Krebsova A, Macek M Sr, Macek M Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC (2002) Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 99:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DM, Yook RM, Moy TF, Blumenthal RS, Becker LC (1998) Markedly high prevalence of coronary risk factors in apparently healthy African-American and white siblings of persons with premature coronary heart disease. Am J Cardiol 82:1046–1051 [DOI] [PubMed] [Google Scholar]
- Blumenthal RS, Becker DM, Moy TF, Coresh J, Wilder LB, Becker LC (1996) Exercise thallium tomography predicts future clinically manifest coronary heart disease in a high-risk asymptomatic population. Circulation 93:915–923 [DOI] [PubMed] [Google Scholar]
- Brunner HR (2001) Experimental and clinical evidence that angiotensin II is an independent risk factor for cardiovascular disease. Am J Cardiol 87:3C–9C [DOI] [PubMed] [Google Scholar]
- Clark LT (1999) Primary prevention of cardiovascular disease in high-risk patients: physiologic and demographic risk factor differences between African American and white American populations. Am J Med 107:22S–24S [DOI] [PubMed] [Google Scholar]
- Denke MA, Adams-Huet B, Nguyen AT (2000) Individual cholesterol variation in response to a margarine- or butter- based diet: a study in families. JAMA 284:2740–2747 [DOI] [PubMed] [Google Scholar]
- Fintel DJ, Links JM, Brinker JA, Frank TL, Parker M, Becker LC (1989) Improved diagnostic performance of exercise thallium-201 single photon emission computed tomography over planar imaging in the diagnosis of coronary artery disease: a receiver operating characteristic analysis. J Am Coll Cardiol 13:600–612 [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Fukino K, Suzuki T, Saito Y, Shindo T, Amaki T, Kurabayashi M, Nagai R (2002) Regulation of angiogenesis by the aging suppressor gene klotho. Biochem Biophys Res Commun 293:332–337 [DOI] [PubMed] [Google Scholar]
- Gavras I, Gavras H (2002) Angiotensin II as a cardiovascular risk factor. J Hum Hypertens Suppl 2 16:S2–S6 [DOI] [PubMed] [Google Scholar]
- Hines LM, Stampfer MJ, Ma J, Gaziano JM, Ridker PM, Hankinson SE, Sacks F, Rimm EB, Hunter DJ (2001) Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Engl J Med 344:549–555 [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH (2002) A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J 144:165–172 [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL (1993) Regression analysis for correlated data. Annu Rev Public Health 14:43–68 [DOI] [PubMed] [Google Scholar]
- McCombs RJ, Marcadis DE, Ellis J, Weinberg RB (1994) Attenuated hypercholesterolemic response to a high-cholesterol diet in subjects heterozygous for the apolipoprotein A-IV-2 allele. N Engl J Med 331:706–710 [DOI] [PubMed] [Google Scholar]
- Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R (2002) In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39:838–843 [DOI] [PubMed] [Google Scholar]
- Morabia A, Bernstein MS, Curtin F, Berode M (2001) Validation of self-reported smoking status by simultaneous measurement of carbon monoxide and salivary thiocyanate. Prev Med 32:82–88 [DOI] [PubMed] [Google Scholar]
- Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M (2000) Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci 57:738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas JM (1999) The genetics of serum lipid responsiveness to dietary interventions. Proc Nutr Soc 58:171–187 [DOI] [PubMed] [Google Scholar]
- Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R (2000) In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 276:767–772 [DOI] [PubMed] [Google Scholar]
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R (1998) Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 248:324–329 [DOI] [PubMed] [Google Scholar]
- Schildkraut JM, Myers RH, Cupples LA, Kiely DK, Kannel WB (1989) Coronary risk associated with age and sex of parental heart disease in the Framingham Study. Am J Cardiol 64:555–559 [DOI] [PubMed] [Google Scholar]
- Shea S, Ottman R, Gabrieli C, Stein Z, Nichols A (1984) Family history as an independent risk factor for coronary artery disease. J Am Coll Cardiol 4:793–801 [DOI] [PubMed] [Google Scholar]
- Shiraki-Iida T, Iida A, Nabeshima Y, Anazawa H, Nishikawa S, Noda M, Kuro-o M (2000) Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med 2:233–242 [DOI] [PubMed] [Google Scholar]
- Talmud PJ, Bujac SR, Hall S, Miller GJ, Humphries SE (2000) Substitution of asparagine for aspartic acid at residue 9 (D9N) of lipoprotein lipase markedly augments risk of ischaemic heart disease in male smokers. Atherosclerosis 149:75–81 [DOI] [PubMed] [Google Scholar]
- Woods MN, Harris KJ, Ahluwalia JS, Schmelzle KH, Mayo MS (2001) Smoking in urban African Americans: behaviors, gender differences, and motivation to quit. Ethn Dis 11:532–539 [PubMed] [Google Scholar]
- Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW (2001) High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 7:853–857 [DOI] [PubMed] [Google Scholar]