Abstract
Speculation has long surrounded the question of whether past exposure to ionizing radiation leaves a unique permanent signature in the genome. Intrachromosomal rearrangements or deletions are produced much more efficiently by densely ionizing radiation than by chemical mutagens, x-rays, or endogenous aging processes. Until recently, such stable intrachromosomal aberrations have been very hard to detect, but a new chromosome band painting technique has made their detection practical. We report the detection and quantification of stable intrachromosomal aberrations in lymphocytes of healthy former nuclear-weapons workers who were exposed to plutonium many years ago. Even many years after occupational exposure, more than half the blood cells of the healthy plutonium workers contain large (>6 Mb) intrachromosomal rearrangements. The yield of these aberrations was highly correlated with plutonium dose to the bone marrow. The control groups contained very few such intrachromosomal aberrations. Quantification of this large-scale chromosomal damage in human populations exposed many years earlier will lead to new insights into the mechanisms and risks of cytogenetic damage.
Introduction
Speculation has long surrounded the question of whether past exposure to ionizing radiation leaves a unique permanent signature in the genome (Sankaranarayanan 1973; UNSCEAR 2000). Such a biomarker would have a strong impact on studies of cancer causation and genetics. We report here a long-lived, low-background, sensitive biomarker of past exposure to densely ionizing radiation. Densely-ionizing radiations include alpha particles such as from radon (Alavanja 2002) or plutonium (Durante and Manti 2002), as well as neutrons, relevant to frequent flyers or flight personnel (Blettner et al. 1998), and occupational neutron exposure (Wolber et al. 1996).
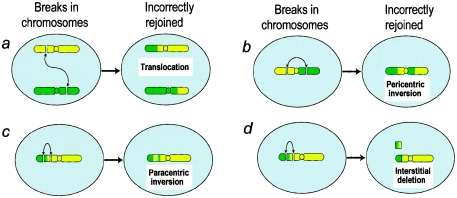
The biomarker relates to the frequency of large (>6 Mb) stable (transmissible to subsequent cell generations) intrachromosomal rearrangements (inversion or deletions), which can be measured in the blood of irradiated individuals, potentially many years after exposure. On the basis of mechanistic considerations (Brenner and Sachs 1994) discussed below, this class of chromosomal aberrations is expected to be highly preferentially induced by alpha particles or neutrons, in comparison to chemical mutagens, x-rays, or endogenous processes such as aging. Figures 1b–1d show schematics of the different types of stable intrachromosomal aberrations (pericentric inversions, paracentric inversions, and intra-arm deletions).
Figure 1.
Schematics of production of stable interchromosomal and intrachromosomal aberrations. a, Interchromosomal aberration produced by misrejoining of chromosome, showing breaks on two different chromosomes. b–d, Intrachromosomal aberrations produced by misrejoining of breaks. Effects of misrejoining of breaks on two different arms of a single chromosome (b) or within a single chromosome arm (c and d) are shown. Intrachromosomal aberrations generally originate from pairs of chromosome breaks that are closer together than those producing interchromosomal aberrations; densely ionizing radiations are more likely than other mutagens to produce such multiple chromosome breaks close together.
The mechanistic background (Brenner and Sachs 1994) relates to the fact that densely ionizing radiations, such as alpha particles or neutrons, produce highly localized DNA damage at the chromosomal level (Prise et al. 2001). Almost all other mutagens, such as chemicals, x-rays, or general aging processes, produce a far more homogeneous spatial distribution of DNA damage. Chromosomal aberrations are primarily produced by the misrepair of pairs of chromosome breaks (Savage 1998). Because alpha particles preferentially produce multiple breaks within single chromosomes—which are themselves confined within localized domains (Cremer and Cremer 2001)—there will be a preference for alpha particles to produce intrachromosomal aberrations (figs. 1b–1d), that is, aberrations within a single chromosome. By contrast, the fact that chemical mutagens, x-rays, and endogenous processes generally produce chromosome breaks distributed across many or all the chromosomes means they are more likely to produce interchromosomal aberrations such as translocations (fig. 1a).
Some supporting evidence (Bauchinger and Schmid 1998) has come from the study of unstable chromosomal aberrations—aberrations involving a lesion that, generally, prevents the cell from dividing. Such aberrations, however, disappear relatively rapidly (Ramalho et al. 1995; International Atomic Energy Agency 2001), so for practical purposes one needs to study stable aberrations that are transmissible to subsequent cell generations. Until recently, stable intrachromosomal aberrations were extremely difficult to measure; early techniques that involved Giemsa staining had very limited resolution; standard modern FISH involves painting whole chromosomes (Speicher et al. 1996; Greulich et al. 2000; Tucker 2001) or whole chromosome arms in a single color (Karhu et al. 2001)—resulting in intra-arm rearrangements being undetectable. However, an efficient chromosome-painting technique, termed “mBAND,” has recently been developed (Chudoba et al. 1999). In mBAND, a series of colored bands are painted along the axis of the chromosome. Thus, any loss or change in the order of these color bands indicates an intrachromosomal rearrangement.
We have applied this technique to healthy former nuclear-weapons workers who were occupationally exposed from 1949 onward in the former Soviet Union (Anspaugh et al. 2002). The radiation workers were employed either in plutonium manufacturing/processing facilities or in a nuclear reactor facility. The plutonium workers, but not the reactor workers, were exposed to densely ionizing alpha particles as a consequence of plutonium inhalation.
Subjects, Material, and Methods
Study Population
Individuals in the study population were occupationally exposed to ionizing radiation from 1949 onward, at the Mayak Production Association near Ozyorsk, Russia (Anspaugh et al. 2002). The plutonium workers were exposed to densely ionizing alpha particles as a consequence of plutonium inhalation and were also exposed to sparsely ionizing gamma rays. The reactor workers were not exposed to plutonium, but they were exposed to sparsely ionizing gamma rays. Both groups were also exposed to a variety of chemical mutagens. Among the Mayak workers, increased risks have been reported for cancers of the lung (Kreisheimer et al. 2000), liver (Gilbert et al. 2000), and bone (Koshurnikova et al. 2000), although all the subjects in the present study appear to be healthy.
We report a study of 31 individuals from the Mayak radiation worker cohort; 11 were exposed to high levels of plutonium (as well as other mutagens, including gamma rays, benzene, and tobacco), and 11 were not exposed to plutonium but were exposed to high doses of gamma rays and chemical mutagens. A small group (n=4) of the plutonium workers who received only moderate exposure and an unexposed control group (n=5) of healthy workers were also assessed. Mean start/end dates of occupational exposure for each of these groups are given in table 1. The study was approved by the appropriate institutional review board, and informed consent was given by each subject.
Table 1.
Dosimetry of the Three Groups with Occupational Exposure to Radiation
| Variable | Highly ExposedPlutonium Workers(n = 11) | Reactor Workers (Zero Plutonium)(n = 11) | Moderately ExposedPlutonium Workers(n = 4) |
| Mean start/end dates of occupational exposure | 1951/1971 | 1949/1977 | 1960/1989 |
| Mean age (range) at time of samplinga | 75 (68–82) | 74 (67–82) | 66 (62–77 ) |
| Mean plutonium dose (range) to bone marrow (Gy) | 1.1 (.4–2.1) | 0 | .19 (.11–.33) |
| Mean gamma dose (range) to bone marrow (Gy) | 1.5 (0–3.1) | 2.3 (1.5–3.8) | .19 (.07–.31) |
Mean age (range) of the control group was 60 years (43–77 years).
Detection of Intra- and Interchromosomal Aberrations
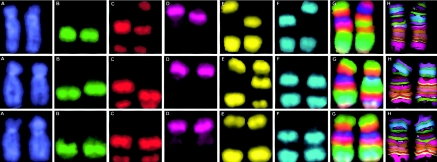
The mBAND technique used to detect intrachromosomal aberrations (Chudoba et al. 1999; Boei et al. 2002) is based on region-specific chromosome paints (RSCPs) combined with quantitative color ratio analysis: Each RSCP is labeled using a unique fluorochrome combination. The partial overlap between adjacent RSCPs results in a “merged color” continuously changing fluorescence pattern along the chromosome axis, quantified as a continuous change of color ratios. “Pseudocolors” are then assigned to chromosome sections with similar color ratios, resulting in a reproducible color banding pattern that is virtually independent of chromosome condensation. The mBAND work reported here used chromosome 5, for which five fluorochromes (Rhodamine 110, Sprectrum Orange [O], DEAC [diethylamino-coumarin {D}], and Biotin, which was detected with Streptavidin-Cy 5 [C]) are used to generate seven RSCPs (two for the p arm and five for the q arm), resulting in 22 color bands. Figure 2 shows some typical images for the individual fluorochromes, as well as merged color images and, finally, pseudocolor images.
Figure 2.
Intrachromosomal aberrations in chromosome 5 detected by use of the mBAND technique. Localization of the RSCP is shown in images captured using six different filters, specifically DAPI (A), FITC (B), Gold (C), Texas Red (D), Cy5 (E), and Aqua (F). Quantitative color ratio analysis of these different chromosome paints yields a “merged color” fluorescence intensity pattern (G) along the chromosome axis, indicating continuously changing color ratios. Finally, “pseudocolors” are assigned to sections of the chromosome with similar color ratios (H). Top, Intrachromosomal interarm aberration (pericentric inversion), as shown in figure 3b. Middle, Intrachromosomal intra-arm aberration (paracentric inversion), as shown in figure 3c. Bottom, Intrachromosomal intra-arm aberration (intra-arm deletion), as shown in figure 3d.
The mFISH technique (Speicher et al. 1996) was used to score stable interchromosomal aberrations (simple translocations) (fig. 1a). The mFISH probes contain chromosome paints specific for each of the 24 human chromosomes. Each paint is labeled with a unique combination of the five fluorochromes (fluorescein isothiocyanate [F], Spectrum Orange [O], Texas Red [R], DEAC [D], and Biotin, which was detected with Streptavidin-Cy 5 [C]). The fluorochrome labeling combinations for the 24 chromosomes are as follows: 1 = C, 2 = D, 3 = R, 4 = F, 5 = O, 6 = FC, 7 = CD, 8 = RC, 9 = OC, 10 = FD, 11 = FR, 12 = FO, 13 = RD, 14 = OD, 15 = OR, 16 = FCD, 17 = FRC, 18 = FOC, 19 = RCD, 20 = OCD, 21 = ORC, 22 = FRD, X = FOD, and Y = ORD. The result is an unequivocal color signature for each of the 24 chromosomes, so that any interchromosomal translocations are observed as color junctions on individual chromosomes. The use of a different color to paint each chromosome a different color significantly improves the precision and accuracy of translocation scoring (Greulich et al. 2000), compared with standard, partial-genome FISH labeling (Tucker 2001).
mBAND/mFISH Assays
Blood samples were collected from the 31 individuals during the year 2001, and intra- and interchromosomal aberrations were scored in peripheral blood lymphocytes. Metaphase cell slide preparations were made at the Southern Urals Biological Institute in Ozyorsk, Russia, using standard protocols (Burak et al. 2001). In the present study, lymphocyte cultures were initiated 4–5 d after the blood was drawn and were incubated for 48 h before metaphase slide preparation. mBAND, mFISH (Speicher et al. 1996; Chudoba et al. 1999), and aberration scoring were subsequently performed in New York. A range of 110–160 metaphase cells were scored for each individual for the mBAND (intrachromosomal) analyses, and a similar number were scored for the mFISH (interchromosomal) analyses.
Chromosome paints, as described above, were obtained from MetaSystems. Microscopic analysis was performed using an Axioplan II imaging microscope (Carl Zeiss) with an HBO-103 mercury lamp and filter sets for FITC, Cy3.5, Texas Red, Cy5, and Aqua. Images were captured, processed, and analyzed using Isis mBAND/mFISH imaging software (MetaSystems).
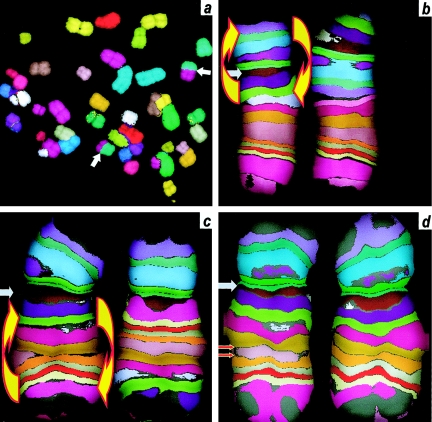
Figure 3 shows some typical chromosomal aberrations, as visualized and scored with mBAND (for intrachromosomal aberrations) and mFISH (for interchromosomal aberrations); pseudocolor images are shown in figure 3, with the underlying mBAND color-filtered images and merged color images illustrated in figure 2.
Figure 3.
Stable inter- and intrachromosomal aberrations in Mayak plutonium workers (pseudocolor images). The panels correspond to the schematics in figure 1. a, Interchromosomal aberration (simple translocation) (white arrows), detected using mFISH. b–d, Intrachromosomal aberrations (gray arrows) in chromosome 5 detected using mBAND, showing centromere. Left chromosome in each pair is normal; right chromosome shows aberration. b, Interarm aberration (pericentric inversion), showing the region of the chromosome that was inverted (yellow arrows). c, Intrachromosomal intra-arm aberration (paracentric inversion), showing the region of the chromosome that was inverted (yellow arrows). d, Intrachromosomal intra-arm aberration (intra-arm deletion), showing the region of the arm that was deleted (red arrows).
Worker Dosimetry
Individual estimates were made of the plutonium doses and the gamma-ray doses to the bone marrow of each individual studied. Currently, plutonium dosimetry estimates are available for ∼8,700 individuals, and gamma-ray dosimetry estimates are available for ∼100,00 individuals (Romanov et al. 2002).
Estimates of plutonium exposure are based on urine sample measurements (Khokhryakov et al. 1998a). An average of about nine measurements per individual are available (Krahenbuhl et al. 2002). To derive organ doses (in particular, for our purposes, red bone marrow doses) from these measurements, several steps are needed: First, a biokinetic model is applied that uses urinary plutonium excretion data to estimate the plutonium accumulation in the respiratory tract (Khokhryakov et al. 2002). A model for lung clearance (Khokhryakov et al. 2000) and another for plutonium excretion (Khokhryakov et al. 1994) are included; information is used regarding the transportability of the particular chemical forms of plutonium to which a given worker was exposed (Khokhryakov et al. 1998b)—transportability determines the biokinetics of the plutonium transport. Finally, autopsy data (Suslova et al. 2002) are used to determine the fraction of the overall plutonium load deposited in different extrapulmonary organs.
Unlike the gamma-ray exposure, which terminated at the end of each individual’s work period, a fraction of the plutonium exposure occurred subsequently, because of long-term retention of a fraction of the plutonium intake. For the plutonium workers studied here, an average of 50% of the bone marrow plutonium dose was deposited after 1983, 25% was deposited after 1993, and 8% was deposited after 1998, as estimated with the dosimetry system described above.
Data about gamma-ray exposure from external sources are based primarily on film badge data (Romanov et al. 2002). Film dosimeters were worn by all workers, beginning in 1948, although the different types of filters used with the film have necessitated the application of a variety of correction factors; these correction factors have been experimentally estimated (Romanov et al. 2002) and applied, on the basis of reconstructions of the gamma-ray energy spectra in different work locations (Vasilenko et al. 2000). Reasonable agreement was found between the gamma-ray dose estimates and experimental electron paramagnetic resonance measurements in tooth enamel of 62 workers at Mayak (Romanyukha et al. 2000).
The reactor workers also had very low levels of neutron exposure. The maximum neutron dose to the bone marrow was estimated to be <0.3% of the gamma-ray dose. Dosimetry details for each of the groups in the present study are given in table 1.
Results
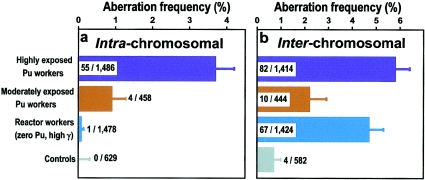
The overall results are given in table 2 and are illustrated in figure 4. Comparably high yields of interchromosomal aberrations (apparently simple translocation between any heterologous pair of chromosome) were seen in both the highly exposed plutonium workers (mean ± SD 5.8 ± 0.6%) and in the reactor workers (mean ± SD 4.7 ± 0.6%), consistent with the fact that the total bone marrow dose was similar in the two groups (2.6 Gy vs. 2.3 Gy, table 1).
Table 2.
Measured Yields of Stable Intrachromosomal and Stable Interchromosomal Aberrations, for the Four Groups Studied
|
No. of Chromosome Aberrations/No. of Metaphase Cells (Mean Frequency ± SD) in |
||||
| Type of Aberration | Highly ExposedPlutonium Workers(n=11) | Reactor Workers(Zero Plutonium)(n=11) | Moderately ExposedPlutonium Workers(n=4) | ControlIndividuals(n=5) |
| Intrachromosomal stablea | 55/1,488b (3.7 ± .5) | 1/1,478c (.1 ± .1) | 4/458d (.9 ± .4) | 0/629 (0 ± .3) |
| Interchromosomal stablee | 82/1,414f (5.8 ± .6) | 67/1,424 (4.7 ± .6) | 10/444 (2.2 ± .7) | 4/582 (.7 ± .3) |
Pericentric inversions, paracentric inversions, and intra-arm deletions in chromosome 5.
P <10−4 (Fisher’s exact test) vs. reactor workers, P = .0006 vs. moderately-exposed plutonium workers, P <10−4 vs. control individuals.
P <10−4 vs. highly-exposed plutonium workers, P = .01 vs. moderately-exposed plutonium workers, P = .7 vs. control individuals.
P = .0006 vs. highly-exposed plutonium workers, P = .01 vs. reactor workers, P = .03 vs. control individuals.
Apparently simple translocations between any heterologous pair of chromosomes.
Unstable interchromosomal aberrations (dicentrics) (7/1,414 [.5%]) were also detected in this group.
Figure 4.
Measured yields of stable chromosomal aberrations in peripheral blood lymphocytes of highly-exposed plutonium workers, moderately-exposed plutonium workers, reactor workers, and unexposed control individuals. Shown on the histograms are the number of aberrations found/number of metaphase cells examined. a, Intrachromosomal aberration (pericentric inversions, paracentric inversions, and intra-arm inversions) measured in chromosome 5. b, Interchromosomal aberrations (apparently simple translocations between any heterologous pair of chromosomes). Note the rarity of intrachromosomal aberrations in the highly exposed reactor workers (who received no plutonium exposure) and in the control group.
Remarkably high frequencies of intrachromosomal aberrations were seen in the highly exposed plutonium workers (mean ± SD 3.7% ± 0.5%), despite the fact that only chromosome 5 was examined. These results are significantly higher than those for the reactor workers (mean ± SD 0.1% ± 0.1%, P<10-4, Fisher’s exact test), although the overall dose to the bone marrow was similar in the two groups. The moderately exposed plutonium workers showed a lower frequency of intrachromosomal aberrations than the highly exposed group (mean ± SD 0.9% ± 0.4%, P<.001) but still significantly higher than the reactor workers (P=.01).
The yield of interchromosomal aberrations in the control population (0.7% ± 0.3%) was reasonably consistent with previous estimates—an estimate of 1.1% for individuals age 60 years (the average age of the control group) has been reported elsewhere (Tucker et al. 1994). No intrachromosomal aberrations were seen in the control population (upper 68% confidence limit 0.3%).
Individual details of the intrachromosomal aberration yields in the 14 plutonium workers are shown in table 3 in relation to the plutonium doses. The number of intrachromosomal aberrations per cell was significantly correlated with the estimated plutonium dose to the bone marrow (P=.002, Kendall’s rank correlation test).
Table 3.
Individual Yields of Stable Intrachromosomal Aberrations in Chromosome 5 for the Plutonium Workers
| Subject | Plutonium Dose toBone Marrow(Gy) | No. of CellsExamined | No. ofIntrachromosomalAberrations |
| 1 | 2.08 | 135 | 9 |
| 2 | 2.00 | 125 | 5 |
| 3 | 1.27 | 115 | 5 |
| 4 | 1.21 | 135 | 5 |
| 5 | 1.13 | 141 | 7 |
| 6 | 1.02 | 110 | 6 |
| 7 | .94 | 147 | 2 |
| 8 | .89 | 145 | 2 |
| 9 | .77 | 126 | 5 |
| 10 | .64 | 157 | 5 |
| 11 | .44 | 152 | 4 |
| 12 | .33 | 111 | 0 |
| 13 | .17 | 127 | 2 |
| 14 | .14 | 110 | 2 |
| 15 | .11 | 110 | 0 |
No evidence of clonal aberrations was found (i.e., the same aberration was never found in more than one cell of the same individual).
Discussion
Long-Term Burden of Stable Intrachromosomal Aberrations
The radiation workers, despite their healthy and long-lived status, maintain a considerable stable chromosomal aberration burden many years after occupational exposure. Specifically, the yield of intrachromosomal aberrations (3.7% in chromosome 5) in the highly exposed plutonium workers (table 2)—when extrapolated, on the basis of DNA content, to the entire genome—implies a detectable intrachromosomal aberration frequency of ∼62%. Thus, more than half of all the cells contain detectable intrachromosomal aberrations. Such large numbers of intrachromosomal aberrations have been predicted on theoretical grounds (Sachs et al. 1997) and have been observed in vitro for unstable (nontransmissible) aberrations (Bauchinger and Schmid 1998), but, until recently, practical probes to detect stable intrachromosomal aberrations were not available.
Specificity of Intrachromosomal Aberrations
We observed a highly significant excess (P<10-4) of intrachromosomal aberrations in the individuals who were highly exposed to plutonium (55/1,486 cells) compared with the highly-exposed reactor workers who were not exposed to plutonium (1/1,478 cells) (fig. 4). Thus, these measurements reveal an unequivocal biomarker of densely ionizing radiation exposure in a human population exposed many years earlier.
Potential Effect of Systemic Exposure to Plutonium
Unlike the gamma-ray exposure, which terminated at the end of each individual’s work period, a fraction of the plutonium exposure occurred subsequently, because of long-term retention of a fraction of the plutonium intake. For the plutonium workers studied here (see the “Subjects, Material, and Methods” section), an average of 50% of the bone marrow plutonium dose was deposited after 1983, 25% was deposited after 1993, and 8% was deposited after 1998. It is therefore important to consider whether the excess of intrachromosomal aberrations in the plutonium workers could be related to the fact that they have been exposed more recently than have most of the reactor workers; this appears unlikely, because of the following considerations:
The observed yield of unstable (nontransmissible) dicentric aberrations in the plutonium workers, which is ∼9% of the stable translocation yield (table 2). These dicentrics are initially produced with about the same frequency as stable translocations (Straume and Lucas 1993), but, because of the relatively rapid disappearance (Ramalho et al. 1995; International Atomic Energy Agency 2001), these dicentrics must have been produced within the past 3 years (i.e., by ∼8% of the total plutonium dose). Thus, it is reasonable to conclude that ∼9% of the measured stable aberration yield in the plutonium workers was formed within the past 3 years by ∼8% of the total plutonium dose, with the remainder of the stable aberrations being produced earlier. Consequently, it is unlikely that the higher yield of stable intrachromosomal aberrations observed in the plutonium workers could be simply a result of their having been produced much more recently than those in the reactor workers.
Potential Use of Intrachromosomal Aberrations as a Biodosimeter of Past Exposure
There is evidence that the frequency of intrachromosomal aberrations is indeed correlated with the plutonium dose to the bone marrow; the results are shown in table 3, and the Kendall rank sum test indicates a highly significant correlation (P=.002). This suggests that intrachromosomal aberrations have the potential to be used for quantitative dose reconstruction of densely ionizing radiation exposure, such as domestic exposure to radon (Alavanja 2002) or exposure of flight personnel to neutrons (Blettner et al. 1998); in each of these two situations, estimation of low-dose epidemiological risk is strongly hindered by current limitations in estimating past radiation exposure on an individual basis (Lubin et al. 1995; Blettner et al. 1998).
There is also good evidence from results in the control group (fig. 4) that the background frequency of intrachromosomal aberrations in an unexposed population is very low, significantly lower than the interchromosomal aberration background (P=.04). This is not unexpected, because the background aberrations are unlikely to be the result of densely ionizing radiation and because other mutagens and endogenous processes would be expected primarily to produce interchromosomal aberrations (Brenner and Sachs 1994). The background aberration rate is an important issue for reconstruction of low doses of radiation, in that it determines the minimum radiation dose that can be reconstructed from measured aberration data (Tucker 2001).
Summary
Intrachromosomal aberrations represent a sensitive, long-lived, quantitative, low-background biomarker of densely ionizing radiation exposure in human populations exposed many years earlier. Even many years after occupational exposure, more than half the blood cells of the healthy plutonium workers contain large (>6 Mb) intrachromosomal rearrangements; blood cells of the various control groups contained very few such intrachromosomal aberrations. Quantification of this large-scale chromosomal damage in human populations exposed many years earlier should lead to new insights into the mechanisms and risks of cytogenetic damage.
Acknowledgments
The authors thank Drs. Rainer Sachs, Nadia Okladnikova, Carl Elliston, Ruth Neta, and Barrett Fountos for useful advice. This work was supported by U.S. Department of Energy grant DE-FC03-01EH01002 and U.S. National Institutes of Health grants RR-11623, ES-07361, and CA-49062.
References
- Alavanja MC (2002) Biologic damage resulting from exposure to tobacco smoke and from radon: implication for preventive interventions. Oncogene 21:7365–7375 [DOI] [PubMed] [Google Scholar]
- Anspaugh LR, Degteva MO, Vasilenko EK (2002) Mayak Production Association: introduction. Radiat Environ Biophys 41:19–22 [DOI] [PubMed] [Google Scholar]
- Bauchinger M, Schmid E (1998) LET dependence of yield ratios of radiation-induced intra- and interchromosomal aberrations in human lymphocytes. Int J Radiat Biol 74:17–25. [DOI] [PubMed] [Google Scholar]
- Blettner M, Grosche B, Zeeb H (1998) Occupational cancer risk in pilots and flight attendants: current epidemiological knowledge. Radiat Environ Biophys 37:75–80 [DOI] [PubMed] [Google Scholar]
- Boei JJ, Vermeulen S, Moser J, Mullenders LH, Natarajan AT (2002) Intrachanges as part of complex chromosome-type exchange aberrations. Mutat Res 504:47–55 [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Sachs RK (1994) Chromosomal “fingerprints” of prior exposure to densely ionizing radiation. Radiat Res 140:134–142 [PubMed] [Google Scholar]
- Burak LE, Kodama Y, Nakano M, Ohtaki K, Itoh M, Okladnikova ND, Vasilenko EK, Cologne JB, Nakamura N (2001) FISH examination of lymphocytes from Mayak workers for assessment of translocation induction rate under chronic radiation exposures. Int J Radiat Biol 77:901–908 [DOI] [PubMed] [Google Scholar]
- Chudoba I, Plesch A, Lorch T, Lemke J, Claussen U, Senger G (1999) High resolution multicolor-banding: a new technique for refined FISH analysis of human chromosomes. Cytogenet Cell Genet 84:156–160 [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2:292–301 [DOI] [PubMed] [Google Scholar]
- Durante M, Manti L (2002) Estimates of radiological risk from a terrorist attack using plutonium. Radiat Environ Biophys 41:125–130 [DOI] [PubMed] [Google Scholar]
- Gilbert ES, Koshurnikova NA, Sokolnikov M, Khokhryakov VF, Miller S, Preston DL, Romanov SA, Shilnikova NS, Suslova KG, Vostrotin VV (2000) Liver cancers in Mayak workers. Radiat Res 154:246–252 [DOI] [PubMed] [Google Scholar]
- Greulich KM, Kreja L, Heinze B, Rhein AP, Weier H-UG, Brückner M, Fuchs P, Molls M (2000) Rapid detection of radiation-induced chromosomal aberrations in lymphocytes and hematopoietic progenitor cells by mFISH. Mutat Res 452:73–81 [DOI] [PubMed] [Google Scholar]
- International Atomic Energy Agency (2001) Cytogenetic analysis for radiation dose assessment: a manual. Technical report 405. International Atomic Energy Agency, Vienna [Google Scholar]
- Karhu R, Ahlstedt-Soini M, Bittner M, Meltzer P, Trent JM, Isola JJ (2001) Chromosome arm–specific multicolor FISH. Genes Chromosomes Cancer 30:105–109 [DOI] [PubMed] [Google Scholar]
- Khokhryakov VF, Kudravtseva TI, Chernikov VI, Suslova KG, Orlova IA, Filipy RE (1998a) A scintillation method for determination of actinide alpha-activity in samples. J Radioanal Nucl Chem 234:293–295 [Google Scholar]
- Khokhryakov VF, Menshikh ZS, Suslova KG, Kudryavtseva TI, Tokarskaya ZB, Romanov SA (1994) Plutonium excretion model for the healthy man. Radiat Protec Dosim 53:235–239 [Google Scholar]
- Khokhryakov V, Suslova K, Aladova E, Vasilenko E, Miller SC, Slaughter DM, Krahenbuhl MP (2000) Development of an improved dosimetry system for the workers at the Mayak Production Association. Health Phys 79:72–76 [DOI] [PubMed] [Google Scholar]
- Khokhryakov VF, Suslova KG, Tseveloyova IA, Aladova EE, Filipy RE (1998b) Classification of alpha-active workplace aerosols based on coefficient of transportability as measured by the dialysis method. J Radioanal Nucl Chem 234:209–212 [Google Scholar]
- Khokhryakov VF, Suslova KG, Vostrotin VV, Romanov SA, Menshikh ZS, Kudryavtseva TI, Filipy RE, Miller SC, Krahenbuhl MP (2002) The development of the plutonium lung clearance model for exposure estimation of the Mayak Production Association, nuclear plant workers. Health Phys 82:425–431 [DOI] [PubMed] [Google Scholar]
- Koshurnikova NA, Gilbert ES, Sokolnikov M, Khokhryakov VF, Miller S, Preston DL, Romanov SA, Shilnikova NS, Suslova KG, Vostrotin VV (2000) Bone cancers in Mayak workers. Radiat Res 154:237–245 [DOI] [PubMed] [Google Scholar]
- Krahenbuhl MP, Slaughter DM, Wilde JL, Bess JD, Miller SC, Khokhryakov VF, Suslova KG, Vostrotin VV, Romanov SA, Menshikh ZS, Kudryavtseva TI (2002) The historical and current application of the FIB-1 model to assess organ dose from plutonium intakes in Mayak workers. Health Phys 82:445–454 [DOI] [PubMed] [Google Scholar]
- Kreisheimer M, Koshurnikova NA, Nekolla E, Khokhryakov VF, Romanow SA, Sokolnikov ME, Shilnikova NS, Okatenko PV, Kellerer AM (2000) Lung cancer mortality among male nuclear workers of the Mayak facilities in the former Soviet Union. Radiat Res 154:3–11 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Boice JD Jr, Samet JM (1995) Errors in exposure assessment, statistical power and the interpretation of residential radon studies. Radiat Res 144:329–341 [PubMed] [Google Scholar]
- Prise KM, Pinto M, Newman HC, Michael BD (2001) A review of studies of ionizing radiation-induced double-strand break clustering. Radiat Res 156:572–576 [DOI] [PubMed] [Google Scholar]
- Ramalho AT, Curado MP, Natarajan AT (1995) Lifespan of human lymphocytes estimated during a six year cytogenetic follow-up of individuals accidentally exposed in the 1987 radiological accident in Brazil. Mutat Res 331:47–54 [DOI] [PubMed] [Google Scholar]
- Romanov SA, Vasilenko EK, Khokhryakov VF, Jacob P (2002) Studies on the Mayak nuclear workers: dosimetry. Radiat Environ Biophys 41:23–28 [DOI] [PubMed] [Google Scholar]
- Romanyukha AA, Ignatiev EA, Vasilenko EK, Drozhko EG, Wieser A, Jacob P, Keirim-Markus IB, Kleschenko ED, Nakamura N, Miyazawa C (2000) EPR dose reconstruction for Russian nuclear workers. Health Phys 78:15–20 [DOI] [PubMed] [Google Scholar]
- Sachs RK, Brenner DJ, Chen AM, Hahnfeldt P, Hlatky LR (1997) Intra-arm and interarm chromosome intrachanges: tools for probing the geometry and dynamics of chromatin. Radiat Res 148:330–340 [PubMed] [Google Scholar]
- Sankaranarayanan K (1973) Recent advances in the evaluation of genetic risks in man from exposure to ionizing radiation. Curr Top Radiat Res Q 9:72–79 [PubMed] [Google Scholar]
- Savage JRK (1998) A brief survey of aberration origin theories. Mutat Res 404:139–147 [DOI] [PubMed] [Google Scholar]
- Speicher MR, Ballard SG, Ward DC (1996) Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nat Genet 12:368–375 [DOI] [PubMed] [Google Scholar]
- Straume T, Lucas JN (1993) A comparison of the yields of translocations and dicentrics measured using fluorescence in situ hybridization. Int J Radiat Biol 64:185–187 [DOI] [PubMed] [Google Scholar]
- Suslova KG, Khokhryakov VF, Tokarskaya ZB, Nifatov AP, Krahenbuhl MP, Miller SC (2002) Extrapulmonary organ distribution of plutonium in healthy workers exposed by chronic inhalation at the Mayak Production Association. Health Phys 82:432–444 [DOI] [PubMed] [Google Scholar]
- Tucker JD (2001) Fish cytogenetics and the future of radiation biodosimetry. Radiat Prot Dosimetry 97:55–60 [DOI] [PubMed] [Google Scholar]
- Tucker JD, Lee DA, Ramsey MJ, Briner J, Olsen L, Moore DH 2nd (1994) On the frequency of chromosome exchanges in a control population measured by chromosome painting. Mutat Res 313:193–202 [DOI] [PubMed] [Google Scholar]
- UNSCEAR (2000) Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2000 report to the General Assembly, with scientific annexes. United Nations, New York [Google Scholar]
- Vasilenko EK, Smetanin MY, Knyazev VA, Miller S, Slaughter M, Jacob P, David J, Fehrenbacher G (2000) Approach to retrospective reconstruction of the photon exposure spectra distribution at technological sites of Mayak PA. Radiat Safety Prob 3:42–50 [Google Scholar]
- Wolber G, Guibbaud Y, Dollo R (1996) Neutron dosimetry in French nuclear power plants: problems and their solutions in 1995. Bull Cancer Radiother Suppl 83:19S–26S [DOI] [PubMed] [Google Scholar]