To the Editor:
“Parental imprinting” refers to an epigenetic marking of genes that results in monoallelic expression. This phenomenon plays a critical role in embryogenesis and development. The epigenetic modification of the genome involves methylation changes and the remodeling of chromatin-associated proteins (Li 2002). Imprints are established during the development of germ cells, and the reprogramming of imprinting occurs within the first days after fertilization (Reik and Walter 2001). The alteration of normal imprinting patterns is implicated in a number of human genetic diseases. Among them, the Beckwith-Wiedemann syndrome (BWS [MIM 130650]) is an overgrowth syndrome secondary to the dysregulation of the imprinted 11p15 region (Maher and Reik 2000). Numerous mechanisms are involved in BWS, and ∼70% of cases of BWS are related to epigenetic abnormalities at the 11p15 locus, mostly demethylation of the KvDMR1 region of the KCNQ1OT (previously called “LIT1”) gene (MIM 604115) (Engel et al. 2000; Bliek et al. 2001; Gaston et al. 2001; Weksberg et al. 2001; DeBaun et al. 2002). KCNQ1OT encodes a noncoding antisense transcript within intron 10 of the KCNQ1 gene (MIM 192500) (Lee et al. 1999; Mitsuya et al. 1999; Smilinich et al. 1999) and might be involved in the regulation of parental imprinting of the centromeric domain of the 11p15 region (Fitzpatrick et al. 2002).
In sheep and cattle, epigenetic abnormalities have been shown to be involved in large offspring syndrome (LOS) (Young et al. 1998). Affected animals exhibit various phenotypes, including large size at birth. In both species, the syndrome is caused by the in vitro exposure of embryos, between fertilization and the blastocyst stage, to various unusual environments. LOS is related to the loss of imprinting of the IGF2 receptor gene (MIM 147280), which ensures internalization and degradation of IGF2 and displays an antiproliferative function (Young et al. 2001). In vitro preimplantation procedures in mice are also responsible for overgrowth, owing to the abnormal expression of various imprinted genes, particularly the genes located at distal chromosome 7 (h19 [MIM 103280] and igf2 [MIM 147470] genes), orthologous to the human 11p15 region (Humpherys et al. 2001; Rideout et al. 2001). In humans, a case of BWS was recently described after in vitro fertilization (IVF) (Olivennes et al. 2001). Moreover, two recent papers (DeBaun et al. 2003; Maher et al. 2003) described an increase in prevalence of assisted reproductive technologies (ART) in patients with BWS. De Baun et al. (2003) reported a sixfold increase (4.6% vs. 0.76% in the general population) and showed that four of the six patients for whom DNA was available exhibited an isolated demethylation of KvDMR1 in the KCNQ1OT gene. Maher et al. (2003) reported a threefold increase (4% vs. 0.997% in the general population) and demonstrated that two of the six patients on whom molecular analysis could be done also exhibited an isolated demethylation of KvDMR1.
Our department is a reference center in France for molecular diagnosis of BWS, and patients are referred from various medical departments (neonatology, pediatrics, genetics, and fetopathology). We studied a series of 149 patients referred for overgrowth syndromes and diagnosed as BWS, since all of them exhibited genetic or epigenetic defects at the 11p15 locus. According to the inclusion criteria described elsewhere (Gaston et al. 2001), 102 patients exhibited a complete form of BWS, and 47 exhibited an incomplete form of BWS. The techniques used to analyze the 11p15 region have been described elsewhere (Gaston et al. 2000, 2001). Epigenetic changes concerned 104 (70%) patients, most of whom (n=90) exhibited a loss of KvDMR1 methylation. Fourteen patients (9.4%) exhibited isolated hypermethylation of the H19 gene. Forty-two patients exhibited a genetic defect: 11p15 uniparental disomy (n=35; 23.5%) and germline CDKN1C (MIM 600856) mutation (n=7; 4.7%). Three patients (2%) had a chromosomal abnormality.
Six of the 149 patients were born following ART. Of note, these six patients exhibited the same epigenetic abnormality (isolated demethylation of KvDMR1 with a demethylation index varying 72%–100%) (fig. 1). All of them were sporadic cases, and one was a DZ twin. The clinical features of these patients and the procedures of ART used for their conception are summarized in table 1. As shown in table 1, the phenotypes of patients born after ART were not different from phenotypes of the other patients (n=84) with isolated demethylation of KVDMR1, with the exception that only one patient born after ART exhibited ear abnormalities. These children were issued from various ART procedures: classical IVF, intracytoplasmic sperm injection (ICSI), embryo freezing, and transfer on day 2, day 3, and day 5. More recent procedures, like ICSI (two of six patients) or blastocyst transfer (one of six patients), did not prevail over other techniques. The representation of ART (4%) in our series is three times higher than that in the general population (1.3%), according to the national report of the French Ministry of Health (9,930 of 770,000 live births resulting in 1,999 from IVF, ICSI, or frozen embryo transfer). On the basis of this report, we would have expected 1.94 of the 149 patients with BWS to be born as a result of ART. To test the significance of this difference of frequencies, we used the Fisher’s exact test (P=.01) as well as the Poisson approximation (two-tailed P=.018; 95% CI 1.5–8.7). Strength of the association between exposure to ART and risk of BWS is expressed by an odds ratio of 3.2 (95% CI 1.4–7.3). This rate is the same as that described by Maher et al. (2003) but lower than that described by DeBaun et al. (2003), which addressed a prospective study. In our series and in Maher’s series, this rate is probably underestimated, as specific questions regarding ART have only been asked systematically in the past year.
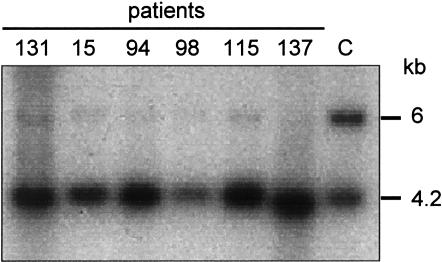
Figure 1.
Methylation analysis of KvDMR1 in liver tissue (patient 131) and leukocytes (patients 15, 94, 98, 115, and 137) from the six patients with BWS born after ART and in leukocytes from a normal control (C). Genomic DNA was digested with BamHI and the methylation-sensitive enzyme NotI. Digested samples were subjected to electrophoresis in a 0.7% agarose gel, blotted onto Hybond XL membranes, and hybridized with the HLHAY79 KvDMR1 probe corresponding to EST 68627 (ATCC; Manassas). The upper band (6 kb) is methylated and corresponds to the maternal allele. The lower band (4.2 kb) is unmethylated and corresponds to the paternal allele.
Table 1.
Clinical Characteristics of the Six Patients with BWS Born Following ART
|
Characteristics of Patient |
||||||||
| 15 | 94 | 98 | 115 | 131 | 137 | Characteristics of Other Patients with Demethylation of KvDMR1 (n=84) | Pa | |
| ART procedure: | ||||||||
| Sperm | Ejaculated | Ejaculated | Ejaculated | Ejaculated | Ejaculated | Ejaculated | ||
| ICSI | Yes | No | No | No | No | Yes | ||
| Frozen embryo | No | No | No | Yes | No | No | ||
| Day of transfer | Day 2 | Day 3 | Day 2 | Day 2 | Day 2 | Day 5b | ||
| Phenotype: | ||||||||
| Sex | F | F | M | F | M | F | 42F/42M | |
| Delivery (weeks) | 40 | 33.5 | 38.5 | 37 | 20c | 32/DZ twind | ||
| Macrosomia | Yes | Yes | Yes | Yes | Yes | No | 72.3% | NSe |
| Birth weight (g)/Birth length (cm) | 4090/51.5 | 2770/48.5 | 4460/53.5 | 4400/55 | ?/480 | 1765/43 | ||
| Macroglossia | Yes | Yes | Yes | Yes | Yes | Yes | 96.4% | NS |
| Organomegaly | No | No | No | Liver | Pancreas | No | 48.7% | NS |
| Abdominal wall | No | Exomphalos | No | Exomphalos | No | Exomphalos | 72.3%f | NS |
| Hemihyperplasia | No | No | No | No | No | No | 26.9% | NS |
| Ear abnormalities | No | No | Yes | No | No | No | 68.9% | P=.02 |
| Hypoglycemia | Yes | No | No | Yes | … | No | 45.6% | NS |
| Facial naevus | Yes | No | No | Yes | No | Yes | 54.5% | NS |
| Other | Macrocephalia, cystic fibrosis | Developmental delay, pyelic dilatation | Inguinal hernia | Adrenal cytomegaly, placental chorioangioma | ||||
χ2 test.
Transfer of three embryos, two at the morula stage and one at the blastocyst stage.
Spontaneous abortion.
DNA from the normal twin was not available.
NS = not significant.
43.4% exomphalos, 24.1% umbilical hernia, 4.8% diastasis recti.
Although the analysis of the imprinting status at chromosome 15q11-13 in children born after ICSI did not reveal an imprinting defect (Manning et al. 2000), two recent papers reported three patients with Angelman syndrome (MIM 105830) born after ICSI (Cox et al. 2002; Ørstavik et al. 2002). All three patients exhibited an imprinting defect, which is a rare cause of Angelman syndrome.
As in the previous two reports (DeBaun et al. 2003; Maher et al. 2003), our data suggest that ART may favor imprinting alterations at the centromeric imprinted 11p15 locus and, consequently, the incidence of BWS. These data highlight the need to carefully follow up children born after ART to test for BWS and other diseases related to imprinted regions. Although no specific procedures of ART appear to be associated with a risk of BWS in our series, these data lend support to the importance of precisely recording these different procedures of ART, particularly the stimulation protocol, the biological technique, the stage of maturation of the gametes, the culture media used at each step, and the timing of embryo transfer.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for BWS, KCNQ1OT, KCNQ1, IGF2 receptor, H19, IGF2, CDKN1C, and Angelman syndrome)
References
- Bliek J, Maas S, Ruijter J, Hennekam R, Alders M, Westerveld A, Mannens M (2001) Increased tumor risk for BWS patients correlates with aberrant H19 and not KCNQ1OT1 hypomethylation in familial cases of BWS. Hum Mol Genet 10:467–476 [DOI] [PubMed] [Google Scholar]
- Cox GF, Bürger J, Lip V, Mau UA, Sperling K, Wu B-L, Horsthemke B (2002) Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet 71:162–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, Feinberg AP (2003) Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 72: 156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, Feinberg AP (2002) Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet 70:604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Smallwood A, Harper A, Higgins M, Oshimura M, Reik W, Schofield P, Maher E (2000) Epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome. J Med Genet 37:921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick GV, Soloway PD, Higgins MJ (2002) Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet 32:426–431 [DOI] [PubMed] [Google Scholar]
- Gaston V, Le Bouc Y, Soupre V, Burglen L, Donadieu J, Oro H, Audry G, Vazquez MP, Gicquel C (2001) Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome. Eur J Hum Genet 9:409–418 [DOI] [PubMed] [Google Scholar]
- Gaston V, Le Bouc Y, Soupre V, Vazquez MP, Gicquel C (2000) Assessment of p57(KIP2) gene mutation in Beckwith-Wiedemann syndrome. Horm Res 54:1–5 [DOI] [PubMed] [Google Scholar]
- Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM 3rd, Biniszkiewicz D, Yanagimachi R, Jaenisch R (2001) Epigenetic instability in ES cells and cloned mice. Science 293:95–97 [DOI] [PubMed] [Google Scholar]
- Lee M, Debaun M, Mitsuya K, Galonek H, Branderburg S, Oshimura M, Feinberg A (1999) Loss of imprinting of a paternally expressed transcript, with antisense orientation to KvLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc Natl Acad Sci USA 96:5203–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673 [DOI] [PubMed] [Google Scholar]
- Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, Macdonald F, Sampson JR, Barratt CL, Reik W, Hawkins MM (2003) Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet 40:62–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Reik W (2000) Beckwith-Wiedemann syndrome: imprinting in clusters revisited. J Clin Invest 105:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Lissens W, Bonduelle M, Camus M, De Rijcke M, Liebaers I, Van Steirteghem A (2000) Study of DNA-methylation patterns at chromosome 15q11-q13 in children born after ICSI reveals no imprinting defects. Mol Hum Reprod 6:1049–1053 [DOI] [PubMed] [Google Scholar]
- Mitsuya K, Meguro M, Lee M, Katoh M, Schulz T, Kugoh H, Yoshida M, Niikawa N, Feinberg A, Oshimura M (1999) LIT1, an imprinted antisense RNA in the human KvLQT1 locus identified by screening for differentially expressed transcripts using monochromosomal hybrids. Hum Mol Genet 8:1209–1217 [DOI] [PubMed] [Google Scholar]
- Olivennes F, Mannaerts B, Struijs M, Bonduelle M, Devroey P (2001) Perinatal outcome of pregnancy after GnRH antagonist (ganirelix) treatment during ovarian stimulation for conventional IVF or ICSI: a preliminary report. Hum Reprod 16:1588–1591 [DOI] [PubMed] [Google Scholar]
- Ørstavik KH, Eiklid K, van der Hagen CB, Spetalen S, Kierulf K, Skjeldal O, Buiting K (2003) Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic sperm injection. Am J Hum Genet 72:218–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Walter J (2001) Genomic imprinting: parental influence on the genome. Nat Rev Genet 2:21–32 [DOI] [PubMed] [Google Scholar]
- Rideout WM 3rd, Eggan K, Jaenisch R (2001) Nuclear cloning and epigenetic reprogramming of the genome. Science 293:1093–1098 [DOI] [PubMed] [Google Scholar]
- Smilinich NJ, Day CD, Fitzpatrick GV, Caldwell GM, Lossie AC, Cooper PR, Smallwood AC, Joyce JA, Schofield PN, Reik W, Nicholls RD, Weksberg R, Driscoll DJ, Maher ER, Shows TB, Higgins MJ (1999) A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc Natl Acad Sci USA 96:8064–8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R, Nishikawa J, Caluseriu O, Fei YL, Shuman C, Wei C, Steele L, Cameron J, Smith A, Ambus I, Li M, Ray PN, Sadowski P, Squire J (2001) Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum Mol Genet 10:2989–3000 [DOI] [PubMed] [Google Scholar]
- Young L, Fernandes K, McEvoy T, Butterwith S, Gutierrez C, Carolan C, Broadbent P, Robinson J, Wilmut I, Sinclair K (2001) Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet 27:153–154 [DOI] [PubMed] [Google Scholar]
- Young LE, Sinclair KD, Wilmut I (1998) Large offspring syndrome in cattle and sheep. Rev Reprod 3:155–163 [DOI] [PubMed] [Google Scholar]