Abstract
Hereditary lymphedema is a developmental disorder characterized by chronic swelling of the extremities due to dysfunction of the lymphatic vessels. Two responsible genes have been identified: the vascular endothelial growth factor receptor 3 (VEGFR3) gene, implicated in congenital lymphedema, or Milroy disease, and the forkhead-related transcription factor gene FOXC2, causing lymphedema-distichiasis. We describe three families with an unusual association of hypotrichosis, lymphedema, and telangiectasia. Using microsatellite analysis, we first excluded both VEGFR3 and FOXC2 as causative genes; we then considered the murine ragged phenotype, caused by mutations in the Sox18 transcription factor, as a likely counterpart to the human disease, because it presents a combination of hair and cardiovascular anomalies, including symptoms of lymphatic dysfunction. Two of the families were consanguineous; in affected members of these families, we identified homozygous missense mutations in the SOX18 gene, located in 20q13. The two amino acid substitutions, W95R and A104P, affect conserved residues in the first α helix of the DNA-binding domain of the transcription factor. In the third family, the parents were nonconsanguineous, and both the affected child and his brother, who died in utero with hydrops fetalis, showed a heterozygous nonsense mutation that truncates the SOX18 protein in its transactivation domain; this substitution was not found in genomic DNA from either parent and hence constitutes a de novo germline mutation. Thus, we show that SOX18 mutations in humans cause both recessive and dominant hypotrichosis-lymphedema-telangiectasia, suggesting that, in addition to its established role in hair and blood vessel development, the SOX18 transcription factor plays a role in the development and/or maintenance of lymphatic vessels.
Introduction
Lymphedema, a chronic swelling of the extremities that is due to impaired lymphatic drainage, causes cosmetic harm, disability, and predisposition to infection and chronic ulceration. Primary lymphedema can be noninherited or hereditary, and, recently, progress has been made in understanding the molecular bases of hereditary forms. Congenital hereditary lymphedema, or Milroy disease (MIM 153100), has been found to be caused by missense inactivating mutations in the kinase domain of vascular endothelial growth factor receptor 3 (VEGFR3) (Irrthum et al. 2000; Karkkainen et al. 2000), and lymphedema associated with distichiasis (MIM 153400), an additional row of eyelashes originating from the meibomian glands, has been shown to be caused by truncating mutations in the forkhead-related transcription factor FOXC2 (Fang et al. 2000; Finegold et al. 2001). Lymphedema is also observed in a few genetic syndromes—such as lymphedema-cholestasis (MIM 214900), or Aagenaes syndrome, mapped to chromosome 15q (Bull et al. 2000), and lymphedema-microcephaly-chorioretinopathy (MIM 152950) (Feingold and Bartoshesky 1992). Finally, nuchal lymphedema and hydrops fetalis are frequently observed in patients with Noonan syndrome (MIM 163950) (Witt et al. 1987) and in patients with a variety of chromosomal abnormalities, including Turner syndrome (Chitayat et al. 1989; Boucher et al. 2001).
The interest in the identification of genes involved in hereditary lymphedema extends beyond the mere understanding of this disorder. Conditions associated with lymphedema, such as Noonan syndrome, may have higher incidences of lymphoproliferative disorders (Choong et al. 1999). In addition, lymphangiogenesis, the development of lymphatic vessels, has recently been linked to tumor growth and metastasis, and lymphedema genes are prime candidates as regulators of this process. In particular, the VEGFR3 gene, implicated in hereditary congenital lymphedema, has attracted much attention as a promising anticancer-drug target (Plate 2001). Overexpression of the VEGFR3 natural ligands VEGFC and VEGFD has been shown to lead to an increase in tumor growth and metastasis via the lymphatic system (Skobe et al. 2001; Stacker et al. 2001), whereas a block of VEGFR3 signaling has been shown to counteract these effects in the mouse (He et al. 2002). Furthermore, the identification of genes causing hereditary lymphedema could lead to therapeutic advances in the management of secondary lymphedema, such as lymphedema resulting from anticancer surgery or parasitic disease.
We describe three families with an unusual hereditary condition in which hypotrichosis, lymphedema, and telangiectasia are associated. After excluding VEGFR3 and FOXC2 as potential disease-causing genes, we identified the murine ragged phenotype as a putative counterpart of the human disorder. We found that SOX18, the human orthologue of the ragged gene, is mutated in these three families and accounts for both a recessive form and a dominant form of the disease.
Subjects and Methods
Subjects
Clinical findings in all patients are summarized in table 1. Written informed consent was obtained from all participants, and the present study was approved by the ethical committee of the medical faculty at the Université catholique de Louvain, Brussels, Belgium.
Table 1.
Summary of Clinical Findings in Patients with SOX18 Mutations
|
Family I |
Family III |
||||
| Boy | Girl | Family IIGirl | Boy | Fetus | |
| Hypotrichosis: | |||||
| Scalp | Very sparse | Very sparse | Sparse | Very sparse | … |
| Eyebrows | Absent | Absent | Absent | Absent | … |
| Eyelashes | Absent | Absent | Absent | Absent | … |
| Onset | Age 0–6 mo | Age 0–2 years | Birth | Age 6 mo | … |
| Lymphedema: | |||||
| Localization | Legs | Legs | Legs | Upper eyelid | Generalized |
| Onset | Age 15 years | Puberty | Age 4 years | Birth | Prenatal |
| Telangiectasia | Not observed | Palm of right hand | Palms and soles | Scalp, scrotum, and legs | … |
| Other findings | Thin and transparent skin on hands and feet, bilateral hydrocele | Thin and transparent skin on hands and feet | Cutis marmorata–like lividity of the skin, small dark-red papular lesions on several toes | Mild eczema on cheeks, scrotal edema, large hydroceles | Nonimmune hydrops fetalis |
| Mode of inheritance | Recessive | Recessive | Recessive | Dominant | Dominant |
| SOX18 mutation | G455C→A104P (homozygous) | G455C→A104P (homozygous) | T428A→W95R (homozygous) | C865A→C240X (heterozygous) | C865A→C240X (heterozygous) |
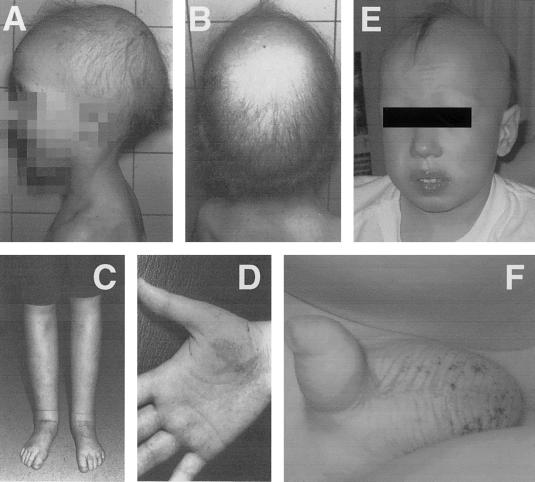
Family I.—The index patient is a boy, the first child born to unaffected parents who are first cousins of Belgian descent (Devriendt et al. 2002). He was born at 32 wk gestation, with birth weight of 1.650 kg (25th–50th percentile), and presented with respiratory distress. A normal amount of scalp hair was present at birth but decreased progressively to the extent of total alopecia at age 6 mo. Some hair growth was noted later (at age 3.5 years), but it remained very sparse (figs. 1A and 1B), with absent eyebrows and eyelashes. Sweating, nails, and teeth were normal. The skin over the hands and feet was thin and transparent, with visible blood vessels. A skin biopsy revealed several abortive hair follicles, some atrophic sebaceous glands, and normal sweat glands. A bilateral hydrocele was surgically corrected at age 12 years. At age ∼15 years, the patient progressively developed lymphedema of the lower limbs. A Doppler ultrasonography of the venous system of the lower limbs done at age 25 years was normal. A lymphatic scintigraphy was performed by interdigital subcutaneous injection of a radio-labeled (Technetium 99m) colloidal tracer into the dorsal part of the foot. No tracer was detected in the groin during a monitoring period of 32.5 min, indicating that there was no detectable lymphatic flow.
Figure 1.
Physical features observed in patients. A and B, Male patient from family I at age 3.5 years. Note the sparse scalp hair and absence of eyebrows. C, Lymphedema of the legs in the female patient from family I, at age 26 years. D, Telangiectasias on the palm in the same patient (also at age 26 years). E, Male patient from family III. Note the sparse scalp hair and absence of eyebrows. F, Scrotal telangiectasias in the same patient.
His younger sister presented a similar phenotype. She was born at 36 wk gestation with birth weight 2.7 kg (50th percentile). Initially, she had normal black hair. A vascular nevus was present on the palm of the right hand and faded during childhood. During infancy, her hair diminished progressively, and, around age 2 years, her hair was very sparse and has remained so ever since. She had neither eyebrows nor eyelashes, and she did not develop axillary or pubic hair at puberty. Around puberty, she developed progressive lymphedema of the lower limbs (fig. 1C). Doppler study of the venous system of the legs was normal. Clinical examination at age 26 years showed, besides the hair abnormalities, normal teeth and nails. Sweating was normal, but her skin was thin. The skin over her hands and feet was transparent, and dilated veins and varicosities were apparent on the palm of her right hand (fig. 1D). The pedigree of family I is shown in figures 2A and 3B.
Figure 2.
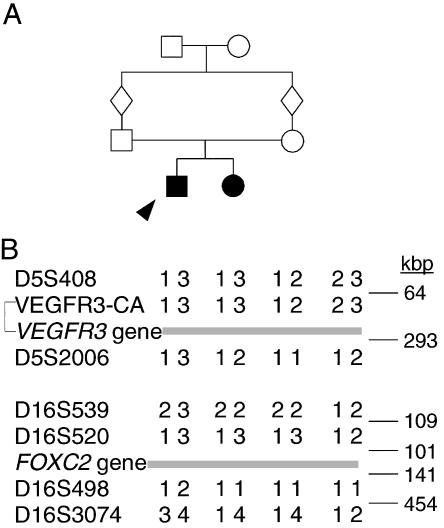
Exclusion of VEGFR3 and FOXC2 as the gene for hypotrichosis-lymphedema-telangiectasia in family I. A, Pedigree of the family. The two affected individuals are represented by blackened symbols, and the index patient is indicated by an arrowhead. Note the consanguinity in the family. B, Microsatellite markers surrounding VEGFR3 and FOXC2. Intermarker physical distances (in kbp), marked on the right, are based on the Human Genome Project sequence draft assembly (UCSC Genome Bioinformatics), as of July 2002.
Figure 3.
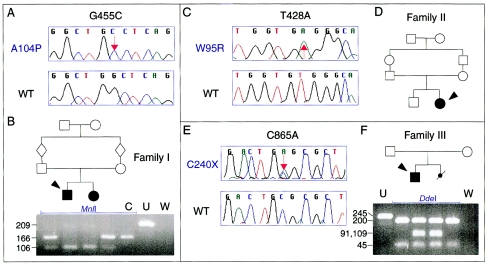
Families with hypotrichosis-lymphedema-telangiectasia and mutations in SOX18. A, C, and E, Electropherograms from affected members of families I, II, and III and unrelated controls, demonstrating mutations G455C, T428A, and C865A (in the protein, A104P, W95R, and C240X, respectively). WT = wild type. B, D, and F, Family pedigrees (with the individual for whom sequence is shown indicated by an arrowhead) and MnlI and DdeI restriction-fragment analysis (for families I and III). C = digested control; U = undigested PCR product; W = water control.
Family II.—The index patient has been described in detail elsewhere (Glade et al. 2001). In summary, she is a 12-year-old child of unaffected, first-cousin, Turkish parents. Her younger brother is unaffected. Lymphedema appeared in the lower limbs at age 4 years. Scalp hair has always been sparse, but with a normal appearance. Eyebrows and eyelashes were missing. Palms and soles showed multiple telangiectasias, with ectatic capillaries and cutis marmorata–like lividity of the skin. Small dark-red papular vascular lesions were seen on several toes. Her nails and teeth were normal. The pedigree of family II is shown in figure 3D. DNA from the parents was not available.
Family III.—The index patient is a boy, born in 1997, with sparse hair. He presented swelling of the upper eyelids, scrotal edema, and very large bilateral hydroceles. Hair loss began at age ∼6 mo, accompanied by a lightening of its color. Now, at age ∼6 years, alopecia is almost complete (fig. 1E), including eyebrows and eyelashes. The patient presented mild eczema on the cheeks and telangiectasias on the scalp, scrotum (fig. 1F), and legs. His nails and teeth were normal.
The brother of the index patient died in utero at 30 wk gestation. The fetus had nonimmune hydrops fetalis, with chylous effusions in the pleural and peritoneal cavities. The lungs presented generalized vascular congestion and a mild dilatation of lymphatic vessels. Parental physical examination showed no abnormalities. The pedigree of family III is shown in figure 3F.
Microsatellite Analysis
Peripheral blood was obtained from participating family members. For the deceased fetus of family III, a tissue sample was used. DNA was extracted using standard laboratory procedures. Microsatellite markers around the VEGFR3, FOXC2, and SOX18 genes were identified on the basis of Entrez Map View (National Center for Biotechnology Information), build 28, and the Unified Database (UDB) (Weizmann Institute of Science [WIS] Bioinformatics and Biological Computing), both as of January 2002. In addition, we used an intronic CA-repeat microsatellite (VEGFR3-CA in fig. 2B) in the VEGFR3 gene (Iljin et al. 2001). Genotyping, using 32P-labeled oligonucleotides, was performed as described elsewhere (Boon et al. 1999). To rule out nonpaternity in family III, in which a de novo mutation was observed, we genotyped the four members of the family for 10 Weber set 8 microsatellites from various chromosomes.
Database Search for Candidate Genes for Hypotrichosis-Lymphedema-Telangiectasia
We searched for potential murine models for the disease in the Jackson Laboratory Mouse Genome Informatics databases, using various available query forms and the keywords “lymphedema,” “lymphatic,” “chylous,” “chylothorax,” “edema,” “edematous,” and “swelling.” Mouse phenotypes retrieved were evaluated for their resemblance to the human condition.
SOX18 Gene Sequencing and Restriction-Enzyme Analyses of Mutations
SOX18 gene structure was obtained from the University of California–Santa Cruz (UCSC) Genome Bioinformatics Web site (Human Genome Project Working Draft, December 2001 freeze). A 1,550-bp PCR fragment containing the full SOX18 coding sequence and the 196-bp intervening intron was amplified using genomic DNA from patients and control individuals, was purified using the Qiagen PCR purification columns (Qiagen), and was sequenced on a CEQ2000 fluorescent capillary sequencer (Beckman Coulter). Sequences were further aligned and analyzed with Sequencher 3.1 (Gene Codes). The identified nucleotide substitutions G455C, T428A, and C865A created additional recognition sites for restriction enzymes MnlI, HphI, and DdeI, respectively. The presence of these RFLPs was tested in the members of the relevant family, as well as in 96 control individuals from the genetically heterogeneous Belgian population. Oligonucleotide sequences and reaction conditions for PCR amplification, sequencing, and restriction analysis are available from the authors on request.
Results
Exclusion of VEGFR3 and FOXC2—and Database Identification of SOX18
In families I and II, the parents were first cousins and presented no signs of either lymphatic or hair anomalies, despite the marked phenotype of their children. This strongly suggested an autosomal recessive mode of inheritance. To assess the possibility that the lymphedema-causing genes VEGFR3 and FOXC2 are involved in the syndrome, we determined the genotypes of the parents of family I and their children for microsatellite markers closely surrounding these genes (fig. 2B). The affected siblings showed heterozygosity for markers at both loci, excluding these genes as causative for the disease under a recessive inheritance model.
A search in mouse databases identified the ragged phenotype, caused by mutations in the Sox18 gene, as a putative murine counterpart of human hypotrichosis-lymphedema-telangiectasia. The order of markers around human SOX18 was inconsistent between integrated (UDB [WIS Bioinformatics and Biological Computing]) and sequence-based (UCSC Genome Bioinformatics) maps of the human genome, both as of January 2002, and genotyping revealed an intermingling of uninformative, recombinant, and potentially linked markers (data not shown). This prompted us to analyze SOX18 by direct sequencing in the patients with hypotrichosis-lymphedema-telangiectasia.
Homozygous SOX18 Mutations in Patients from Families I and II
Sequencing of the SOX18 gene in family I, presenting consanguinity, revealed a G455C transversion in the coding sequence (GenBank accession number NM_018419) (fig. 3A). This nucleotide substitution caused the replacement of alanine at position 104 in the SOX18 protein (GenBank accession number NP_060889) by a proline (A104P). The mutation was present at homozygous state in the two affected children and at heterozygous state in the unaffected parents (fig. 3B), in accordance with a recessive inheritance pattern in a consanguineous family.
In consanguineous family II, we identified a T428A transversion in the coding sequence, resulting in the replacement of tryptophan at position 95 in the protein by an arginine (W95R). This mutation was present at homozygous state in the affected patient (fig. 3C).
Neither mutation was observed in the genomic DNA from 96 control individuals (data not shown).
Heterozygous SOX18 Mutation in Patients from Family III
In family III, we identified a C865A transversion in the coding sequence (fig. 3E), transforming the codon for cysteine at position 240 of the protein (TGC) into a premature stop codon (TGA). The mutation was detected at heterozygous state in DNA extracted from the blood of the patient and the tissue of the deceased fetus and was not present in genomic DNA from the parents (fig. 3F). This mutation, which truncates the transcription factor in its transactivating domain, was not observed in genomic DNA from 96 control individuals. The genotypes of the four members of the family, for 10 microsatellite markers, were compatible with paternity (data not shown).
Discussion
To identify the gene causing hypotrichosis-lymphedema-telangiectasia in three families, we first investigated the possible involvement of VEGFR3 and FOXC2, the two genes associated with lymphedema in humans (Fang et al. 2000; Irrthum et al. 2000; Karkkainen et al. 2000; Finegold et al. 2001). FOXC2, in particular, could be considered to be an interesting candidate, because it causes lymphedema, with variable age at onset, associated with distichiasis (the presence of an additional row of eyelashes). A hair abnormality was also seen in the three families described here, and lymphedema was not always present at birth. However, we were able to exclude the two genes in one of the consanguineous families by using microsatellite analysis.
We then searched for an animal model of the disease in public databases. The chy mouse has recently been identified as an animal model for congenital hereditary lymphedema. As do human patients, the chy mouse has an inactivating mutation in the VEGFR3 tyrosine kinase domain and exhibits leg edema due to hypoplastic lymphatic vessels (Karkkainen et al. 2001). An anomaly frequently observed in the chy mouse is the occurrence of chylous ascites, which may be caused by dysfunction of intestinal lymphatic vessels. Similarly, the murine ragged phenotype, caused by a mutation in the gene encoding the transcription factor Sox18, presents chylous ascites, in addition to sparse fur (Pennisi et al. 2000b). Thus, we considered SOX18 to be a remarkable candidate for the human hypotrichosis-lymphedema-telangiectasia syndrome.
In adults, the SOX18 gene is expressed in a broad range of tissues, including brain, heart, skeletal muscle, spleen, kidney, liver, and lung (Pennisi et al. 2000c; Hosking et al. 2001). It belongs to the SOX (Sry-type high-mobility group [HMG] box) gene family, encoding transcription factors required in diverse developmental processes, such as lens formation, sex and neural determination, spermatogenesis, chondrogenesis, and cardiac development (Wegner 1999). These transcription factors are highly similar to each other in the HMG domain, a 79-amino-acid DNA-binding motif that specifically binds to the heptameric sequence (A/T)(A/T)CAA(A/T)G (Wegner 1999). Three members of the SOX family are already associated with a human disease. Mutations in SRY cause sex reversal and gonadal dysgenesis (Berta et al. 1990; Jager et al. 1990), mutations in SOX9 cause campomelic dysplasia with autosomal sex reversal (Foster et al. 1994; Wagner et al. 1994), and mutations in SOX10 cause Waardenburg-Hirschsprung syndrome (Pingault et al. 1998).
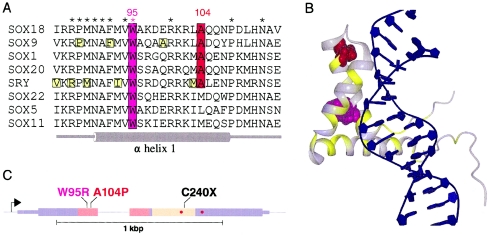
We identified recessive SOX18 missense mutations in affected individuals from two consanguineous families (I and II) with hypotrichosis-lymphedema-telangiectasia. The first recessive mutation, A104P, affects an alanine that is conserved in most members of the SOX family (fig. 4A). Because SOX18 and SRY are homologous and present high sequence similarity in their respective HMG domains, we used the nuclear-magnetic-resonance model of SRY (Werner et al. 1995) to visualize the positions of the two recessive mutations (fig. 4B). The alanine at position 104 (shown in red in the model) is located in the first α helix of the DNA-binding domain, and its replacement by a proline, an amino acid that produces significant bends in α helices, is very likely to affect the structure of this domain. The second recessive mutation, W95R, substitutes a tryptophan residue (shown in magenta in fig. 4) that is conserved in all SOX proteins. The three-dimensional structure of SRY reveals that this tryptophan belongs to a cluster of conserved aromatic and aliphatic residues that maintain orientation of the three α helices of the DNA-binding domain through their packing (Werner et al. 1995), and the mutation thus probably destabilizes the domain.
Figure 4.
Positions of SOX18 mutations in patients with hypotrichosis-lymphedema-telangiectasia. A, Multiple alignment of protein sequences around mutated residues W95 and A104, including representative members of the different SOX protein subfamilies. * = Amino acids conserved in all 20 known SOX proteins. B, Superimposition of SOX18-mutated amino acids over the nuclear-magnetic-resonance model of the homologous HMG domain of SRY (Protein Data Bank PDB ID 1J46), with W95 shown in magenta and A104 shown in red. The structure was drawn with DINO. Positions of disease-causing amino acid substitutions in SOX9, SOX10, and SRY (obtained from Swiss-Prot [entries P48436, P56693, and Q05066, respectively]) are shown in yellow in panels A and B. C, Schematic representation of SOX18, showing positions of the three mutations. Thin boxes represent 5′ and 3′ UTRs, and thick boxes represent translated sequences. Regions corresponding to the DNA-binding domain and the transactivation domain are shown in pink and beige, respectively. Positions corresponding to murine ragged mutations (single-nucleotide deletions) are indicated by red circles.
The C240X SOX18 mutation in family III, located in the vicinity of the murine ragged frameshift mutations (fig. 4C), causes a most-likely-dominant form of the syndrome, since the patient and the fetus are heterozygous for the substitution and since sequencing of the complete coding region and splice sites did not reveal additional genetic alterations. Moreover, the nucleotide substitution was not found in DNA extracted from the blood of the parents, and nonpaternity was ruled out, implying that this mutation arose de novo. This is similar to the situation for the other SOX genes associated with human disorders (i.e., SRY, SOX9, and SOX10), in which usually-de-novo mutations are observed at the heterozygous state (Berta et al. 1990; Jager et al. 1990; Foster et al. 1994; Wagner et al. 1994; Pingault et al. 1998). Like the autosomal SOX9 and SOX10 genes, which presumably exert their effect through haploinsufficiency (Foster et al. 1994; Wagner et al. 1994; Pingault et al. 1998), the C240X mutation may result in a null allele. In this case, the proteins with the recessive mutations should retain some activity. However, a dominant-negative effect cannot be ruled out, because the resulting protein is truncated in its transactivation domain and, if still present, may bind the promoters of target genes via its intact DNA-binding domain.
The first component of the syndrome, early-onset hypotrichosis with absence of eyebrows and eyelashes, is present in all patients with SOX18 mutations. During murine embryonic development, Sox18 is transiently expressed in the mesenchyme underlying the vibrissae and pelage hair follicles, and its role in hair development has been demonstrated by its implication in the murine ragged phenotype (Pennisi et al. 2000b). Its two closest paralogues, Sox7 and Sox17, have been postulated to carry overlapping functions (Pennisi et al. 2000a). Human SOX7 and Xenopus Sox17 physically interact with β-catenin and interfere with Wnt/β-catenin signaling (Zorn et al. 1999; Takash et al. 2001), a pathway essential for hair morphogenesis (Gat et al. 1998). β-Catenin controls fate decision of the skin stem cells toward epidermal or follicular keratinocyte differentiation (Huelsken et al. 2001), and the observed hypotrichosis could be due to abnormal interference with this process by Sox18. Intriguingly, mice with conditional inactivation of β-catenin after hair-follicle formation show complete loss of pelage after the first hair cycle, a phenotype reminiscent of the human patients with SOX18 mutations.
The second component of the syndrome, lymphedema, presented differently in the three families. The age at onset of the lower-limb lymphedema was highly variable, ranging from 4 to 15 years. The affected boy from family III currently has no lower-limb lymphedema, but he presented with edema of the upper eyelids. A more severe failure of the lymphatic system presented in his late brother, who had nonimmune hydrops fetalis. This severe presentation was also observed in two patients in a family with hereditary lymphedema-distichiasis caused by a mutation in the FOXC2 gene (Fang et al. 2000). The frequent involvement of the lymphatic system among patients implies SOX18 in development and/or maintenance of lymphatic vessels. Because SOX proteins constitute a family of ⩾20 members in humans (Schepers et al. 2002), have overlapping expression patterns, and bind to the same DNA heptamer motif, they are supposed to achieve specificity through combinatorial associations with other factors (Wilson and Koopman 2002). Interaction with, for example, FOXC2 or the lymphatic vessel regulator PROX1 (Wigle and Oliver 1999) might enable SOX18 to specifically activate lymphatic-related target genes. Such candidate targets are the genes encoding vascular endothelial growth factors VEGFC and VEGFD, as well as their receptor, VEGFR3. Indeed, direct regulation of the VEGFC and VEGFD genes might be possible, because they contain the heptameric binding site for SOX proteins in their promoters, as revealed by examination of current sequence databases (data not shown).
The third component of the syndrome is an anomaly of peripheral blood vessels, manifesting as telangiectasia in three of the four patients. Involvement of blood vessels is not surprising, since Sox18 mRNA is transiently detected in endothelial cells during mouse embryonic development and since the Sox18-mutated ragged mouse exhibits conspicuous vascular anomalies, such as hematomas and dilation or rupture of peripheral blood vessels (Pennisi et al. 2000b); however, no signs of comparable vascular abnormalities were observed in the patients. The telangiectasias, as well as the other observed anomalies—such as thinness and transparency of the skin, hydrocele, and small cutaneous papular vascular lesions—were observed only in some patients. The phenotypic heterogeneity is reminiscent of that seen in lymphedema-distichiasis (Finegold et al. 2001) and could be explained by the presence of modifier genes. In fact, the influence of modifier genes has been demonstrated in the Sox18-deficient ragged mouse, in which the accumulation of chyle in the peritoneum depends on the genetic background (Wallace 1979). Thus, SOX18 mutations are expected to present as isolated alopecia in some patients—especially young children, before the onset of lymphedema—and as nonimmune hydrops fetalis of unknown etiology in others.
Acknowledgments
We thank the family members for their invaluable collaboration. Studies were supported by grants from the Fonds National de Recherche Scientifique (F.N.R.S.) and the Belgian Federal Service for Scientific, Technical, and Cultural Affairs. M.V. is a Chercheur qualifié du F.N.R.S. K.D. is Senior Clinical Investigator of the Fund for Scientific Research–Flanders (F.W.O.-Vlaanderen), Belgium, and is supported by a grant from the Belgian Foundation for Research in Paediatric Cardiology. M.A.M.V.S. is supported by ZON-MW grant 920-083, grant 02/94 from the Stichting Drie Lichten, and a grant from Multigen BV, The Netherlands, and by a fellowship from the Berliner Stiftung für Dermatologie. A.I. was supported by a fellowship from the Fonds pour la formation à la recherche dans l’industrie et dans l’agriculture (F.R.I.A.) and a fellowship from the Fonds du Patrimoine de la Faculté de Médecine of Université catholique de Louvain. We thank Ms. Ana Gutierrez for her excellent technical assistance.
Note added in proof.—
While the present article was in press, the finding of SOX2 mutations in patients with anophthalmia was reported by Fantes et al. (2003).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- DINO, http://cobra.mih.unibas.ch/dino/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for SOX18 coding sequence [accession number NM_018419] and SOX18 protein [NP_060889])
- Mouse Genome Informatics, http://www.informatics.jax.org/
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/ (for Entrez Map View)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Milroy disease, lymphedema-distichiasis, lymphedema-cholestasis, lymphedema-microcephaly-chorioretinopathy, and Noonan syndrome)
- Protein Data Bank, The, http://www.rcsb.org/pdb/ (for SRY HMG domain [PDB ID 1J46])
- Swiss-Prot, http://us.expasy.org/sprot/ (for amino acid substitutions in SOX9, SOX10, and SRY [entries P48436, P56693, and Q05066, respectively])
- UCSC Genome Bioinformatics, http://genome.ucsc.edu/ (for Human Genome Project Working Draft)
- WIS Bioinformatics and Biological Computing, http://bioinformatics.weizmann.ac.il/index.html (for UDB)
References
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M (1990) Genetic evidence equating SRY and the testis-determining factor. Nature 348:448–450 [DOI] [PubMed] [Google Scholar]
- Boon LM, Brouillard P, Irrthum A, Karttunen L, Warman ML, Rudolph R, Mulliken JB, Olsen BR, Vikkula M (1999) A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21–22. Am J Hum Genet 65:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher CA, Sargent CA, Ogata T, Affara NA (2001) Breakpoint analysis of Turner patients with partial Xp deletions: implications for the lymphoedema gene location. J Med Genet 38:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull LN, Roche E, Song EJ, Pedersen J, Knisely AS, van Der Hagen CB, Eiklid K, Aagenaes Ø, Freimer NB (2000) Mapping of the locus for cholestasis-lymphedema syndrome (Aagenaes syndrome) to a 6.6-cM interval on chromosome 15q. Am J Hum Genet 67:994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitayat D, Kalousek DK, Bamforth JS (1989) Lymphatic abnormalities in fetuses with posterior cervical cystic hygroma. Am J Med Genet 33:352–356 [DOI] [PubMed] [Google Scholar]
- Choong K, Freedman MH, Chitayat D, Kelly EN, Taylor G, Zipursky A (1999) Juvenile myelomonocytic leukemia and Noonan syndrome. J Pediatr Hematol Oncol 21:523–527 [PubMed] [Google Scholar]
- Devriendt K, Vikkula M, Irrthum A, Matthijs G, Mertens A, Fryns JP (2002) Autosomal recessive alopecia and lymphedema. Genet Couns 13:74–75 [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW (2000) Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet 67:1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, Van Heyningen V, FitzPatrick DR (2003) Mutations in SOX2 cause anophthalmia. Nat Genet 33:461–463 [DOI] [PubMed] [Google Scholar]
- Feingold M, Bartoshesky L (1992) Microcephaly, lymphedema, and chorioretinal dysplasia: a distinct syndrome? Am J Med Genet 43:1030–1031 [DOI] [PubMed] [Google Scholar]
- Finegold DN, Kimak MA, Lawrence EC, Levinson KL, Cherniske EM, Pober BR, Dunlap JW, Ferrell RE (2001) Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum Mol Genet 10:1185–1189 [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525–530 [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95:605–614 [DOI] [PubMed] [Google Scholar]
- Glade C, van Steensel MA, Steijlen PM (2001) Hypotrichosis, lymphedema of the legs and acral telangiectasias—new syndrome? Eur J Dermatol 11:515–517 [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K (2002) Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 94:819–825 [DOI] [PubMed] [Google Scholar]
- Hosking BM, Wyeth JR, Pennisi DJ, Wang SC, Koopman P, Muscat GE (2001) Cloning and functional analysis of the Sry-related HMG box gene, Sox18. Gene 262:239–247 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (2001) β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545 [DOI] [PubMed] [Google Scholar]
- Iljin K, Karkkainen MJ, Lawrence EC, Kimak MA, Uutela M, Taipale J, Pajusola K, Alhonen L, Halmekytö M, Finegold DN, Ferrell RE, Alitalo K (2001) VEGFR3 gene structure, regulatory region, and sequence polymorphisms. FASEB J 15:1028–1036 [DOI] [PubMed] [Google Scholar]
- Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M (2000) Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet 67:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager RJ, Anvret M, Hall K, Scherer G (1990) A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature 348:452–454 [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN (2000) Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet 25:153–159 [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla-Herttuala S, Finegold DN, Ferrell RE, Alitalo K (2001) A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA 98:12677–12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, Bowles J, Nagy A, Muscat G, Koopman P (2000a) Mice null for Sox18 are viable and display a mild coat defect. Mol Cell Biol 20:9331–9336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, Abbott C, Koopman P (2000b) Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet 24:434–437 [DOI] [PubMed] [Google Scholar]
- Pennisi D, James KM, Hosking B, Muscat GE, Koopman P (2000c) Structure, mapping, and expression of human SOX18. Mamm Genome 11:1147–1149 [DOI] [PubMed] [Google Scholar]
- Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M (1998) SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet 18:171–173 [DOI] [PubMed] [Google Scholar]
- Plate K (2001) From angiogenesis to lymphangiogenesis. Nat Med 7:151–152 [DOI] [PubMed] [Google Scholar]
- Schepers GE, Teasdale RD, Koopman P (2002) Twenty pairs of Sox: extent, homology, and nomenclature of the mouse and human Sox transcription factor gene families. Dev Cell 3:167–170 [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7:192–198 [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 7:186–191 [DOI] [PubMed] [Google Scholar]
- Takash W, Cañizares J, Bonneaud N, Poulat F, Mattéi MG, Jay P, Berta P (2001) SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res 29:4274–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120 [DOI] [PubMed] [Google Scholar]
- Wallace ME (1979) Analysis of genetic control of chylous ascites in ragged mice. Heredity 43:9–18 [DOI] [PubMed] [Google Scholar]
- Wegner M (1999) From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27:1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner MH, Huth JR, Gronenborn AM, Clore GM (1995) Molecular basis of human 46X,Y sex reversal revealed from the three-dimensional solution structure of the human SRY-DNA complex. Cell 81:705–714 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98:769–778 [DOI] [PubMed] [Google Scholar]
- Wilson M, Koopman P (2002) Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Curr Opin Genet Dev 12:441–446 [DOI] [PubMed] [Google Scholar]
- Witt DR, Hoyme HE, Zonana J, Manchester DK, Fryns JP, Stevenson JG, Curry CJ, Hall JG (1987) Lymphedema in Noonan syndrome: clues to pathogenesis and prenatal diagnosis and review of the literature. Am J Med Genet 27:841–856 [DOI] [PubMed] [Google Scholar]
- Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE (1999) Regulation of Wnt signaling by Sox proteins: XSox17 α/β and XSox3 physically interact with β-catenin. Mol Cell 4:487–498 [DOI] [PubMed] [Google Scholar]