Abstract
The transcription factor p53 plays a key role in the cellular defense against cancer development. It is inactivated in virtually every tumor, and in every second tumor this inactivation is due to a mutation in the TP53 gene. In this perspective, we show that this diverse mutational spectrum is unique among all other cancer-associated proteins and discuss what drives the selection of TP53 mutations in cancer. We highlight that several factors conspire to make the p53 protein particularly vulnerable to inactivation by the mutations that constantly plague our genome. It appears that the TP53 gene has emerged as a victim of its own evolutionary past that shaped its structure and function towards a pluripotent tumor suppressor, but came with an increased structural fragility of its DNA-binding domain. TP53 loss of function - with associated dominant-negative effects - is the main mechanism that will impair TP53 tumor suppressive function, regardless of whether a neomorphic phenotype is associated with some of these variants.
Subject terms: Gene regulation, Protein folding, Tumour-suppressor proteins
Introduction—Mutational patterns in cancer-associated proteins
Established more than 30 years ago, the concept that tumor development relies on the activation of oncogenes and the loss of function of tumor suppressor genes is still prevailing, but this binary view needs revising as genes associated with DNA replication and repair or immune escape were shown to be important players as well [1–5].
Alterations in oncogenes associated with hyperactivity (hypermorphic variants) or with a gain of function (neomorphic variants) are usually single nucleotide substitutions (SNS) creating missense variants in the functional domain of the protein. These oncogenic mutations, therefore, have a skewed distribution along the protein sequence and cluster at very specific positions. The limited number of hotspot variants in oncogenes such as KRAS (codons 12 and 13), the phosphatidylinositol 3-kinase catalytic subunit, PIK3CA (codons 542 or 545), BRAF (codon 600), EGFR (codon 858), isocitrate dehydrogenase 1, IDH1 (codon 132) or Kit (codon 816) are perfect examples of this restriction [6]. This observation is also supported by multiple saturation mutagenesis analysis showing that cancer-associated variants in oncogenes are restricted to a few residues [7].
In contrast, loss-of-function alterations in tumor suppressor genes (amorphous or hypomorphic variants) are predominantly events that will prevent protein expression such as indels, splicing or nonsense mutations that can be distributed across the protein sequence. This is reflected by the great diversity of some of those events (indels of different sizes) and their large distribution along the protein for most tumor suppressor genes. Fortunately, indels occur far less frequently than missense mutations in both normal or tumoral genomes by about one order of magnitude (Supplementary Fig. 1) [8, 9]. Furthermore, as both alleles of these genes need to be inactivated, this lowers the probability for the selection of a pathogenic loss of function.
A single gene, the so-called TP53 tumor suppressor gene, does not fit into this picture as the vast majority of mutational events are missense mutations spread across more than half of the protein. Missense mutations predominate in the central DNA-binding domain (DBD), whereas frameshift variants are more frequent in the N- and C-terminal regions of the protein (Fig. 1A, B) [10–12]. It is generally assumed that this very specific mutational landscape in TP53 is linked to a specific selection of variants with a potential gain of novel antimorphic or neomorphic functions [10, 13]. Without excluding the possibility that the high expression of heterogenous missense p53 variants has a clinical relevance in human tumors, we show below that this mutational landscape is predominantly shaped by the extreme fragility of the p53 protein, as demonstrated by systematic functional analyses and structural studies.
Fig. 1. Analysis of somatic TP53 variants in tumor tissues according to their frequency in the UMD_TP53 database (2024 release, 248,363 patients).
A Somatic missense variants are localized predominantly in the DNA-binding domain (central panel) of the multidomain p53 protein and have a much lower frequency in the flanking N- and C-terminal regions (left and right panel, respectively). B Frequent cancer-associated somatic variants are found predominantly in the DNA-binding domain, whereas low frequency variants are more predominant in the N- and C-terminal regions and correspond to indels. TD = tetramerization domain (C) TP53 loss of activity for missense variants localized in the DNA-binding domain of the p53 protein. p53 activities ranging from 0 (inactive) to 1 (full activity) were taken from the work of Kotler et al. [39]. Red circles: variants frequently found in human cancer (at least 75 cases in the database); green circles: ultra rare variants (1 or 2 cases) or variants that have not been reported in cancer yet. For each residue, the average activity of all frequent or rare variants is given. Violin plots illustrate the distribution of TP53 activity for the two classes of TP53 variants. The structure of the DNA-binding domain is shown on the right as a cartoon representation (PDB entry 2XWR) [77].
Early discoveries hinting at p53’s structural fragility and its implications
The first hint that p53 mutants sustain a change of conformation originated from the observation that they could be recognized by the Hsc70/Hsp70 chaperone [14, 15]. In addition, some p53 monoclonal antibodies were shown to react with alternative conformational forms of p53 [16]. Generation and epitope mapping of novel monoclonal antibodies revealed that this conformational flexibility affects the central region of the protein, which was not yet defined as a DBD at the time [17, 18]. Functional analysis of the major TP53 hotspot variants revealed that TP53 variants with mutations at codon 175, such as R175H or R175P, were associated with an unfolded conformation and chaperone binding, whereas variations at codon 248 or 273 (e.g., R248L or R273H) did not induce any change [19]. The elucidation of the crystal structure of the central region of p53 and the identification of this domain as a specific DBD then provided an explanation for the above findings and led to this simple binary classification of common cancer mutations as contact mutations that disrupt amino acids directly contacting DNA (at codons 273 and 248) and structural mutations that destabilize the structure/conformation of the DBD (at codons 175, 249, or 282) [20–22]. As it later turned out, the R175H variant actually belongs to a special subgroup of structural mutations that directly impair zinc binding, which is key for the overall thermodynamic and kinetic stability of the DBD and the structural integrity of the DNA-binding surface [23–25]. Further evidence for the intrinsic instability of p53 came from the discovery of thermosensitive variants in both mouse and human cells, a very rare observation for a mammalian gene [26, 27]. These variants, such as the A138V and V143A mutants initially studied (human numbering), have a wild-type-like conformation and activity at permissive temperature (32 °C), whereas the protein unfolds when shifted to non-permissive temperature (37 °C), leading to a loss of function and an association with the chaperone Hsc70. Since then, many more temperature-sensitive p53 cancer mutants have been identified and characterized [28–31]. A similar behavior upon temperature shift was also found for the p53 homolog from the cold-blooded clawed frog Xenopus laevis, although in that case for the wild-type protein. When expressed at 37 °C, a temperature above the physiological body temperature of the animal, the X. laevis p53 DBD adopts a mutant-like conformation, binds to chaperone Hsc70, and the full-length protein loses all its growth suppressive activity, whereas it behaves normally at 32 °C or below [32, 33]. A similar observation has been reported for Drosophila p53 [34].
The above observations on the p53 folding state in cells are consistent with structural and biophysical studies on the p53 protein that described its DBD as intrinsically unstable [35]. This domain is therefore very sensitive to local perturbation induced by any mutation, leading in many cases to global unfolding at body temperature, which is accompanied by the loss of its DNA-binding ability [23]. In other words, its low intrinsic stability makes p53 susceptible to inactivation by destabilizing mutations that would result in a benign phenotype in the context of a more stable structural scaffold [36].
Insights from large-scale functional analyses of TP53 variants
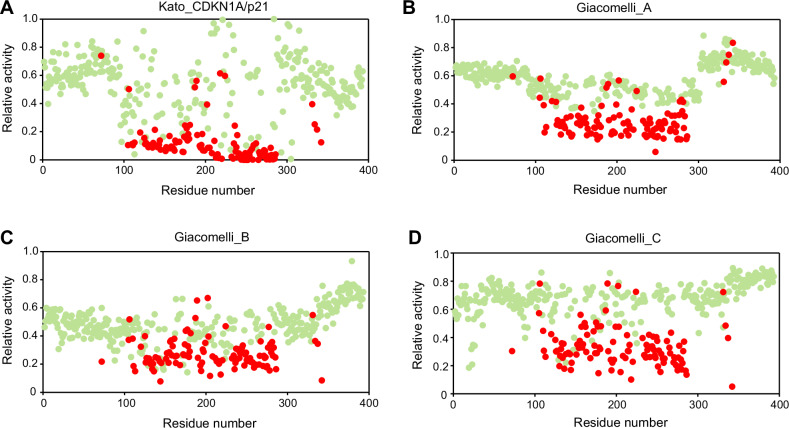
In a landmark paper published by the group of C. Ishioka in 2003, the authors performed a saturation mutagenesis of every TP53 position and demonstrated that most missense mutations in the central DBD of TP53 lead to a loss of the transactivation activity of the protein [37]. There is a strong correlation between the mutations found in human tumors and inactive variants from this artificial library [38]. Recent studies have extended these observations with the analysis of TP53 saturation mutagenesis screens in mammalian cells, using either cell death or growth arrest as a readout of TP53 activity [39–41]. One of the great strengths of saturation mutagenesis of TP53 is the unbiased generation of variants, irrespective of whether they were selected in human cancer. These three studies highlighted two important features: First, in stark contrast to other tumor suppressor genes, more than half of all possible TP53 missense variants in the DBD lead to a loss of function that for most missense and indel variants is indistinguishable. This alleviates the need to select for less frequent indel mutations, which is supported by the observation that the frequency of frameshift TP53 mutations in human cancer (10–15%) is similar to the frequency observed in the human genome (Supplementary Fig. S1). Second, whatever assays have been used to record TP53 loss of function, missense variants that are not found in human cancer usually displayed a significant residual activity (Figs. 1C and 2). Outliers, i.e., missense mutations that are recurrently found in cancer but failed to show evidence for loss of function, often induce splice alterations as revealed by RNA analysis of tumor tissues or knock-in screens [41, 42]. Together, the strong correlation of loss of function and occurrence in cancer emphasizes the need for a loss of function to generate variants selected during neoplasia transformation.
Fig. 2. Distribution of loss-of-activity missense variants in the p53 protein.
p53 activities ranging from 0 (inactive) to 1 (full activity) were taken from the work of Kato et al. (CDKN1A/p21 promoter) [37] (A) or from the three different readouts described by Giacomelli et al. [40] (B–D). Mutation frequencies in tumors (somatic count) are taken from the upcoming release of the UMD_TP53 database (2024 release, 248,363 patients). Red circles: variants frequently found in human cancer (more than 75 cases in the database); green circles: ultra rare variants (only 1 or 2 cases in the database) or variants that have never been reported in cancer. For each residue, the average activity of all frequent or rare variants is given. The outlier at codon 72 is due to the R72P polymorphism.
Several dominant-negative effect mechanisms exacerbate the effects of loss-of-function mutations
Compared with other tumor suppressors, such as Retinoblastoma protein or APC, p53 is at a further disadvantage when it comes to inactivation by mutation: it forms tetramers [43, 44]. As shown initially for the test case of the DNA-contact mutant R273H and later by saturating mutagenesis, missense mutants of the p53 DBD, but not outside of it, exert a dominant-negative effect over the wild-type protein through the formation of mixed tetramers with weakened DNA-binding [45, 46], which drastically reduces the amount of fully active wild-type homotetramers in heterozygous cells to a level that may not be sufficient for efficient tumor suppression.
The cell’s misery is compounded by the fact that p53 exposes a series of aggregation-prone sequence motifs upon unfolding. As if opening Pandora’s box, unfolding of thermolabile mutants unleashes a wave of destruction, resulting in coaggregation not only with the wild-type protein in heterozygous cells but also with other cellular proteins, including the paralogs p63 and p73 [47]. DBD-mediated coaggregation with the tumor suppressor p73 effectively kills off a potential salvage pathway [33, 48–50], and binding to p73 was postulated to be one of the possible gain-of-function mechanisms of mutant p53 [51, 52], although this is in essence a dominant-negative effect. So at least some neomorphic phenotypes may be directly linked to p53-mediated aggregation, in addition to the mere loss of p53 transcriptional activity. From a molecular viewpoint, many so-called gain of function phenotypes may be alternatively viewed as the phenotypic manifestation of a loss of multiple functions rather than the creation of a mutant protein with novel functions.
Another important aspect is that there is an overabundance of p53 protein in p53-mutated tumor cells, due to perturbation of its normal proteasomal degradation pathways (usually referred to as stabilization of mutant p53 - but not to be confused with the conformational stability of the protein discussed elsewhere in this perspective) [53]. Dysregulated, highly abundant mutant p53 proteins have the potential to exert an active role in tumor cells, not only via DBD-mediated coaggregation but also via their promiscuous interaction modules in the disordered N- and C-terminal regions, and this may be one of the contributing factors why mutant p53 tumors have a poorer prognosis than wild-type tumors in certain cancers [54, 55]. However, the overabundance of mutant p53 protein in tumors is usually directly linked to secondary genetic alterations such as aneuploidy and loss of p16INK4A or Mdm2 [56–59], in addition to TP53 loss of function, and may therefore contribute to tumor progression only at later stages [41]. Moreover, p53 transcriptional activity serves as a more effective stratifier of cancer patient outcomes than mutation status [10–12], suggesting that prognosis is primarily driven by p53 loss of function.
What drives the selection of TP53 mutations in cancer?
Based on the above observations, all deleterious TP53 missense variants in the DBD would be expected with a similar prevalence in cancer patients. However, the TP53 mutation spectrum shows clear mutational hotspots, somewhat reminiscent of the mutation profile of classical oncogenes. While this might suggest that some variants display a specific oncogenic gain of function that is actively selected in cancers, there are alternative explanations. First, the mutational hotspots are characterized by DNA sequences with elevated intrinsic susceptibility to the most prevailing mutagenic mechanisms that drive genomic instability in cancer cells [40]. For example, the six most frequent missense variants occur at methylated CpG sites that are particularly prone to aging-related mutagenic processes, and the R249S hotspot mutation, which is prevalent in hepatocellular carcinoma in sub-Saharan Africa and Southeast Asia, has been directly linked to exposure to the crop contaminant aflatoxin B1 [60]. Importantly, the combination of functional data obtained by saturating mutagenesis with mutational probabilities derived from the most common mutagenic processes in cancer provided a near-perfect model of the observed spectrum of TP53 missense variants, without the need to postulate additional oncogenic gain-of-function effects [40]. Second, unlike most indel mutations, missense mutations produce mutant peptides that can be recognized by the immune system as neoantigens [61]. These neoantigens have the therapeutic potential to be utilized in directing immune responses against the tumor [62]. Interestingly, the most prevalent hotspot mutants seem to be less immunogenic than non-hotspot mutants [63]. Insufficient clearance of poorly antigenic variants by the immune system may therefore further contribute to their prevalence in cancer samples.
Therefore, in contrast to oncogenes that have a limited number of residues that can lead to a gain of function and tumor suppressor genes that need more drastic modifications such as indel events to display a loss of function, 55% of potential missense variants that can occur in the DBD of the p53 protein have been found in human cancer (at least 10x) and display a loss of activity, either complete or partial. Prevalence differences between individual missense variants are explained well by mutational probabilities and immunogenic properties. TP53 loss of function (and associated dominant-negative effects) remains the main mechanism that will impair TP53 tumor suppressive function, regardless of whether a neomorphic phenotype is associated with some of these variants. In support of this, a recent study by Wang et al. demonstrated that removal of mutant p53 genes that had been associated with oncogenic gain-of-function properties by CRISPR/Cas9 had no effect on cancer cell growth in vitro or in vivo [64, 65].
It is important to consider that the frequency of TP53 mutations differs widely between different tumor types [11, 12, 66] and that the inherent genetic background of the individual could also play a role [67, 68]. For instance, TP53 mutations are almost universal in small-cell lung cancer, which originates from rare neuroendocrine lung cells, but are much less frequent in lung adenocarcinomas, which arise from alveolar type II or club cells. Additionally, mutant p53 can enhance the activity of co-mutated proto-oncogenes. For example, by inducing the splicing regulator hnRNPK, mutant p53 alters the splicing of mRNAs encoding GTPase-activating proteins, resulting in heightened KRAS signaling [69]. Conversely, oncogenic KRAS signaling increases CREB1 phosphorylation, enabling its binding to mutant p53. Together, they hyperactivate the expression of the pro-metastatic transcription factor FOXA1 [70]. Thus, the selection of TP53 mutations in cancer is strongly influenced by cell- or tissue-specific factors and the presence of cooperating oncogenes.
TP53: a victim of its evolutionary past?
It appears that the diversity of oncogenic TP53 variants is predominantly due to the misfortune of TP53 to encode an excessively fragile and extended functional domain that can be easily targeted by the high frequency of SNS that plague normal and tumor cells. Although in vitro analyses and mouse models suggest various heterogenous antimorphic or neomorphic activities for several variants, confirmation of the clinical relevance of these findings is still pending.
Protein structures are usually remarkably robust to changes in the primary sequence, but they have evolved for optimal function in cells and not necessarily the greatest stability [71, 72]. The extreme fragility of p53 may therefore be necessary to provide the structural plasticity to adapt its conformation to sense and react to local perturbation in the cellular environment, and also allow for rapid p53 turnover [73, 74]. Intriguingly, unlike its more stable paralogs, p63 and p73, which have retained more ancestral features, the p53 protein appears to have evolved at a much faster rate, which also affected its stability [74]. In vertebrates, there is a good correlation between the thermodynamic stability of the p53 DBD and the body temperature of a particular organism or the optimal temperature of the habitat in the case of cold-blooded animals, suggesting that human p53 may have evolved to be only marginally stable and now pays the price for its evolutionary past when faced with mutations [73–76]. Unlucky, indeed.
Supplementary information
Acknowledgements
A.C.J. is funded by German Research Foundation (DFG) grant JO 1473/1–3. A.C.J. is also grateful for support by the Structural Genomics Consortium (SGC), a registered charity (No:1097737) that received funds from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], EU/EFPIA/OICR/McGill/KTH/Diamond Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA, Pfizer, and Takeda. T.St. is funded by the German Research Foundation (STI 182/15-1, GRK2573), Wilhelm Sander-Stiftung (2022.129.1), Federal Ministry of Education and Research (BMBF, German Center for Lung Research, DZL) and Hessian Ministry of Higher Education, Research, Science and the Arts (HMWK, LOEWE iCANx). T.So. is supported by the Cancéropole Île-de-France (convention no. 2019-1-EMERG-22-INSERM 6–1) and by Hadassah France.
Author contributions
A.C.J., T.St., and T.So. conceived ideas for this perspective and wrote the manuscript. T.So. performed the statistical analysis of the upcoming release of the UMD TP53 mutation database, with input from A.C.J. and T.St.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andreas C. Joerger, Email: joerger@pharmchem.uni-frankfurt.de
Thorsten Stiewe, Email: stiewe@uni-marburg.de.
Thierry Soussi, Email: thierry.soussi@igp.uu.se.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-024-01391-6.
References
- 1.Klein G. Oncogenes and tumor suppressor genes. Acta Oncol. 1988;27:427–37. [DOI] [PubMed] [Google Scholar]
- 2.Friend SH, Dryja TP, Weinberg RA. Oncogenes and tumor-suppressing genes. N. Engl J Med. 1988;318:618–22. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 6.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Jiménez F, Muiños F, Sentís I, Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, et al. A compendium of mutational cancer driver genes. Nat Rev Cancer. 2020;20:555–72. [DOI] [PubMed] [Google Scholar]
- 8.Sahakyan AB, Balasubramanian S. Single genome retrieval of context-dependent variability in mutation rates for human germline. BMC Genom. 2017;18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. 2015;22:1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, et al. Integrated analysis of TP53 gene and pathway alterations in the cancer genome Atlas. Cell Rep. 2019;28:1370–84.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Andrade KC, Lee EE, Tookmanian EM, Kesserwan CA, Manfredi JJ, Hatton JN, et al. The TP53 database: transition from the international agency for research on cancer to the US National Cancer Institute. Cell Death Differ. 2022;29:1071–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2:a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturzbecher HW, Chumakov P, Welch WJ, Jenkins JR. Mutant p53 proteins bind hsp 72/73 cellular heat shock-related proteins in SV40-transformed monkey cells. Oncogene. 1987;1:201–11. [PubMed] [Google Scholar]
- 15.Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gannon JV, Greaves R, Iggo R, Lane DP. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990;9:1595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legros Y, Lafon C, Soussi T. Linear antigenic sites defined by the B-cell response to human p53 are localized predominantly in the amino and carboxy-termini of the protein. Oncogene. 1994;9:2071–6. [PubMed] [Google Scholar]
- 18.Legros Y, Meyer A, Ory K, Soussi T. Mutations in p53 produce a common conformational effect that can be detected with a panel of monoclonal antibodies directed toward the central part of the p53 protein. Oncogene. 1994;9:3689–94. [PubMed] [Google Scholar]
- 19.Ory K, Legros Y, Auguin C, Soussi T. Analysis of the most representative tumour-derived p53 mutants reveals that changes in protein conformation are not correlated with loss of transactivation or inhibition of cell proliferation. EMBO J. 1994;13:3496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–55. [DOI] [PubMed] [Google Scholar]
- 21.Pavletich NP, Chambers KA, Pabo CO. The DNA-binding domain of p53 contains the four conserved regions and the major mutation hot spots. Genes Dev. 1993;7:2556–64. [DOI] [PubMed] [Google Scholar]
- 22.Bargonetti J, Manfredi JJ, Chen X, Marshak DR, Prives C. A proteolytic fragment from the central region of p53 has marked sequence-specific DNA-binding activity when generated from wild-type but not from oncogenic mutant p53 protein. Genes Dev. 1993;7:2565–74. [DOI] [PubMed] [Google Scholar]
- 23.Bullock AN, Henckel J, Fersht AR. Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: definition of mutant states for rescue in cancer therapy. Oncogene. 2000;19:1245–56. [DOI] [PubMed] [Google Scholar]
- 24.Blanden AR, Yu X, Blayney AJ, Demas C, Ha JH, Liu Y, et al. Zinc shapes the folding landscape of p53 and establishes a pathway for reactivating structurally diverse cancer mutants. Elife. 2020;9:e61487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joerger AC, Fersht AR. Structure-function-rescue: the diverse nature of common p53 cancer mutants. Oncogene. 2007;26:2226–42. [DOI] [PubMed] [Google Scholar]
- 26.Michalovitz D, Halevy O, Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990;62:671–80. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Guo XY, Hu GY, Liu WB, Shay JW, Deisseroth AB. A temperature-sensitive mutant of human p53. EMBO J. 1994;13:2535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dearth LR, Qian H, Wang T, Baroni TE, Zeng J, Chen SW, et al. Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis. 2007;28:289–98. [DOI] [PubMed] [Google Scholar]
- 29.Hu W, Feng Z. Hypothermia is a potential new therapy for a subset of tumors with Mutant p53. Cancer Res. 2021;81:3762–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song H, Wu J, Tang Y, Dai Y, Xiang X, Li Y, et al. Diverse rescue potencies of p53 mutations to ATO are predetermined by intrinsic mutational properties. Sci Transl Med. 2023;15:eabn9155. [DOI] [PubMed] [Google Scholar]
- 31.Balourdas DI, Markl AM, Krämer A, Settanni G, Joerger AC. Structural basis of p53 inactivation by cavity-creating cancer mutations and its implications for the development of mutant p53 reactivators. Cell Death Dis. 2024;15:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soussi T, Caron de Fromentel C, Sturzbecher HW, Ullrich S, Jenkins J, May P. Evolutionary conservation of the biochemical properties of p53: specific interaction of Xenopus laevis p53 with simian virus 40 large T antigen and mammalian heat shock proteins 70. J Virol. 1989;63:3894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bensaad K, Le Bras M, Unsal K, Strano S, Blandino G, Tominaga O, et al. Change of conformation of the DNA-binding domain of p53 is the only key element for binding of and interference with p73. J Biol Chem. 2003;278:10546–55. [DOI] [PubMed] [Google Scholar]
- 34.Waddell S, Jenkins JR, Proikas-Cezanne TA. “no-hybrids” screen for functional antagonizers of human p53 transactivator function: dominant negativity in fission yeast. Oncogene. 2001;20:6001–8. [DOI] [PubMed] [Google Scholar]
- 35.Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53. Annu Rev Biochem. 2008;77:557–82. [DOI] [PubMed] [Google Scholar]
- 36.Joerger AC, Ang HC, Fersht AR. Structural basis for understanding oncogenic p53 mutations and designing rescue drugs. Proc Natl Acad Sci USA. 2006;103:15056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA. 2003;100:8424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soussi T, Kato S, Levy PP, Ishioka C. Reassessment of the TP53 mutation database in human disease by data mining with a library of TP53 missense mutations. Hum Mutat. 2005;25:6–17. [DOI] [PubMed] [Google Scholar]
- 39.Kotler E, Shani O, Goldfeld G, Lotan-Pompan M, Tarcic O, Gershoni A, et al. A systematic p53 mutation library links differential functional impact to cancer mutation pattern and evolutionary conservation. Mol Cell. 2018;71:178–90.e8. [DOI] [PubMed] [Google Scholar]
- 40.Giacomelli AO, Yang X, Lintner RE, McFarland JM, Duby M, Kim J, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet. 2018;50:1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funk J, Klimovich M, Pavlakis E, Neumann M, Drangenstein D, Noeparast M, et al. Functional diversity of the TP53 mutome revealed by saturating CRISPR mutagenesis. bioRxiv. 2023; 10.1101/2023.03.10.531074.
- 42.Carbonnier V, Leroy B, Rosenberg S, Soussi T. Comprehensive assessment of TP53 loss of function using multiple combinatorial mutagenesis libraries. Sci Rep. 2020;10:20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joerger AC, Wilcken R, Andreeva A. Tracing the evolution of the p53 tetramerization domain. Structure. 2014;22:1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gencel-Augusto J, Lozano G. p53 tetramerization: at the center of the dominant-negative effect of mutant p53. Genes Dev. 2020;34:1128–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Natan E, Hirschberg D, Morgner N, Robinson CV, Fersht AR. Ultraslow oligomerization equilibria of p53 and its implications. Proc Natl Acad Sci USA. 2009;106:14327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boettcher S, Miller PG, Sharma R, McConkey M, Leventhal M, Krivtsov AV, et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science. 2019;365:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol. 2011;7:285–95. [DOI] [PubMed] [Google Scholar]
- 48.Kravchenko JE, Ilyinskaya GV, Komarov PG, Agapova LS, Kochetkov DV, Strom E, et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci USA. 2008;105:6302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stiewe T, Putzer BM. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nat Genet. 2000;26:464–9. [DOI] [PubMed] [Google Scholar]
- 50.Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strano S, Munarriz E, Rossi M, Cristofanelli B, Shaul Y, Castagnoli L, et al. Physical and functional interaction between p53 mutants and different isoforms of p73. J Biol Chem. 2000;275:29503–12. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26:2220–5. [DOI] [PubMed] [Google Scholar]
- 53.Sadagopan A, Garaffo N, Chang H-J, Schreiber SL, Meyerson M, Gibson WJ. p53 protein abundance is a therapeutic window across TP53 mutant cancers and is targetable with proximity inducing small molecules. bioRxiv.2024; 10.1101/2024.07.27.605429.
- 54.Robles AI, Harris CC. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb Perspect Biol. 2010;2:a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stiewe T, Haran TE. How mutations shape p53 interactions with the genome to promote tumorigenesis and drug resistance. Drug Resist Updat. 2018;38:27–43. [DOI] [PubMed] [Google Scholar]
- 56.Redman-Rivera LN, Shaver TM, Jin H, Marshall CB, Schafer JM, Sheng Q, et al. Acquisition of aneuploidy drives mutant p53-associated gain-of-function phenotypes. Nat Commun. 2021;12:5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexandrova EM, Yallowitz AR, Li D, Xu S, Schulz R, Proia DA, et al. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature. 2015;523:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lü Y, Cho T, Mukherjee S, Suarez CF, Gonzalez-Foutel NS, Malik A, et al. Genome-wide CRISPR screens identify novel regulators of wild-type and mutant p53 stability. Mol Syst Biol. 2024;20:719–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouas D, Shi H, Hainaut P. The aflatoxin-induced TP53 mutation at codon 249 (R249S): biomarker of exposure, early detection and target for therapy. Cancer Lett. 2009;286:29–37. [DOI] [PubMed] [Google Scholar]
- 61.Malekzadeh P, Pasetto A, Robbins PF, Parkhurst MR, Paria BC, Jia L, et al. Neoantigen screening identifies broad TP53 mutant immunogenicity in patients with epithelial cancers. J Clin Investig. 2019;129:1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsiue EH, Wright KM, Douglass J, Hwang MS, Mog BJ, Pearlman AH, et al. Targeting a neoantigen derived from a common TP53 mutation. Science. 2021;371:eabc8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoyos D, Zappasodi R, Schulze I, Sethna Z, de Andrade KC, Bajorin DF, et al. Fundamental immune-oncogenicity trade-offs define driver mutation fitness. Nature. 2022;606:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lane DP. Mutant p53 gain-of-function in the spotlight: are we suffering a GOF Delusion. Cancer Discov. 2024;14:211–3. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Burigotto M, Ghetti S, Vaillant F, Tan T, Capaldo BD, et al. Loss-of-function but not gain-of-function properties of mutant TP53 are critical for the proliferation, survival, and metastasis of a broad range of cancer cells. Cancer Discov. 2024;14:362–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leroy B, Fournier JL, Ishioka C, Monti P, Inga A, Fronza G, et al. The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic Acids Res. 2013;41:D962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnoud T, Parris JLD, Murphy ME. Common genetic variants in the TP53 pathway and their impact on cancer. J Mol Cell Biol. 2019;11:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grochola LF, Zeron-Medina J, Meriaux S, Bond GL. Single-nucleotide polymorphisms in the p53 signaling pathway. Cold Spring Harb Perspect Biol. 2010;2:a001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Escobar-Hoyos LF, Penson A, Kannan R, Cho H, Pan CH, Singh RK, et al. Altered RNA Splicing by Mutant p53 Activates oncogenic RAS signaling in pancreatic cancer. Cancer Cell. 2020;38:198–211.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim MP, Li X, Deng J, Zhang Y, Dai B, Allton KL, et al. Oncogenic KRAS recruits an expansive transcriptional network through mutant p53 to drive pancreatic cancer metastasis. Cancer Discov. 2021;11:2094–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taverna DM, Goldstein RA. Why are proteins so robust to site mutations. J Mol Biol. 2002;315:479–84. [DOI] [PubMed] [Google Scholar]
- 72.Tokuriki N, Tawfik DS. Stability effects of mutations and protein evolvability. Curr Opin Struct Biol. 2009;19:596–604. [DOI] [PubMed] [Google Scholar]
- 73.Khoo KH, Andreeva A, Fersht AR. Adaptive evolution of p53 thermodynamic stability. J Mol Biol. 2009;393:161–75. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q, Balourdas DI, Baron B, Senitzki A, Haran TE, Wiman KG, et al. Evolutionary history of the p53 family DNA-binding domain: insights from an Alvinella pompejana homolog. Cell Death Dis. 2022;13:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandt T, Kaar JL, Fersht AR, Veprintsev DB. Stability of p53 homologs. PLoS ONE. 2012;7:e47889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joerger AC, Fersht AR. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu Rev Biochem. 2016;85:375–404. [DOI] [PubMed] [Google Scholar]
- 77.Natan E, Baloglu C, Pagel K, Freund SM, Morgner N, Robinson CV, et al. Interaction of the p53 DNA-Binding Domain with Its N-Terminal extension modulates the stability of the p53 Tetramer. J Mol Biol. 2011;409:358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.