Abstract
The spore coat of Dictyostelium is formed de novo from proteins secreted from vesicles and cellulose synthesized across the plasma membrane as differentiating spores rise up the stalk. The mechanism by which these events are coordinated is not understood. In the course of experiments designed to test the function of the inner layer coat protein SP85 (PsB), expression of a specific partial length fragment was found to interrupt coat assembly after protein secretion and prior to cellulose synthesis in 85% of the cells. This fragment consisted of SP85's N-terminal domain, containing prespore vesicle targeting information, and its Cys-rich C1 domain. The effect of the NC1 fusion was not cell autonomous in interstrain chimeras, suggesting that it acted at the cell surface. SP85-null spores presented an opposite phenotype in which spores differentiated prematurely before reaching the top of the stalk, and cellulose was slightly overproduced in a disorganized fashion. A similar though less severe phenotype occurred when a fusion of the N and C2 domains was expressed. In a double mutant, absence of SP85 was epistatic to NC1 expression, suggesting that NC1 inhibited SP85 function. Together, these results suggest the existence of an outside-in signaling pathway that constitutes a checkpoint to ensure that cellulose synthesis does not occur until coat proteins are properly organized at the cell surface and stalk formation is complete. Checkpoint execution is proposed to be regulated by SP85, which is in turn under the influence of other coat proteins that interact with SP85 via its C1 and C2 domains.
Cell walls of plants, animals, and fungi and other microbial eukaryotes are composed of polysaccharides and proteins. Long, linear polysaccharides such as cellulose, chitin, β-1,3-glucans, and hyaluronan are typically synthesized at the cytoplasmic face of the plasma membrane and simultaneously translocated to the cell surface. In contrast, the proteins and shorter or branched polysaccharides are primarily secreted via exocytosis from post-Golgi vesicles of the secretory pathway. To ensure appropriate interactions between these two groups of molecules, their delivery systems are regulated relative to one another. For example, the quantity and organization of cellulose, hemicellulose, pectins, and proteins are distinct in the primary, secondary, and tertiary cell walls of plants (5, 10). In Saccharomyces cerevisiae, the synthesis of polysaccharides such as chitin and β-1,3-linked glucans is coordinated at the bud tip with secretion of mannoproteins, β-1,6-linked glucans, and cross-linking enzymes (6, 18). Evidence is accumulating that remodeling of the cell walls of both plants and fungi is influenced by outside-in signaling pathways that involve cell surface transmembrane proteins (17, 25).
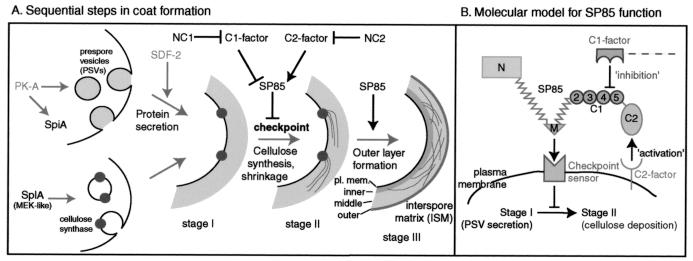
In Dictyostelium, the spore coat is formed de novo at the surface of prespore cells at the end of the fruiting body developmental cycle, about 24 h after starvation (34). The spore coat is a relatively simple cell wall consisting of about 50% cellulose and 50% protein, and 2% is a galuran polysaccharide containing Gal, GalNAc, and possibly GalUA (39). Cellulose, which is distributed throughout most of the thickness of the coat, is sandwiched between an outer, protein-rich layer, which comprises a permeability barrier toward exogenous macromolecules, and a more diminutive, proteinaceous inner layer near the plasma membrane (34) (see summary diagram in Fig. 9A). Ultrastructural analysis of differentiating wild-type and cellulose-null spores (34, 38) suggests that coat assembly involves an ordered series of events including (i) secretion of the proteins and the galuran, which are previously stored in prespore vesicles (PSVs) (stage I), (ii) cell shrinkage and deposition of cellulose (stage II), and (iii) formation of the electron-dense outer layer (stage III) (see Fig. 9A). This sequence makes it possible for secreted proteins to influence later steps, including cellulose synthesis.
FIG. 9.
Model for the terminal checkpoint and its regulation by SP85. (A) Delivery of the two major components of the coat, protein and cellulose, are proposed to be under control of separate signaling pathways which depend upon PK-A (20) and SplA (23), respectively. SDF-2 (32) is suggested to initiate coat formation by stimulating exocytosis of future coat proteins from PSVs (upper arm; stage I). The pathway and timing of arrival of cellulose synthase (lower arm) at the plasma membrane are not known. Subsequent stage II events, including expulsion of water and cellulose synthesis await checkpoint execution. The checkpoint is suppressed from the time of PSV formation by SP85, a resident PSV protein that is later secreted to contribute to the coat. SP85 itself is regulated by the opposing activities of a C2 factor, which supports SP85-mediated suppression, and a C1 factor, which overcomes SP85 suppression, resulting in checkpoint activation. The effects of expressing NC1 and NC2 are interpreted as competitive inhibition of the actions of the C1 and C2 factors, respectively. Deposition of cellulose results in organization of the outer and inner layers (stage III), which depends on a second function of SP85. Locations of the inner, middle, and outer layers and the plasma membrane (pl. mem.) are noted. (B) SP85 is postulated to inhibit the checkpoint via a plasma membrane sensor, which transduces the inhibition signal intracellularly. The M or N domain of SP85 may bind the receptor directly or indirectly. Regulation of SP85 by the C2 and C1 factors are represented as locational. The deinhibiting influence of the C1 factor, itself presumably connected to a coat protein network, is proposed to relocate SP85 away from the sensor as a result of overall hydration. Inhibition of the checkpoint is relieved, and sporulation continues to stage II.
SP85 is an abundant protein that localizes to the inner layer of the coat near the plasma membrane and was previously suggested to perform a cross-bridging role in spore coat structure (22, 39, 40). Biochemical studies show that SP85 specifically binds cellulose and another coat protein, SP65 (39), and that its recombinant C-terminal domain can bind both simultaneously (40). The N-terminal region contains information for targeting SP85 to the PSV (40). Biochemical analysis of SP85 from crude extracts of prespore cells suggests that SP85 also interacts with other coat proteins, including SP60, SP70, and SP96, and possibly cellulose, by direct and indirect contacts (22). Genetic ablation of SP85 results in a functionally deficient coat based on increased coat permeability, decreased buoyant density, failure to incorporate SP65, and reduced germination time (40). Thus, SP85 contributes substantially to the assembly and structure of the coat, possibly via interactions with cellulose, SP65, and other coat proteins.
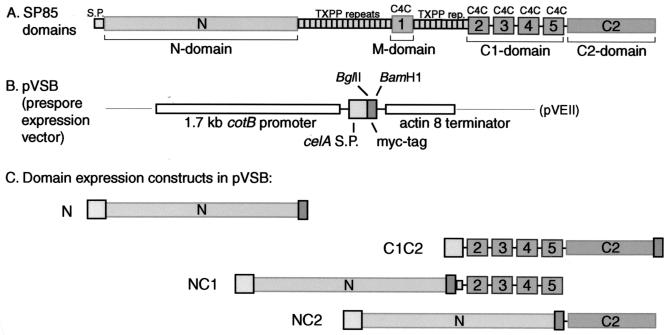
To further examine the role of SP85, we carried out a domain expression study in vivo to augment the biochemical and gene deletion findings. The C-terminal domain of SP85, 197 amino acids, is separated from the remainder of the protein by a series of 10 TXPP tetrapeptide repeats (Fig. 1A). It consists of a Cys-rich C1 region of 118 amino acids and a C2 region of 79 amino acids that lacks Cys residues. The C1 region contains four tandem copies of the so-called C4C repeat, which contains predicted β-turns and four conserved Cys residues similar to features of the N-terminal subdomain of the epidermal growth factor (EGF) module (39). The predicted C1 and C2 domains were fused to the N-terminal domain because the N domain is by itself targeted to the PSV but is not incorporated into the coat (40) and might facilitate folding of the short C1 and C2 domains. This study examines the expression of these NC1 and NC2 domain fusions and focuses on an early specific phenotype of NC1-expressing cells: suppression of terminal sporulation. The findings suggest the existence of a novel signaling pathway, originating at the cell surface, that regulates terminal steps of sporulation including cellulose synthesis. A role for SP85 and interacting coat proteins in this signaling pathway explains many of the defects of SP85-null spores and the dominant negative effects of the partial length fragments.
FIG. 1.
Domain motifs in SP85 and expression constructs. (A) Domain model for SP85 based on amino acid sequence motifs and functional expression studies (39, 40). S.P., cleavable signal sequence. TXPP refers to the sequence of the tandem tetrapeptide repeats, which are likely to be O-glycosylated. Boxes numbered 1 to 5 are EGF-like C4C repeats. (B) Sequence organization of a general expression construct for myc-tagged secretory proteins in prespore cells (40) derived originally from pVEII. (C) Expression constructs examined in this study. N and C1C2 were previously described (40). N was expressed by ligating a cDNA for the N-domain into the BglII site of pVSB, yielding pVSBN. The NC1 and NC2 domain fusion constructs were created by ligating cDNAs encoding C1 or C2 into the BamHI site of pVSBN.
MATERIALS AND METHODS
Cell manipulations.
Cell strains are listed in Table 1. Cells were suspension grown in axenic medium (HL-5) and induced to develop by washing in 10 mM potassium phosphate (KP), pH 6.5, and depositing in KP on nonnutrient agar plates as described previously (28). To induce synchronous terminal differentiation in dcsA-null strains, cell aggregates (22 to 26 h) were dissociated into single cells and resuspended in 20 mM 8-bromocyclic AMP (8-Br-cAMP) (Sigma Chemical Co., St. Louis, Mo.) in KPS (80 mM sucrose in KP) as described previously (21, 38) and incubated on coverslips placed in six-well plates. These were monitored on an inverted Nikon Diaphot phase contrast microscope.
TABLE 1.
Strains employed
| Strain | Informal namea | Parental strain | Description | Reference |
|---|---|---|---|---|
| Ax3 | NC-4 | Normal | 19 | |
| HW60 | AH | Ax3 | Expresses NC1C2 | 40 |
| HW61 | AC | Ax3 | Expresses C1C2 | 40 |
| HW62 | AN | Ax3 | Expresses N | 40 |
| HW65 | ANC1 | Ax3 | Expresses NC1 | This study |
| HW66 | ANC2 | Ax3 | Expresses NC2 | This study |
| DG1099 | D1 | Ax3 | dcsA (cellulose synthase) null | 4 |
| HW67 | DNC1 | DG1099 | dcsA null, expresses NC1 | This study |
| HW68 | DNC2 | DG1099 | dcsA null, expresses NC2 | This study |
| HW70 | B1 | Ax3 | pspB (SP85) null | 40 |
| HW71 | BNC1 | HW70 | SP85−, expresses NC1 | This study |
| HW72 | BNC2 | HW70 | SP85−, expresses NC2 | This study |
| TL56 | Ax3 | cotABC null | 12 |
Strains are named informally by using an acronym in which the first letter denotes the parental strain (A, Ax3; B, B1; D, DG1099) and the subsequent letters denote the SP85 domains expressed.
To quantitate spore differentiation and cellulose production, 10-cm-diameter nonnutrient agar plates were inoculated with 1.2 × 108 cells. To produce interstrain chimeras, cells were mixed at the indicated ratios prior to plating. After 36 to 48 h, culminants were scraped off with a spatula and resuspended in 0.5% (vol/vol) NP-40 (to lyse nonencapsulated cells)-20 mM EDTA in KP buffer (pH 6.5) by vortexing. For mutant strains that produced sticky spores, cell suspensions were subjected to mild probe sonication to generate a single-cell suspension while avoiding spore lysis as determined by phase contrast microscopy. Stalks were removed by filtration through a screen. Spores were centrifuged, resuspended with vortexing in 0.1% Calcofluor White ST in KP buffer, and counted in a hemacytometer under epifluorescence illumination through the DAPI (4′,6′-diamidino-2-phenylinole) filter channel, to visualize Calcofluor-induced fluorescence of cellulose-encased spores or spore coats (to account for apparent autogermination). Cell suspensions were plated in association with Klebsiella aerogenes on SM agar to examine germination efficiency (36).
Cells were collected in a similar manner for Western blot analysis, except that nonnutrient agar plates were scraped in 20 mM EDTA in KP supplemented with 90 mM sucrose (to protect dcsA-null cells) and cells were dissociated by repeated pipetting. Cell pellets were separated from the soluble fraction (interspore matrix) by centrifugation at 13,000 × g for 15 s. In some cases, cells were resuspended in 1 M ammonium acetate (NH4Ac) and centrifuged again in an attempt to remove loosely associated protein. These supernatants were dried in a vacuum centrifuge, resuspended in water, and dried again to remove NH4Ac.
For viewing spore differentiation, individual sorocarps (fruiting bodies) were lifted from their base with a scalpel blade and transferred into an 18-μl drop of 0.1% Calcofluor White ST in KPS on a glass slide and covered with a 0.17-mm-thick coverslip. For viewing many sori, the coverslip was touched to the tops of a lawn of sorocarps and then deposited with the adsorbed sori onto a drop of Calcofluor on a slide.
Expression of NC1 and NC2.
PCR primers were designed to amplify the C1 region, amino acids 309 to 437, and the C2 region, amino acids 437 to 512, from pspB cDNA in p14E6Pst73#1 (39). Primer sequences were as follows, where underlining denotes sequences homologous to SP85-encoding DNA: C1 upper primer, 5′-TCTCGCGGATCCGAATTCACACAACCACCAAGAGCATCA; C1 lower primer, 5′-TCTCGCGGATCCTGGTCTAACGTATACACAATGGAG; C2 upper primer, 5′-TCTGGAAGATCTCCATGGCCAACAGGTAGATGGGGTGA; and C2 lower primer, 5′-AGACCTAGATCTAAAACCATTGAGATCGTTTACGTC. BamHI or BglII sites located at the 5′ ends of the primers were used to ligate the resulting fragments into the BamHI site of pVSBN (40), a previously described prespore cell expression plasmid for the N-terminal region of SP85 whose BamHI site resided after the C-terminal c-myc epitope tag (Fig. 1). The encoded NC1 fusion protein consisted of, starting at the N terminus, the cleavable celA signal peptide (for rough endoplasmic reticulum [rER] targeting), the SP85 N-terminal region (for PSV targeting), the c-myc epitope (for detection), amino acids GSGFTQPP, the C1 region, and amino acids GS. NC2 was similar except that, following the c-myc epitope, it contained amino acids GSPW, the C2 region, and amino acids RS. Expected sequences were confirmed in both directions.
pVSBNC1 and pVSBNC2 were introduced into strains Ax3, B1, and DG1099 by electroporation, and transformants were selected in 5 to 10 μg of G418/ml in HL-5 as previously described (24). Clonal isolates expressing maximal levels of NC1 and NC2 in slugs based on Western blot analysis were examined further.
Cellulose assays.
Spore samples to be analyzed for cellulose were centrifuged at 13,000 × g for 10 min, resuspended in 8 M urea-50 mM dithiothreitol in KP buffer, boiled for 3 min, and washed twice in water by centrifugation. Anhydrous trifluoroacetolysis has been used previously to convert wood and spore cellulose into Glc (11, 39). For trifluoroacetolysis, cell pellets were taken to dryness in a vacuum centrifuge, resuspended in undiluted trifluoroacetic acid (TFA) (Pierce), and heated for 1 h at 100°C followed by dilution with 2 volumes of H2O and continued heating at 100°C for 3 h. The hydrolyzed samples were then dried in a vacuum centrifuge. For cellulase digestion, cell pellets were resuspended in 100 μl of 100-U/ml cellulase enzyme complex from Trichoderma reesei (Sigma Chemical Co.) in 26 mM KP, pH 5.0, and incubated at 37°C for 1 h. Total Glc was assayed amperometrically on a Dionex PA-10 column as described previously (39). Controls showed that cellulase contributed negligible Glc and that washed spore coats contained negligible free Glc (<2% of the level seen in cellulose) (data not shown).
To test the susceptibility of total glucan to mild acid hydrolysis, aliquots were incubated in 4 M TFA for 4 h at 100°C and Glc was assayed as described above.
Immunofluorescence.
Cells were processed for immunofluorescence as previously described (38). Antibodies are listed in Table 2. Strain comparisons were carried out on samples processed in parallel. Examples shown are representative of two or more independent trials.
TABLE 2.
Abs employed
Western blot analysis of coat protein expression.
Cell pellets and supernatants from 0.5 × 106 to 2 × 106 input cell or spore equivalents were separated on a sodium dodecyl sulfate (SDS)-polyacrylamide (7 to 15% linear gradient) gel, electroblotted to 0.45-μm-pore-size nitrocellulose, probed sequentially with primary antibodies (Abs) and alkaline phosphatase-conjugated secondary antibodies, and developed colorimetrically in the presence of 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium as described previously (40). Anti-SpiA was adsorbed against an excess of a particulate fraction from strain Ax3 cells grown in HL-5 for 18 h at 4°C and recovered as the supernatant after centrifugation at 100,000 × g for 1 h.
RESULTS
Expression of NC1 and NC2 domain fusions of SP85.
The predicted domains of SP85 are depicted in Fig. 1A. In a previous study (40), expression of DNA encoding the N domain with the celA signal peptide and a c-myc epitope tag, under control of the prespore-specific cotB promoter (pVSBN) (Fig. 1B and C), had resulted in a protein that was targeted properly to the PSV and subsequently secreted but was not incorporated into the coat. To examine the functional properties of the C1 and C2 domains, they were separately fused downstream of the N domain and its C-terminal myc tag (Fig. 1C), to direct targeting to the PSV as for native SP85. An alternative approach to express C1 and C2 alone has thus far been unsuccessful in vegetative cells, with most protein remaining intracellular and insoluble in extracts (P. Zhang and C. M. West, unpublished data). The resulting plasmids, pVSBNC1 and pVSBNC2, were electroporated as described in Materials and Methods into the normal strain Ax3, the SP85-null strain B1, and the cellulose synthase (dcsA)-null strain DG1099. The results from one representative high-expressing clone of each strain are presented here.
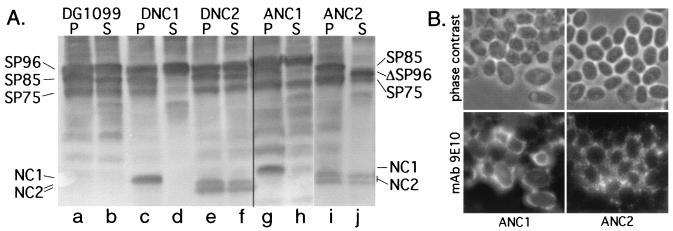
When induced to terminally differentiate, clonal strains derived from transfections of strain Ax3 with pVSBNC1 expressed a novel, developmentally regulated protein with an apparent Mr of 38,000 that was similar to the predicted value of 37,400 after removal of the signal peptide (data not shown). This new protein could be detected by polyclonal Ab (PAb) CT7 and PAb NT7 against SP85, and monoclonal Ab (MAb) 9E10 against the myc epitope tag (Table 2). When cells from terminally differentiated culminants were dissociated and centrifuged to separate cell-associated proteins from soluble proteins, the NC1 protein was almost exclusively associated with the cells (Fig. 2A, lanes g and h). Strain names are defined in Table 1. Cell-associated NC1 was resistant to extraction with high salt (1 M NH4Ac) (data not shown). The level of NC1 was similar to that of endogenous SP85 based on labeling intensity with PAb NT7. Similar results were obtained when NC1 was expressed in the dcsA-null strain DG1099 (Fig. 2A, lanes c and d) and the SP85-null strain B1 (data not shown). Thus, cell association did not depend on cellulose or endogenous SP85 as previously observed for other coat proteins (38, 40).
FIG. 2.
Expression of the NC1 and NC2 domain fusions. (A) Western blot analyses. Strains were allowed to terminally differentiate for 26 h (strains ANC1 and ANC2) or 40 h (strains DG1099, DNC1, and DNC2), dissociated into single cells in the presence of EDTA, and centrifuged to separate the cells (P, pellet) from soluble proteins (S, supernatant or interspore matrix). Aliquots of the pellets and supernatants from equivalent fractions of the original sample were compared by SDS-polyacrylamide gel electrophoresis and Western blotting. Blots were probed with a mixture of PAb NT7 to detect NC1, NC2, and SP85, and MAb 83.5 to detect the coat proteins SP96 and SP75, so as to compare all the proteins on the same blot. Protein bands are identified in the margin. The same NC1 and NC2 bands seen with PAb NT7 were also detected with MAb 9E10 against the c-myc epitope tag (data not shown). In ANC2, SP96 was replaced by a more-rapidly migrating species postulated to be derived from SP96, referred to as ΔSP96. (B) Immunofluorescence localization of NC1 and NC2 after terminal differentiation. Culminants were adsorbed onto coverslips, fixed, permeabilized, and immunoprobed with MAb 9E10 to the c-myc epitope associated with expressed NC1 or NC2, as described in Materials and Methods. Negligible labeling was seen in the absence of MAb 9E10 (not shown). Note that MAb labeling is generally confined to the cell surface and not in intracellular vesicles.
Expression of the NC2 domain fusion in strain Ax3 resulted in a novel protein doublet with apparent Mr values of 35,000 and 34,000 (Fig. 2A, lanes i and j) compared to the predicted value of 30,900. The two NC2 bands might differ because of variable posttranslational modification or truncation at the C terminus. The NC2 proteins were slightly enriched in the cell pellet compared to the soluble interspore matrix fraction, and pellet-associated NC2 was also resistant to extraction with 1 M NH4Ac (data not shown). A similar NC2 doublet was seen when expressed in strains DG1099 (Fig. 2A, lanes e and f) and B1 (data not shown).
To determine if cell-associated NC1 or NC2 was intracellular or at the cell surface, differentiating cells were examined by immunofluorescence. Cells were adsorbed to coverslips, fixed in paraformaldehyde, permeabilized with an organic solvent, and probed with the anti-myc tag MAb 9E10 for NC1 or NC2. Dissociated 18-h ANC1 and ANC2 slug cells displayed punctate labeling in the cytoplasm (data not shown), similar to that seen for the N domain alone in PSVs (40). In contrast, terminally differentiated cells showed exclusively cell surface labeling (Fig. 2B). NC2 appeared to be more diffusely associated with the cell surface than NC1, consistent with the Western blot analysis showing up to 50% of NC2 in the cell wash fraction (Fig. 2A, lanes i and j). Corresponding results were obtained with strains DNC1, DNC2, BNC1, and BNC2 (data not shown). The results suggest that NC1 and NC2 were sufficiently properly folded to traverse the secretory pathway and incorporate efficiently at high levels into cell surface structures via their C1 or C2 domains.
NC1 inhibits cellulose synthesis.
In normal strains such as Ax3, sporulation occurs as cells rise up the forming stalk and is completed as the last prestalk cells enter its apical end. The terminally differentiated spores, which are no longer self adherent, are suspended contiguously in a spherical droplet of interspore matrix, which is collectively referred to as the sorus (Fig. 3A). Spores have a characteristic ellipsoid shape and a high level of refractility and, in the presence of Calcofluor White ST, are outlined by a blue fluorescent ring indicative of cellulose.
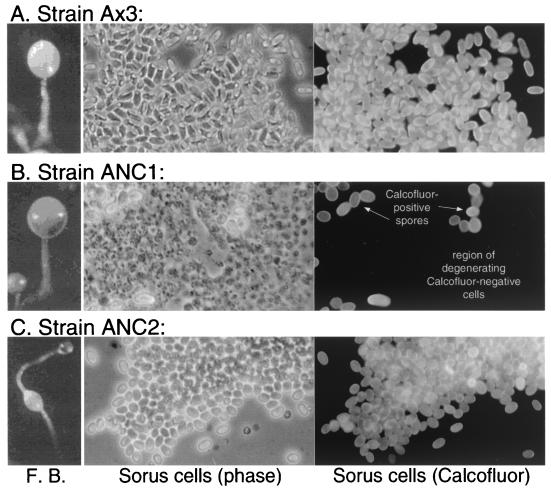
FIG. 3.
Sporulation in NC1 and NC2 domain expression strains. Representative culminants from strains Ax3 (parental), ANC1, and ANC2 are shown in the panels on the left. Ax3 forms a normal fruiting body with a sorus, consisting of spores, perched on top of the cellular stalk. The unusual morphology of strain ANC2 varied in frequency from 50 to 90%, with the remaining culminants having a more normal appearance as shown in panel A. Spores were found only in the lower sorus when present. Panels on the right show images from the same field of view of a representative squash preparation of a single sorus. The field was imaged using either phase contrast (middle panels) or epifluorescence to reveal labeling with Calcofluor White ST (right panels) to show cellulose. Note that most ANC1 cells were Calcofluor negative and tended to lyse, that both ANC1 and ANC2 Calcofluor-positive cells tended to be less elongate than parental Ax3 cells (Table 3), and that ANC2 spores did not disperse as well under the coverslip owing to abnormal self-cohesiveness.
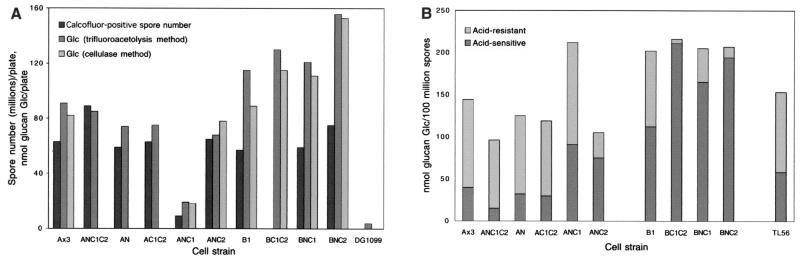
Strain ANC1 formed normal appearing sori except that they were abnormally transparent (Fig. 3B). In addition, the great majority of cells were not labeled with Calcofluor, suggesting an absence of cellulose, and tended to lyse under the coverslip. Some sori completely lacked spores, whereas others contained occasional Calcofluor-positive spores. After an additional 24 h, most of the previously Calcofluor-negative cells tended to acquire faint outlines of Calcofluor-induced fluorescence, possibly overcoming inhibitory effects of NC1 (data not shown). To quantitate Calcofluor-positive spore formation, fruiting bodies were suspended in 0.5% NP-40 to lyse all other cells and filtered to remove stalks. Strain ANC1 produced only about 15% of the number of Calcofluor-positive spores formed by strain Ax3 (Fig. 4A). If harvested within 12 h of differentiation, most of these spores were resistant to detergent and able to germinate as determined by plating in association with K. aerogenes (data not shown).
FIG. 4.
Calcofluor-positive spore numbers and cellulose production. For each strain, a plate of culminants originally seeded with 1.2 × 108 cells was suspended in 0.5% NP-40-KP to lyse nonspore cells, filtered to remove stalks, and centrifuged to recover sedimentable material. (A) Spores (or coats due to autogermination) were counted in a hemacytometer after labeling with Calcofluor White ST. After centrifugation, the pellet was extracted with hot urea-dithiothreitol and aliquots were degraded by trifluoroacetolysis or digestion with cellulase. Free Glc was assayed by high-pH anion-exchange chromatography. Data shown are averages of two independent determinations. (B) Aliquots were subjected to mild acid hydrolysis, and released Glc was assayed as described above. Data are given as fractions of total Glc assayed by trifluoroacetolysis (from panel A), which is plotted on a per Calcofluor-positive spore basis. Data are averaged from two independent trials.
Inhibition of Calcofluor-positive spore formation was specific to NC1 as the effect was not seen when NC2 was expressed (Fig. 3C). However, the majority of NC2 fruiting bodies exhibited an abnormal morphology in which prespore cells failed to ascend to the apex of the stalk, instead differentiating into Calcofluor-positive spores before stalk formation was complete as determined by time course studies (data not shown). In addition, although all the spores were Calcofluor positive, they were abnormally cohesive and did not disperse under the coverslip as did parental spores. Furthermore, they exhibited a less elongated morphology on average (Table 3). Production of normal spore numbers was confirmed when quantitated as described above (Fig. 4A). Instead of inhibiting cellulose formation as for NC1, NC2 might cause premature sporulation, resulting in stranding the spores partway up the stalk with negative consequences for cell dissociation and spore shape.
TABLE 3.
Mutant spore shapesa
| Strain | Ratio of axes (no. of spores measured)a |
|---|---|
| Ax3 | 2.12 (64) |
| AC1C2 | 1.99 (35) |
| ANC1b | 1.76 (44) |
| ANC2 | 1.52 (66) |
| B1 | 1.25 (54) |
| BNC1 | 1.37 (25) |
| BNC2 | 1.28 (34) |
Axial ratios were measured from enlargements of phase contrast micrographs taken of dispersed spores after settling in a hemacytometer.
Only Calcofluor-positive spores included.
Expression of the N domain alone had no effect on fruiting body morphology, Calcofluor-positive spore number, or cellulose deposition (Fig. 4A). Although N is not incorporated into the coat, expression of this domain does cause permanent changes in the coat, including decreased buoyant density and barrier functions (40; C. M. West, K. Kelley, and G. W. Erdos, unpublished data). Furthermore, the C1C2 construct had no effect on these properties (Fig. 4A), including spore shape (Table 3), although previous work showed that C1C2 was incorporated into the coat (40). Thus, the effect of NC1 could be attributed specifically to C1, and C1 needed to be isolated from C2. The role of N is likely to target C1 to the PSV and subsequently to the cell surface and possibly to promote folding and or stability, but an additional, more direct role for N in NC1 action cannot be excluded.
To confirm that strain ANC1 underproduced cellulose during sporulation, cells from the sorus were assayed for cellulose content both chemically and enzymatically (Fig. 4A). The samples were first extracted with urea to remove interfering material (39), and parallel aliquots were subjected to either anhydrous trifluoroacetolysis or cellulase digestion. The released Glc was measured by high-pH anion-exchange chromatography on a Dionex PA-10 column and quantitated by pulsed amperometry. Using either method, similar levels of Glc were assayed from aliquots of strain Ax3 spores (Fig. 4A). This demonstrated that nearly all urea-insoluble Glc-containing polymer in these cells released by the acid treatment was cellulose-like. Consistent with this interpretation, negligible Glc was recovered from the dcsA-null strain DG1099, which lacks the catalytic subunit of cellulose synthase. Strains expressing the N domain, the C1C2 fusion, or NC2 produced levels of cellulose per spore similar to that of Ax3. In contrast, ANC1 produced a low level of cellulose corresponding roughly to the small number of Calcofluor-positive spores produced. Thus, presumptive spores of strain ANC1 were inhibited in cellulose synthesis, and inhibition appeared to be an all-or-nothing effect at the level of the individual cell.
NC1 does not inhibit spiA expression or PSV exocytosis.
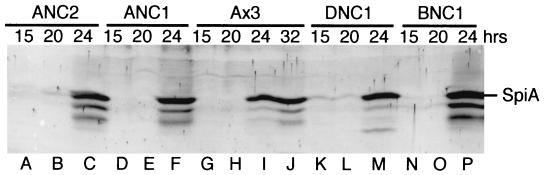
To investigate the step at which sporulation was blocked by NC1, expression of the late sporulation marker gene spiA, which is induced before cellulose synthesis, was examined. SpiA is thought to encode a prespore cell surface transmembrane protein that is not necessary for coat formation but is required for spore stability (26). Western blot analysis of differentiating cells showed that the SpiA protein is expressed similarly in ANC1 compared to strain Ax3 (Fig. 5, compare lanes D to F and A to C). Thus, the arrest in ANC1 cells does not affect induction of SpiA and may occur afterwards.
FIG. 5.
Expression of the early sporulation marker SpiA. Cells were developed for 15, 20, 24, or 32 h as indicated and harvested for analysis by SDS-polyacrylamide gel electrophoresis and Western blotting with a preabsorbed antiserum against the SpiA protein. Culminants derived from plating 106 cells were loaded per lane. The position of the SpiA protein at an Mr of 31,000 is shown in the margin. The lower Mr value bands are likely to be degradation products seen previously (26). As expected, cells did not accumulate SpiA until after 20 h of development.
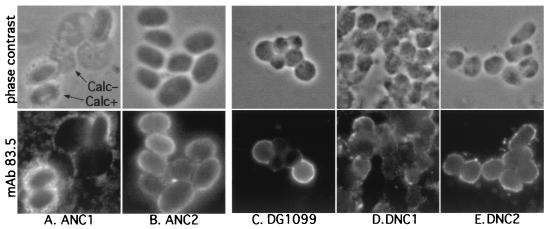
To examine the first step of coat assembly per se, exocytosis of coat proteins from the PSV, coat proteins were localized by indirect immunofluorescence as described above for NC1 and NC2. MAb 83.5, which recognizes the outer-layer-associated proteins SP96, SP80, and SP75, immunolabels the surface of normal spores (13), as also seen for the ANC2 spores here (Fig. 6B). Both cellulose-positive ANC1 spores, recognized by their smooth contours, and cellulose-negative ANC1 cells showed surface labeling (Fig. 6A). The absence of internal labeling, which would have been evident given the absence of a cellulose coat and acetone permeabilization, showed that coat proteins had been secreted. Labeling of cellulose-negative ANC1 cells was less intense, however, suggesting a more diffuse distribution at the cell surface. Thus, NC1 inhibited cellulose synthesis at some step after spiA expression and PSV exocytosis.
FIG. 6.
Immunofluorescence localization of coat proteins in terminally differentiated cells. Cells from the indicated strains were processed as described in the legend to Fig. 2B except that they were immunoprobed with MAb 83.5 to localize the coat proteins SP96, SP80, and SP75. Shown are phase contrast (upper panels) and immunofluorescence images (lower panels) of the same fields. Note that MAb labeling is generally confined to the cell surface and not in intracellular vesicles. For ANC1 cells (A), labeling is seen around both Calcofluor-positive (Calc+) cells, recognized by their more oval, phase-bright profiles, and Calcofluor-negative (Calc−) cells, which are more flattened against the coverslip.
The dominant negative action of NC1 does not depend on cellulose.
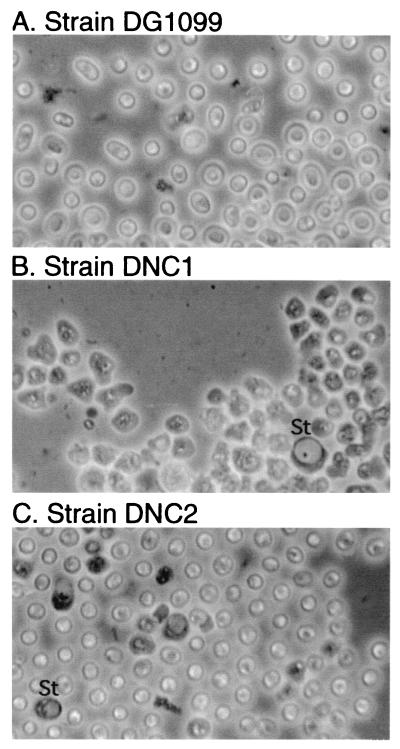
As described in the Discussion, the C1 domain exhibits cellulose-binding activity, which might be involved in regulating cellulose synthesis. To test for this, NC1 was expressed in the dcsA-null strain DG1099, which lacks the cellulose synthase catalytic subunit (4). Although true spores are not formed by the parental strain DG1099 owing to the lack of cellulose, they produce spherical, highly refractile, osmotically sensitive pseudospores enveloped by a diffuse precoat of protein (38). Sporulation was induced by dissociating 24-h slug cells in 20 mM 8-Br-cAMP on coverslips, as sporulation of DG1099 in vivo is delayed and asynchronous compared to that of wild-type cells (38). The appearance of normal pseudospores is shown in Fig. 7A. Expression of NC2 in DG1099 (strain DNC2) did not affect pseudospore differentiation (Fig. 7C). In contrast, DNC1 produced only amoeboid cells that remained attached to the coverslip and occasional stalklike cells recognized by their vacuolation (Fig. 7B). The cells exhibited only slight swelling when incubated in water for 15 min (data not shown), suggesting that they are more like prespore cells than pseudospores, which swell extensively under these conditions (38). The somewhat more amoeboid appearance of DNC1 (Fig. 7B) than of ANC1 cells (Fig. 3B) might have been because DNC1 cells were differentiated in osmotically balanced buffer rather than in the hypertonic environment of the sorus (8). As in strain ANC1, SpiA expression was normal (Fig. 5, lanes K to M), and immunolabeling showed that coat proteins were localized exclusively at the cell surface (Fig. 6D) as seen in DG1099 and DNC2 pseudospores (Fig. 6C and E). Therefore, DNC1 cells exocytosed their PSVs normally but were unable to form pseudospores, which involves cell shrinkage, suppression of osmotic regulation, and acquisition of a spherical shape (38). Thus, NC1 appeared to interrupt the entire terminal differentiation program that occurs after exocytosis (i.e., stage II [see Fig. 9A]) not just cellulose synthesis per se. NC1 action does not depend on cellulose binding and does not act by inhibiting intracellular cAMP accumulation, as the effect was observed even though the cells were bathed in the membrane permeant cAMP analog 8-Br-cAMP.
FIG. 7.
Effects of NC1 and NC2 on terminal differentiation in the dcsA-null strain DG1099. Strains DG1099, DNC1, and DNC2 were induced to sporulate on coverslips in the presence of 20 mM 8-Br-cAMP and examined by phase contrast microscopy. Note that strains DG1099 and DNC2 formed normal appearing pseudospores, characterized by their small, spherical, phase-bright shape, while DNC1 cells remained amoeboid. Occasional vacuolated stalk cells are labeled St.
NC1 inhibits cellulose deposition transcellularly.
To investigate whether NC1 inhibits cellulose deposition and other stage II sporulation events before or after it is secreted, the cell autonomy of the NC1 phenotype was examined. If the phenotype were not cell autonomous, this would support action at the cell surface that had transferred to neighboring cells, as a previous study showed that many coat proteins, including probably SP85, intermingle between neighboring spores during coat assembly (35). This was tested by mixing DNC1 cells (which express NC1) with normal Ax3 cells during development. Since DNC1 cannot produce cellulose-positive spores because they are dcsA null and cellulose deposition is cell autonomous (38), all cellulose-positive spores would have derived from strain Ax3. Chimeras between dcsA-null DG1099 and Ax3 cells were used as a control. A 9:1 mixture of DG1099:Ax3 produced six times as many Calcofluor-positive spores as the DNC1:Ax3 mixture (Ax3, 5.2 × 107 spores/108 input cells; DG1099, 0.00 × 106 spores/108 input cells; DNC1, 0.00 × 106 spores/108 input cells; DG1099:Ax3 (9:1), 4.0 × 106 spores/108 input cells; DNC1:Ax3 (9:1), 0.70 × 106 spores/108 input cells [data are from a single representative experiment]). The simplest interpretation is that the suppressive effect of DNC1 cells on cellulose deposition by neighboring normal spores was mediated by NC1 diffusing between cells. This suggests that NC1 also inhibits cellulose deposition by the cells in which it is expressed from the outside in.
SP85 is required for orderly cellulose deposition.
The effects of NC1 implicated its parent protein, SP85, in the normal timing of terminal (stage II) sporulation events, including cellulose synthesis. To evaluate the role of full-length SP85, sporulation was reexamined in the SP85-null mutant B1 (40). Unlike strain ANC1, strain B1 produced normal numbers of cellulose-positive spores (Fig. 4A and 8A). However, the spores differentiated before stalk formation was complete and were self cohesive, effectively stranding them at half mast (Fig. 8A) as seen in strain ANC2 above (Fig. 3C). Furthermore, B1 spores tended to be spherical in contrast to the ellipsoid shape of wild-type parental spores (Table 3), suggesting a defect in cellulose as cellulose is required to maintain the initial elongate shape of spores during sporulation (38). In B1 sori that were less than 1 day old, empty spore coats were frequently seen, suggesting premature germination or spore lysis consistent with a defective coat (data not shown). B1 spores were also observed to fragment more easily than Ax3 spores when exposed to probe sonication (data not shown). Since the level of cellulose per cell was not reduced in these coats (Fig. 4), the organization of coat cellulose might be aberrant.
FIG. 8.
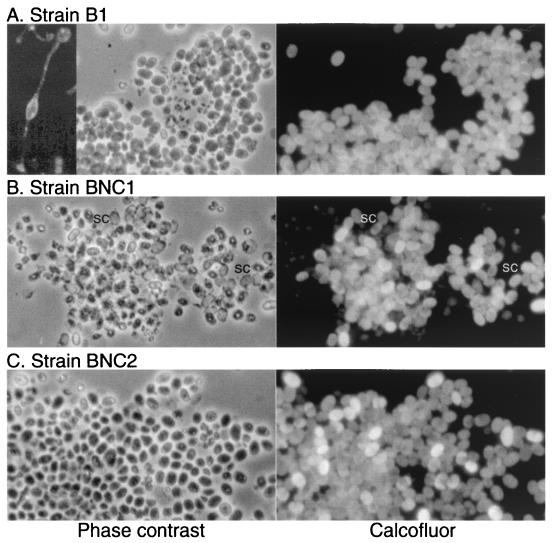
Effects of NC1 and NC2 on terminal differentiation in SP85-null strain B1. Strains B1 (SP85-null), BNC1, and BNC2 were adsorbed to a coverslip, bathed in 0.1% Calcofluor White ST in KP buffer, and examined by phase contrast (left panels) or epifluorescence illumination (right panels) to identify cellulose-positive spores. Inset for strain B1 shows the appearance of a typical B1 fruiting body. Spores are present only in the lower sorus. Note that B1-derived spores tended to be round and did not disperse well under the coverslip owing to self cohesion and that there was a tendency for autogermination as evidenced by the presence of Calcofluor-positive, phase-empty coats (two examples are labeled sc in panel B).
Spore coat cellulose is normally resistant to hydrolysis in aqueous 4 M TFA (39), consistent with crystalline packing of its linear β-1,4-glucan chains in microfibrils. This suggested that susceptibility to hydrolysis in 4 M TFA might be used to assess the organization of the glucan chains in the mutants. Aliquots of spores used to measure total cellulose above were hydrolyzed in 4 M TFA for 4 h at 100°C. Less than 25% of the cellulose from strain Ax3 was converted to Glc under these relatively mild conditions (Fig. 4B). The greater fraction of acid-labile Glc than previously reported (39) may be due to the pretreatment with hot urea to ensure removal of interfering substances. In contrast to Ax3 cellulose, greater than half of the B1 cellulose was hydrolyzed in 4 M TFA, suggesting that the glucan chains of B1 cellulose were more solvent exposed. In addition, cellulose was slightly overexpressed on a per spore basis (Fig. 4). Increased synthesis of improperly organized cellulose might have resulted from premature cellulose synthesis, as suggested by the morphology of B1 fruiting bodies. As detailed in the Discussion, we speculate that coat cellulose is more acid labile because cellulose synthesis was initiated prior to the proper spatial organization of cellulose synthase in the membrane and of potential cofactors at the cell surface. Thus, absence of SP85 appears to have an effect opposite to that of NC1, to promote rather than delay terminal sporulation.
NC1 acts upstream of SP85.
The inhibitory effect of NC1 on terminal sporulation might result from an interfering effect on SP85 function, or represent a novel function. To distinguish between these possibilities, the phenotype of the double mutant in which NC1 was expressed in the SP85-null (B1) background was examined. In contrast to when NC1 was expressed in the Ax3 background, all cells in the (lower) sorus were Calcofluor positive (Fig. 8B) and normal numbers of Calcofluor-positive spores were produced (Fig. 4A), indicating that the absence of SP85 was epistatic to NC1. As seen also for B1 spores, BNC1 spores were defective based on the appearance of empty coats in the sorus, suggesting spontaneous germination (Fig. 8B), a roundish shape (Table 3), and increased levels of cellulose which was also acid labile (Fig. 4B). The further increase in the fraction of cellulose that was acid labile (Fig. 4B), seen also when NC2 and C1C2 were expressed, indicated that these constructs had additional effects in the absence of SP85. With respect to checkpoint execution, NC1 thus appears to act primarily by interfering with a natural function of SP85 itself, possibly by competing with other proteins with which SP85 normally interacts (see Discussion).
DISCUSSION
The two main conclusions of this study are that the timing of coat protein secretion and cellulose deposition are coordinated by a checkpoint, and that checkpoint execution is influenced by the coat protein SP85. Based on previous evidence and summarized in Fig. 9A, coat assembly is a multistep process, beginning with protein secretion (stage I), followed by cell shrinkage and cellulose synthesis (stage II), and subsequently, formation of the outer proteinaceous layer (stage III). Protein and cellulose are synthesized by separate mechanisms that are likely to be under separate regulation, with evidence that cellulose synthesis is selectively controlled by the MEK-like kinase SplA (23; C. M. West, unpublished data). As depicted in Fig. 9A, we propose that the synthesis of cellulose is also influenced by a cell surface checkpoint in which the coat structural protein SP85 exerts an inhibitory effect until proteins rearrange after secretion. Two postulated factors, C1 and C2, are proposed to inhibit and enhance SP85 function, respectively, and NC1 and NC2, two partial SP85 sequences, affect checkpoint execution by interfering with their regulation of SP85 (Fig. 9A). The evidence is as follows.
A cell surface checkpoint in coat assembly: evidence from NC1.
Previous time course studies indicated that exocytosis of coat proteins occurs before cellulose synthesis (34), and this was reinforced by the recent observation that exocytosis does not depend on cellulose in a cellulose synthase (dcsA)-null strain (38). In cells expressing the NC1 domain fusion of SP85, a sporulation arrest or delay occurs after exocytosis (Fig. 6) but before cellulose synthesis (Fig. 3 and 4A). Arrested cells express the last known gene to be up-regulated during sporulation, spiA (Fig. 5), which is normally transcribed as prespore cells rise up the extending stalk (27), and culmination is completed normally. However, the arrested cells are fragile and tend to lyse when removed from the sorus for examination (Fig. 3B). A phenotype is also detected when NC1 is expressed in dcsA-null cells: when such cells are induced to sporulate, they remain amoeboid (Fig. 7B), fail to shrink, and are resistant to hypotonic lysis. This suggests that in the sorus, arrested cells might be unstable because of the hypertonic environment of the interspore matrix (8) or because they attempt to germinate in situ (30).
Inhibition by NC1 occurs at a discrete step in the sporulation program, between stages I and II (Fig. 9A), which we refer to as a cell surface checkpoint. The existence of a checkpoint during normal sporulation would allow time for (i) coat proteins and galuran to hydrate into conformations suitable for interaction with each other and nascent cellulose, (ii) final assembly of cellulose synthase subunits into a multisubunit complex and organization of the terminal complex (5, 10, 14) to support deposition of crystalline microfibrils, and (iii) the differentiating spores to reach the stalk apex. Checkpoint execution may be actively suppressed in the PSV even before exocytosis of the coat proteins.
The NC1-dependent arrest seems to be mediated by an extracellular pathway, as an excess of DNC1 cells can arrest cellulose synthesis by normal spores in interstrain chimeras (see Results). Since the arrest is not rescued by incubation of DNC1 cells in 8-Br-cAMP, NC1 expression does not appear to interfere with activation of protein kinase A (PK-A) by the spore differentiation factor 2 (SDF-2)→DhkA pathway (32). The arrest may affect a target of PK-A that is regulated by SplA, the MEK-like kinase cited above.
The effect of NC1 appears to be attributable to native functions of the C1 and N domains and not to misfolded or otherwise aberrant protein configurations. NC1 is expressed at a level comparable to that of other coat proteins and accumulates at the cell surface coordinately with other coat proteins (Fig. 2). The C1 domain confers retention of the fusion protein at the cell surface since the N domain, expressed alone, is released in soluble form (40). The fraction of NC1 that is secreted in soluble form by induced dcsA-null cells is competent to bind Avicel cellulose (L. Kaplan and C. M. West, unpublished data), indicating that its folding is not grossly aberrant. Moreover, C4C motifs are expressed in a variety of sequence contexts in other coat proteins (36), suggesting that their local environments might not be critical for folding. Indeed, C4C-folding may be intrinsically driven as the absence of N-glycosylation motifs indicates that it is not subject to glycosylation-dependent quality control in the rER (16), and independent folding seems to be a general feature of EGF-like modules (15). NC1-mediated arrest occurs at a discrete step many hours after accumulation of NC1, arguing that NC1 does not nonspecifically interfere with rER or Golgi processes. Finally, the effect of NC1 depends on the presence of endogenous SP85 (Fig. 8), which also argues that it is not simply due to an accumulation of denatured NC1.
Checkpoint model.
A biochemical mechanism to explain the SP85 mutant phenotypes is proposed in Fig. 9B. According to this model, an unidentified plasma membrane sensor protein, the checkpoint sensor, transfers inhibitory information intracellularly. When SP85 binds to the sensor, inhibition is exerted and the cell cannot proceed to stage II. SP85 itself is regulated, positionally or conformationally, in a positive sense by a hypothetical C2 factor and in a negative sense by a hypothetical C1 factor, which bind to the C2 and C1 domains, respectively. The C2 factor aids in positioning SP85 with respect to the checkpoint sensor, while the C1 factor prevents SP85 from binding to the sensor as a result of hydration-dependent positional or conformational changes following its exocytosis. NC2 and NC1 are interpreted to interfere with checkpoint regulation by competing with the activities of the C2 and C1 factors, respectively.
SP85 is thought to inhibit passage through the checkpoint because in its absence, cellulose synthesis appears to occur precociously. The earliest SP85-null phenotype is that spores deposit cellulose before stalk formation is complete, leaving them stranded below the stalk apex (Fig. 8A). This phenotype is consistent with evidence from myosin light chain mutants that the migration of prespore cells up the stalk appears to require active cell motility (7), which would be blocked by premature completion of the coat. SP85-null spores are also abnormally self-cohesive (Fig. 8A), less elongated (Table 3), and more permeable (40), and they contain excess, improperly organized cellulose (Fig. 4) and tend to break down in the fruiting body (data not shown). These defects are not seen in other coat protein deletion mutants such as strain TL56 (Fig. 4B). The simplest interpretation is that stages II and III of coat assembly are executed prematurely, resulting in stranded spores with defective coats. Thus, SP85 appears to normally sustain the checkpoint as a negative effector.
The NC1-induced arrest acts upstream of SP85 as it does not occur in SP85-null cells (Fig. 8B). This suggests that NC1 interferes with the normal down-regulation of SP85 to sustain blockage of the checkpoint. The checkpoint-inhibiting effect of NC1 is not mimicked by NC2 or C1C2 (Fig. 4A), indicating that the phenotype is not the result of simply trapping the N domain in the coat and that it depends on the isolation of C1 from the C2 domain of the protein. The C1 domain of NC1 may competitively interfere with the normal inhibitory binding of the hypothetical C1 factor to the C1 domain of SP85 (Fig. 9B).
In contrast to that of NC1, NC2 expression mostly mimics SP85-null phenotypes. NC2 cells differentiate into sticky spores that fail to rise to the apex of the stalk (Fig. 3C). Although they produce normal levels of cellulose, the wall is partially defective (Fig. 4B) and spores tend to be round (Table 3). It is essential for these effects that C2 is expressed separately from C1, as strain AC (expressing C1C2) produces normal appearing fruiting bodies and spores (Fig. 4, Table 3, and data not shown). The dominant negative nature of NC2's action suggests that NC2 interferes with activation of SP85, leading us to propose the existence of an activating C2 factor. As illustrated in the model (Fig. 9B), the C2 factor might sequester SP85 near the plasma membrane for optimal presentation to the checkpoint sensor, similar to the role postulated for syndecan or other heparan sulfate proteoglycans in the presentation of growth factors to animal cells (3). The somewhat milder phenotype of ANC2 spores than of SP85-null spores suggests that the dominant negative mode of NC2 action is not as deleterious as the complete absence of SP85. Expression of NC2 in SP85-null cells produces a stronger cellulose defect (Fig. 4B) than the absence of SP85 alone, however, indicating that NC2 has additional targets.
SP85 ligands.
The distinct sequence motifs present in the C1 and C2 regions (Fig. 1) suggest that they fold separately and exhibit discrete binding activities. Binding studies utilizing secretions from DNC1 and DNC2 cells induced in suspension in the presence of 8-Br-cAMP suggest that the cellulose and SP65 binding activities of the C1C2 fusion (40) map to NC1, whereas NC2 binds two unidentified coat proteins (Zhang et al., unpublished data). SP65 is thus the prime candidate for the C1 factor that is proposed to relieve SP85-mediated inhibition of checkpoint execution (Fig. 9B). Additional receptor candidates for SP85 are other coat proteins, including SP70 and SP60 (22), and several proteins specifically expressed on the prespore cell plasma membrane, including SpiA (26), gp150/LagC (31), SP29/PsA (37), and WGA80B (33). However, SP70 and SP60 might not be involved in checkpoint regulation, as cellulose synthesis is nearly normal in strain TL56 (Fig. 4B), which is genetically deficient in these and another coat proteins (12).
The checkpoint model predicts the existence of a sensor in the plasma membrane and an intracellular pathway to transduce the signal to regulate cell volume and cellulose synthesis. Two-component histidine kinases regulate other late developmental events in Dictyostelium (9, 41) and the induction of cellulose synthase in a strain of Rhizobium (2), and a related protein might be involved here. Interaction of SP85 with the proposed sensor might be mediated by its M or N domain. A transient role for the N domain in NC1 action, in addition to targeting to the PSV (40), is possible, and the missing tandem tetrapeptide repeats normally present between N and C1, which were excluded owing to difficulties in expression in Escherichia coli, may also be important (39).
Implications for cell wall assembly.
The SP85 model provides an example of how an external structural protein can coordinate polysaccharide synthesis with delivery of a wall component to the cell surface. The checkpoint pathway may be related to the cell wall integrity pathway of yeast, which is activated by unknown ligands acting on genetically defined transmembrane sensor proteins that influence mitogen-activated protein kinase pathways (6, 25). This correlates with a possible role of SplA in Dictyostelium (23; West, unpublished data). Vascular plants have wall-associated transmembrane Ser/Thr kinases implicated in cell wall expansion that might use pectins and a Gly-rich protein as ligands (17), suggesting that outside-in signaling might be general in cellulose-rich walls. The outcome of mutating SP85 on overproduction and acid lability of cellulose is reminiscent of the rsw1 mutation in the catalytic subunit of an Arabidopsis cellulose synthase (1) and the effects of triathiazine herbicides (11), suggesting that these perturbations of cellulose synthase might interrupt a related checkpoint regulation pathway in plants. Other deficiencies in SP85 mutant coats, such as increased permeability and decreased buoyant density (40), might result from checkpoint misregulation, although SP85's cellulose-binding activity, apparently not involved in checkpoint regulation, indicates that this protein also has additional functions in coat formation. Derangement of the checkpoint may explain the spore-defective phenotypes of mutations of other late-acting genes including, e.g., acrA (29). Further understanding of SP85's role in checkpoint regulation is likely to come from identification of other coat proteins that interact with its C1 and C2 domains, refined mutagenesis of full-length SP85, and additional knowledge about the regulation of cellulose synthase.
Acknowledgments
We are grateful to Scott McMillen of the Protein Analysis Core of the University of Florida Interdisciplinary Center for Biotechnology Research for the Glc determinations. Anti-SpiA was generously provided by W. F. Loomis and D. Fuller. T. Mullins provided valuable perspective.
This project was partially supported by a grant (MCB-9730036) from the National Science Foundation. A.C.M. was a University of Florida undergraduate University Scholar.
REFERENCES
- 1.Arioli, T., L. Peng, A. S. Betzner, J. Burn, W. Wittke, W. Herth, C. Camilleri, H. Hofte, J. Plazinski, R. Birch, A. Cork, J. Glover, J. Redmond, and R. E. Williamson. 1998. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279:717-720. [DOI] [PubMed] [Google Scholar]
- 2.Ausmees, N., H. Jonsson, S. Hoglund, H. Ljunggren, and M. Lindberg. 1999. Structural and putative regulatory genes involved in cellulose synthesis in Rhizobium leguminosarum bv. trifolii. Microbiology 145:1253-1262. [DOI] [PubMed] [Google Scholar]
- 3.Baeg-Hun, B., and N. Perrimon. 2000. Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila. Curr. Opin. Cell Biol. 12:575-580. [DOI] [PubMed] [Google Scholar]
- 4.Blanton, R. L., D. Fuller, N. Iranfar, M. J. Grimson, and W. F. Loomis. 2000. The cellulose synthase gene of Dictyostelium. Proc. Natl. Acad. Sci. USA 97:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brett, C. T. 2000. Cellulose microfibrils in plants: biosynthesis, deposition, and integration into the cell wall. Int. Rev. Cytol. 199:161-199. [DOI] [PubMed] [Google Scholar]
- 6.Cabib, E., D.-H. Roh, M. Schmidt, L. B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679-19682. [DOI] [PubMed] [Google Scholar]
- 7.Chen, T.-L. L., W. A. Wolf, and R. L. Chisholm. 1998. Cell-type-specific rescue of myosin function during Dictyostelium development defines two distinct cell movements required for culmination. Development 125:3895-3903. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, D. A., A. J. Dunbar, S. D. Buconjic, and J. F. Wheldrake. 1999. Ammonium phosphate in sori of Dictyostelium discoideum. Microbiology 145:1891-1901. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, D. A., D. C. Mahadeo, D. N. Cervi, Y. Kishi, K. Gale, T. Sands, and M. Sameshima. 2000. Environmental regulation of pathways controlling sporulation, dormancy and germination utilizes bacterial-like signaling complexes in Dictyostelium discoideum. Protist 151:111-126. [DOI] [PubMed] [Google Scholar]
- 10.Delmer, D. P. 1999. Cellulose biosynthesis: exciting times for a difficult field of study. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:245-276. [DOI] [PubMed] [Google Scholar]
- 11.Fengel, D., and G. Wegner. 1979. Hydrolysis of cellulose: mechanisms of enzymatic and acid catalysis. Adv. Chem. Ser. 181:145-158. [Google Scholar]
- 12.Fosnaugh, K. L., D. Fuller, and W. F. Loomis. 1995. Structural roles of the spore coat proteins in Dictyostelium discoideum. Dev. Biol. 166:823-825. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Yanes, B., R. B. Mandell, M. Girard, S. Henry, O. Aparicio, M. Gritzali, R. D. Brown, G. W. Erdos, and C. M. West. 1989. The spore coat of a fucosylation mutant in Dictyostelium discoideum. Dev. Biol. 133:576-587. [DOI] [PubMed] [Google Scholar]
- 14.Grimson, M. J., C. H. Haigler, and R. L. Blanton. 1996. Cellulose microfibrils, cell motility, and plasma membrane protein organization change in parallel during culmination in Dictyostelium discoideum. J. Cell Sci. 109:3079-3087. [DOI] [PubMed] [Google Scholar]
- 15.Handford, P. A., A. K. Downing, D. P. Reinhardt, and L. Y. Sakai. 2000. Fibrillin: from domain structure to supramolecular assembly. Matrix Biol. 19:457-470. [DOI] [PubMed] [Google Scholar]
- 16.Helenius, A., and M. Aebi. 2001. Intracellular functions of N-linked glycans. Science 291:2364-2369. [DOI] [PubMed] [Google Scholar]
- 17.Kohorn, B. D. 2001. WAKs; cell wall associated kinases. Curr. Opin. Cell Biol. 13:529-533. [DOI] [PubMed] [Google Scholar]
- 18.Lipke, P. N., and R. Ovalle. 1998. Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol. 180:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomis, W. F. 1971. Sensitivity of Dictyostelium discoideum to nucleic acid analogues. Exp. Cell Res. 64:484-486. [DOI] [PubMed] [Google Scholar]
- 20.Loomis, W. F. 1998. Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev. 62:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, M. 1992. Efficient induction of sporulation of Dictyostelium prespore cells by 8-bromocyclic AMP under both submerged- and shaken-culture conditions and involvement of protein kinase(s) in its action. Dev. Growth Differ. 34:263-275. [DOI] [PubMed] [Google Scholar]
- 22.McGuire, V., and S. Alexander. 1996. PsB multiprotein complex of Dictyostelium discoideum: demonstration of cellulose binding activity and order of protein subunit assembly. J. Biol. Chem. 271:14596-14603. [DOI] [PubMed] [Google Scholar]
- 23.Nuckolls, G. H., N. Osherov, W. F. Loomis, and J. A. Spudich. 1996. The Dictyostelium dual-specificity kinase splA is essential for spore differentiation. Development 122:3295-3305. [DOI] [PubMed] [Google Scholar]
- 24.Pang, K. M., M. A. Lynes, and D. A. Knecht. 1999. Variables controlling the expression level of exogenous genes in Dictyostelium. Plasmid 41:187-197. [DOI] [PubMed] [Google Scholar]
- 25.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson, D. L., and W. F. Loomis. 1992. Disruption of the sporulation-specific gene spiA in Dictyostelium discoideum leads to spore instability. Genes Dev. 6:1058-1070. [DOI] [PubMed] [Google Scholar]
- 27.Richardson, D. L., W. F. Loomis, and A. R. Kimmel. 1995. Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development 120:2891-2900. [DOI] [PubMed] [Google Scholar]
- 28.Sassi, S., M. Sweetinburgh, J. Erogul, P. Zhang, P. Teng-Umnuay, and C. M. West. 2001. Analysis of Skp1 glycosylation and nuclear enrichment in Dictyostelium. Glycobiology 11:283-295. [DOI] [PubMed] [Google Scholar]
- 29.Soderbom, F., C. Anjard, N. Iranfar, D. Fuller, and W. F. Loomis. 1999. An adenylyl cyclase that functions during late development of Dictyostelium. Development 126:5463-5471. [DOI] [PubMed] [Google Scholar]
- 30.Virdy, K. J., T. W. Sands, S. H. Kopko, S. van Es, M. Meima, P. Schaap, and D. A. Cotter. 1999. High cAMP in spores of Dictyostelium discoideum: association with spore dormancy and inhibition of germination. Microbiology 145:1883-1890. [DOI] [PubMed] [Google Scholar]
- 31.Wang, J., L. Hou, D. Awrey, W. F. Loomis, R. A. Firtel, and C.-H. Siu. 2000. The membrane glycoprotein gp150 is encoded by the lagC gene and mediates cell-cell adhesion by heterophilic binding during Dictyostelium development. Dev. Biol. 227:734-745. [DOI] [PubMed] [Google Scholar]
- 32.Wang, N., F. Soderbom, C. Anjard, G. Shaulsky, and W. F. Loomis. 1999. SDF-2 induction of terminal differentiation in Dictyostelium discoideum is mediated by the membrane-spanning sensor kinase DhkA. Mol. Cell. Biol. 19:4750-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West, C. M., and W. F. Loomis. 1985. Absence of a carbohydrate modification does not affect the level of subcellular localization of three membrane glycoproteins in modB mutants of Dictyostelium discoideum. J. Biol. Chem. 260:13801-13809. [PubMed] [Google Scholar]
- 34.West, C. M., and G. W. Erdos. 1990. Formation of the Dictyostelium spore coat. Dev. Genet. 11:492-506. [DOI] [PubMed] [Google Scholar]
- 35.West, C. M., and G. W. Erdos. 1992. Incorporation of protein into spore coats is not cell-autonomous in Dictyostelium. J. Cell Biol. 116:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West, C. M., J. Mao, H. van der Wel, G. W. Erdos, and Y. Zhang. 1996. SP75 is encoded by the DP87 gene, and belongs to a family of modular Dictyostelium outer layer spore coat proteins. Microbiology 142:2227-2243. [DOI] [PubMed] [Google Scholar]
- 37.Zachara, N. E., N. H. Packer, M. D. Temple, M. B. Slade, D. R. Jardine, P. Karuso, C. J. Moss, B. C. Mabbutt, P. M. G. Curmi, K. L. Williams, and A. A. Gooley. 1996. Recombinant prespore-specific antigen from Dictyostelium discoideum is a β-sheet glycoprotein with a spacer peptide modified by O-linked N-acetylglucosamine. Eur. J. Biochem. 238:511-518. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, P., A. C. McGlynn, W. F. Loomis, R. L. Blanton, and C. M. West. 2001. Spore coat formation and timely sporulation depend on cellulose in Dictyostelium. Differentation 67:72-79. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Y., R. D. Brown, and C. M. West. 1998. Two proteins of the Dictyostelium spore coat bind to cellulose in vitro. Biochemistry 37:10766-10779. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y., P. Zhang, and C. M. West. 1999. A linking function for the cellulose-binding protein SP85 in the spore coat of Dictyostelium discoideum. J. Cell Sci. 112:4667-4677. [DOI] [PubMed] [Google Scholar]
- 41.Zinda, M. J., and C. K. Singleton. 1998. The hybrid histidine kinase dhkB regulates spore germination in Dictyostelium discoideum. Dev. Biol. 196:171-183. [DOI] [PubMed] [Google Scholar]