Abstract
Ovarian failure (OF) at age <40 years occurs in ∼1% of all women. Other than karyotype abnormalities, very few genes are known to be associated with this ovarian dysfunction. We studied eight patients who presented with premature OF and white-matter abnormalities on magnetic resonance imaging. Neurological signs may be absent or present after OF. In seven patients, we report for the first time mutations in three of the five EIF2B genes (EIF2B2, -4, and -5) that were recently shown to cause childhood ataxia with central nervous system hypomyelination/vanishing white-matter disease leukodystrophy. The correlation we observed between the age at onset of the neurological deterioration and the severity of OF suggests a common pathophysiological pathway.
Ovarian failure (OF) can be expressed as primary amenorrhea or as secondary amenorrhea lasting >6 mo, associated with elevated gonadotrophin levels at age <40 years. Premature OF affects 1% of all women and occurs in 0.1% at age <30 years (Coulam et al. 1986). OF has been associated with karyotype abnormalities, including various X chromosome aberrations (POFX [MIM 31360]; Shelling 2000), such as Turner syndrome, which causes depletion of ovarian follicles during development. Although such conditions as autoimmune diseases or diabetes mellitus are also associated with OF, the cause is unknown in ∼95% of cases. However, since many affected women have a family history of the condition, predisposition to OF may be inherited (Conway 1997). To date, mutations associated with OF have been identified in a small number of genes (Schlessinger et al. 2002), including those that encode the inhibin alpha (INHA [MIM 147380]) (Shelling et al. 2000), the follicle-stimulating hormone receptor (FSHR; MIM 136435) (Aittomäki et al. 1995), the luteinizing hormone/choriogonadotrophin receptor (LHCGR [MIM 152790]) (Latronico et al. 1996), and the forkhead transcription factor 2 (FOXL2 [MIM 605597]) (Harris et al. 2002). In FOXL2 mutations, OF is associated with blepharophimosis-ptosis-epicanthus inversus syndrome (BPES [MIM 110100]) (Harris et al. 2002). Mutations in these various genes are present in <10% of patients with OF (Harris et al. 2002).
Elsewhere, we have described four patients with the unusual association of OF with white-matter abnormalities observed on cerebral magnetic resonance imaging (MRI), and we have termed the condition “ovarioleukodystrophy” (Schiffmann et al. 1997). Similarities of the cerebral abnormalities to those in patients with childhood ataxia with CNS hypomyelination (CACH)/vanishing white-matter leukodystrophy (VWM [OMIM 603896]; Schiffmann et al. 1994; van der Knaap et al. 1997) led us to test eight patients with ovarioleukodystrophy for mutations in the five subunits (α–ɛ) of the eukaryotic translation initiation factor 2B (eIF2B), which was recently found to be mutated in CACH/VWM (Leegwater et al. 2001; van der Knaap et al. 2002).
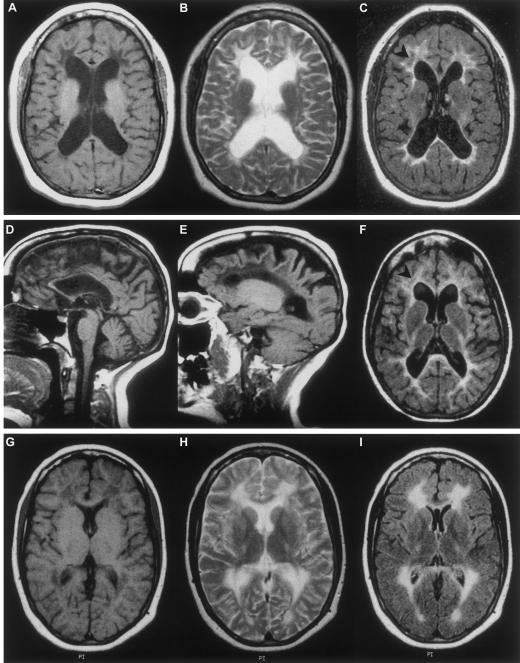
Eight patients from seven families were identified on the basis of unusual association of OF with white-matter abnormalities. The diagnosis of OF has been confirmed by findings of high basal gonadotrophin levels and low estrogen and progesterone levels. All the patients had a normal karyotype. Clinical characteristics of patients are reported in table 1. Only patient 871 had consanguineous parents (first cousins from Algeria). In the three patients with primary amenorrhea (941-1, 992, and 943), school difficulties, together with poor fine motor performance, were present prior to the development of a slowly progressive neurological disease in adolescence. Only patient 943 presented with rapid cognitive decline, including a frontal lobe syndrome. The age at menarche was normal in the five patients with secondary amenorrhea (941-2, 944, 871, 948, and 1014). Two patients (941-2 and 944), who never acquired regular menstrual cycles, had school difficulties and developed progressive motor deterioration after adolescence. In contrast, three other patients (871, 948, and 1014), who had regular menstrual cycles, attended high school with no difficulty; two presented with neurological dysfunction in their 30s (patients 871 and 948), and the third (patient 1014) had normal neurological and cognitive functions when recurrent headaches at 16 years of age led to the incidental discovery of white-matter abnormalities during cerebral MRI. In all instances, except patient 943, an abnormal diffuse signal in the hemispheric white matter was observed during MRI (fig. 1). Six patients (941-1, 941-2, 944, 992, 871, and 948) had abnormal signal (similar to that found in cerebrospinal fluid) within the abnormal white matter when tested by use of fluid-attenuated inversion recovery (FLAIR) sequence imaging. These abnormalities were identical to those seen in patients with CACH/VWM, although the cavitation process was less extensive than in the typical form of CACH/VWM and was restricted to the frontal region in four patients (941-1, 941-2, 944, and 948) (fig. 1). In contrast to the typical form of CACH/VWM, atrophy was observed in the cerebral cortex, hemispheric white matter, and corpus callosum; atrophy extended to the brain stem or the cerebellum in two patients (941-1 and 941-2) (fig. 1). The MRI abnormalities did not significantly change during the 8-year follow-up of patients 941-1, 941-2, and 992.
Table 1.
Clinical Characteristics of Patients and Mutations Detected in EIF2B2, EIF2B4, and EIF2B5[Note]
|
Patient Number |
||||||||
| Characteristic | 941-1a | 941-2a | 944 | 992 | 943a | 871 | 948 | 1014 |
| Age at examination | 23 | 24 | 33 | 16 | 29 | 31 | 48 | 16 |
| Development: | ||||||||
| Walking at age (mo) | 18 | 12 | 12 | 12 | 12 | 12 | 17 | 12 |
| Speech | Delayed | Normal | Normal | Normal | Delayed | Normal | Normal | Normal |
| Education | DS | DS | DS | DS | DS | HS graduate | HS teacher | HS student |
| Ovarian function: | ||||||||
| Menarche at age | NA | 16b | 14c | NA | NA | 13d | 14 | 14 |
| Amenorrhea at age | NA | NA | 26 | NA | NA | 27 | 31 | 16 |
| Size of ovariese | Atrophied | Atrophied | Atrophied | ND | Atrophied | ND | Normal | ND |
| Neurologic signs: | ||||||||
| Spasticity | + | + | + | ++ | − | + | + | − |
| Gait instability at age | 10 | 15 | 25 | 10 | NO | 27 | 30 | NO |
| Walker use at age | NO | 16 | 30 | 16 | NO | NO | NO | NO |
| Dysarthria at age | NO | 20 | NO | 14 | NO | NO | NO | NO |
| Sphincter dysfunction at age | NO | 20 | 26 | NO | NO | NO | 30 | NO |
| Optic atrophy | + | + | + | − | − | − | − | − |
| Other | +f | − | − | − | +g | − | − | − |
| Acute episodes at age | NO | NO | 28 (visual loss) | NO | NO | 31 (recurrent headaches) | 3 (simple seizures) | NO |
| Cognitive function | IQ = 77 at age 15; IQ = 79 at age 23; VMD; speed processing | IQ = 60 at age 16; IQ = 66 at age 24; VMD; attention deficit | IQ = 60 at age 29; IQ= 65 at age 30; VMD; poor executive function | IQ not evaluated; no deterioration | IQ = 80 at age 6; IQ = 52 at age 28; frontal lobe syndrome | IQ not evaluated; slow with anxiety and depression at age 27 | Mini mental status = 21/30 at age 45 | IQ = 96 at age 16 |
| Alive | Yes | Yes | Yes | Yes | Yes | Noh | Yes | Yes |
| Mutated gene | EIF2B4 | EIF2B4 | EIF2B2 | EIF2B2 | NI | EIF2B5 | EIF2B5 | EIF2B5 |
| Nucleotide changesi | C1393T, C1465T | C1393T, C1465T | C512T, 607-12del/insTG | C547T, A638G | NI | G338A, G338A | G338A, G338A | G338A, C583T |
| Amino acid changesj | C465R, Y489H | C465R, Y489H | S171F, M203fs | R183stop, E213G | NI | R113H, R113H | R113H, R113H | R113H, R195C |
Note.— Age is shown in years, except as otherwise noted. + = spasticity; ++ = severe spasticity; − = none; DS = difficulties in school; fs = frameshift; HS = high school; NA = not applicable; ND = not determined; NI = not identified; NO = not observed; VMD = visual motor difficulties.
Three patients were reported elsewhere (Schiffmann et al. 1997); patient 941-1 reported as patient 1, patient 941-2 as patient 2, and patient 943 as patient 3.
Patient 941-1 had irregular menses at ∼6-mo intervals.
Patient 944 had with irregular menses and was treated with a combination of estrogen and progesterone.
Patient 871 had a first-trimester spontaneous abortion at age 22 years.
Ovaries observed by use of pelvic ultrasonography.
Patient 941-1 experienced fine motor difficulties at age 23 years.
Patient 943 experienced left facial weakness.
Patient 871 died of status epilepticus at age 32 years.
For the nucleotide changes, numbering starts with A of ATG start codon.
For the amino acid changes, numbering starts with first methionine. R113H = mutation of arginine to histidine.
Figure 1.
Cerebral MRI of affected patients. Images of patients 941-2 (A, B, and C), 944 (D, E, and F), and 1014 (G, H, and I), obtained when patients were, respectively, 24, 33, and 16 years of age. In the hemispheric white matter, the following anomalies were observed: an abnormal diffuse signal, characterized by a decreased signal on T1 weighted sequence imaging (A, D, E, and G) and an increased signal on T2 (B and H) and on fluid attenuated inversion recovery (FLAIR) sequences imaging (C, F, and I). Patients 941-2 and 944, who had early school difficulties, have cerebral atrophy involving the cerebral cortex, hemispheric white matter (with ventricular dilatation), and corpus callosum. Abnormal signal within the abnormal white matter on FLAIR sequence imaging is extensive in the frontal white matter of patient 941-2 (C, arrowhead), limited in patient 944 (F, arrowhead), and absent in patient 1014, who experienced secondary amenorrhea and episodes of headache but had normal results on neurological examination at age 16 years.
The similarities of the cerebral MRI abnormalities observed in our patients with OF to those in patients with CACH/VWM led us to test the five EIF2B genes recently found to be mutated in patients with CACH/VWM (EIF2B1 [GenBank accession number NM 001414], EIF2B2 [MIM 606454; GenBank accession number NM 014239; region 14q24.3], EIF2B3 [GenBank accession number NM 020365], EIF2B4 [MIM 606687; GenBank accession number NM 015636; region 2p23], and EIF2B5 [MIM 603945; GenBank accession number XM 029136; region 3q28]) (Leegwater et al. 2001; van der Knaap et al. 2002). We sequenced all the coding regions of the five EIF2B genes (including 39–173 nt of the intronic regions) in these eight affected patients, as described elsewhere (van der Knaap et al. 2002; modified primer sequences available upon request). All the identified mutations are reported in table 1. Mutations were found in EIF2B genes in all the patients except patient 943: four mutations were in the EIF2B2 gene (patients 944 and 992); two were in the EIF2B4 gene (patients 941-1 and 941-2, who are sisters); and two were in the EIF2B5 gene (patients 948, 871, and 1014). These mutations were not found in a control group composed of 320 chromosomes of individuals from the same populations, which were of northern European and North African descent. Patients 871 and 948 had an R113H homozygous missense mutation in the ɛ subunit of eIF2B. In the four other families, patients were compound heterozygotes for different mutations: six were missense mutations (including one patient with the R113H mutation), and one was a frameshift mutation.
The eIF2B protein is composed of five subunits (α–ε) and converts the protein synthesis initiation factor 2 (eIF2) from an inactive GDP-bound form to an active eIF2-GTP complex (Gomez and Pavitt 2000), allowing the formation of the 43S complex, precursor of the protein translation initiation. Although eIF2B is a ubiquitous protein, its mutations were previously described as affecting only the brain white matter. In the present study, we report OF due to eIF2B mutations in seven patients. The only patient without identified EIF2B mutations (patient 943) had a distinctive neurological presentation that included cognitive deterioration without motor signs and with white-matter abnormalities restricted to the frontal lobes. Further analyses are needed to rule out mutations in the intronic and regulatory regions of the five EIF2B genes. However, the distinctive neurological presentations suggest that another gene could be involved in this patient’s OF.
The seven patients with OF and eIF2B mutations have diffuse white-matter abnormalities, which are detected by use of cerebral MRI. Six developed neurological deterioration with progressive urinary, speech, and gait disturbances. The age at onset of the neurological deterioration correlated positively with the severity of the ovarian dysfunction, and OF can precede the neurological decline, as was observed in patient 948. Further analyses are needed to determine if EIF2B genes mutations are responsible for a significant percentage of apparently isolated OF.
For EIF2B2 and EIF2B4, the combinations of heterozygous mutations found in our patients with ovarioleukodystrophy were different from those described in a pool of patients with CACH/VWM (van der Knaap et al. 2002). Five mutations have not been reported before: S171F and R183stop (EIF2B2), C465R and Y489H (EIF2B4), and R195C (EIF2B5) (table 1).
Three of the six families with OF carry an R113H mutation in the ɛ subunit of eIF2B, which is the guanine nucleotide exchange factor of the protein. Patients from these families have the mildest forms of the disease. The R113H mutation in EIF2B5 has also been found in 22% of chromosomes of 41 patients with CACH/VWM (Leegwater et al. 2001). Because the arginine at position 113 is not conserved among species and because histidine is found at this position in the rat and mouse, the homozygous R113H mutation observed in humans (patients 871 and 948) may not strongly affect the eIF2Bɛ protein function. The third patient (1014), who had early secondary amenorrhea, is a compound heterozygote of R113H and an unreported R195C mutation. Since the arginine at position 195 of the ɛ subunit of eIF2B is highly conserved among species (from yeast to humans), its substitution by cytidine may more profoundly disturb the function of this protein. We recently found that individuals from two indigenous North American populations, the Cree and the Chippewa, have a particularly severe form of leukodystrophy and are homozygous for a mutation at the same arginine 195 residue but substituted by histidine (R195H) (Fogli et al. 2002). In this severe form of the eIF2B mutation, as well as in the classical form of CACH/VWM, patients do not survive to puberty and therefore do not express OF. However, several reports in the literature suggest that ovarian dysgenesis may be present in these patients. Two children with neuropathological abnormalities suggestive of CACH/VWM were also found at autopsy to have “ovarian dysgenesis” (Boltshauser et al. 2002) or “bilateral streak ovaries” (van der Knaap 1997). In addition, OF was reported in two sisters who presented with primary amenorrhea and behavior problems at ages >30 years, with subsequent neurological deterioration, white-matter abnormalities (detected during cerebral MRI), and pigmentary orthochromatic leukodystrophy (observed at autopsy) (Verghese et al. 2002).
The upper limit of reproductive life span is predetermined at birth, because all of the follicles that a woman will have in adult life are formed in utero. The number of primordial follicles reaches a maximum of 7 million at 5 mo gestation, whereas it declines to ∼2 million at birth and to ∼400,000 at puberty (Christin-Maitre et al. 1998). That pool supplies the ovulatory follicles after menarche and is depleted until it falls below a threshold value of 1,000 follicles, and menopause ensues (Faddy 2000). Therefore, >99% of the follicles from the initial pool will undergo atresia, with apoptosis being the most important phenomenon controlling this atresia (Christin-Maitre et al. 1998). Premature OF could result from a decrease in the number of follicles formed, an increase in the rate of follicle loss, an alteration in the recruitment of the follicle, or an interruption in the maturation of the follicle. In BPES due to FOXL2 mutation, the ovarian dysfunction and the eyelid phenotype can be explained by the restricted expression of the mutated FOXL2 gene in these cell types during development (Crisponi et al. 2001). Although eIF2B is a ubiquitous protein, its mutations, for still unexplained reasons, seem to affect only the CNS and ovary in the patients we have reported here. The correlation observed between the age at onset of the neurological deterioration and the severity of OF suggests a common pathophysiological pathway. The potential role of eIF2B in the formation of follicles and glial cells has not yet been reported. In both tissues, successive programmed cell death occurs during development, and defects in regulation of intracellular death effectors, such as caspase-2, can affect both tissues (Bergeron et al. 1998). The role of eIF2B in apoptosis is presently unknown. However, in the brains of patients with CACH/VWM, apoptosis of mature oligodendrocytes, as determined by use of morphological criteria, has been reported only once (Brück et al. 2001), whereas an increase in the number of mature oligodendocytes that have foamy aspects but lack apoptotic features has been frequently reported (Rodriguez et al. 1999; Wong et al. 2000). In addition, in a particularly severe form of CACH/VWM, no abnormalities have been found by use of TUNEL and P53 labeling (Francalanci et al. 2001). eIF2B is an important regulatory pathway for the prevention of synthesis of denatured proteins during cellular stress, and it functions in parallel with the production of heat shock proteins (Proud et al. 2002). In patients with eIF2B mutations, a high susceptibility to cellular stress is suggested by the acute phase of neurological deterioration observed after minor head trauma or common viral infections. Knockout mice for the heat shock factor 2 (Hsf2), a transcriptional regulator of heat shock gene expression, are infertile because of the increased apoptosis of ovarian follicles that is associated with brain abnormalities, an observation suggestive of defects in glial cell development (Kallio et al. 2002). Similarly, eIF2B dysfunction in humans may be responsible for increased apoptosis of ovarian follicles (leading to OF) and for a defect in glial cell development (causing abnormal formation of white-matter structures). This abnormal CNS development would increase susceptibility of eIF2B-mutated cells to cellular stress, with subsequent progressive neurological deterioration and white-matter cavitation. Estrogen deprivation due to OF may accelerate this deterioration by increasing the vulnerability of neurons to injury (Liu et al. 2001).
In conclusion, we report an association of white-matter disease with OF in seven patients with mutations in the EIF2B genes. Further investigation may show that defects in this gene are the basis of some cases of OF of unknown cause. The correlation we observed between the age at onset of the neurological deterioration and the severity of the OF suggests a common pathophysiological pathway in these two tissue types that is mediated by eIF2B and result from dysgenesis during development and/or abnormal response to stress in the adult.
Acknowledgments
We gratefully acknowledge the participation of the patients’ families and the patient referral by Dr. Mark Lipson. We thank Dr. S. Matsuzaki for her comments on this manuscript. We also thank P. Combes, F. Gauthier, and G. Giraud for technical help in processing blood samples and in sequencing. This work was supported by grants from the European Leukodystrophy Association, the Fondation pour la Recherche Médicale (grant ARS 2000), and the Jean Pierre and Nancy Boespflug Myopathic Research Foundation.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human EIF2B1 mRNA [accession number NM 001414], EIF2B2 mRNA [accession number NM 014239], EIF2B3 mRNA [accession number NM 020365], EIF2B4 mRNA [accession number NM 015636] , and EIF2B5 mRNA [accession number XM 029136])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CACH/WVM, XPOF, BPES, EIF2B1, EIF2B2, EIF2B3, EIF2B4, EIF2B5, INHA, FSHR, FOXL2, and LHCGR)
References
- Attomäki K, Lucena JL, Pakarinen P, Sistonen P Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lahvaslaiho H, Engel AR (1995) Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82:959–968 [DOI] [PubMed] [Google Scholar]
- Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JCM, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J (1998) Defects in regulation of apoptosis in caspase-2–deficient mice. Genes Dev 12:1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltshauser E, Barth PG, Troost D, Martin E, Stallmach T (2002) “vanishing white matter” and ovarian dysgenesis in an infant with cerebro-oculo-facio-skeletal phenotype. Neuropediatrics 33:57–62 [DOI] [PubMed] [Google Scholar]
- Brück W, Herms J, Brockmann K, Schulz-Schaeffer W, Hanefeld F (2001) Myelinopathia centralis diffusa (VWM disease): evidence of apoptotic oligodendrocyte degeneration in early lesion development. Ann Neurol 50:532–536 [DOI] [PubMed] [Google Scholar]
- Christin-Maitre S, Vasseur C, Portnoi MF, Bouchard P (1998) Genes and premature ovarian failure. Mol Cell Endocrinol 145:75–80 [DOI] [PubMed] [Google Scholar]
- Conway GS (1997) Premature ovarian failure. Curr Opin Obstet Gynecol 9:202–206 [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF (1986) Incidence of premature ovarian failure. Obstet Gynecol 67:604–606 [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- Faddy MJ (2000) Follicle dynamics during ovarian ageing. Mol Cell Endocrinol 163:43–48 [DOI] [PubMed] [Google Scholar]
- Fogli A, Wong K, Eymard-Pierre E, Wenger J, Bouffard JP, Goldin E, Black DN, Boespflug-Tanguy O, Schiffmann R (2002) Cree leukoencephalopathy and CACH/VWM disease are allelic at the EIF2B5 locus. Ann Neurol 52:506–510 [DOI] [PubMed] [Google Scholar]
- Francalanci P, Eymard-Pierre E, Dionisi-Vici C, Boldrini R, Piemonte F, Virgili R, Fariello G, Bosman C, Santorelli FM, Boespflug-Tanguy O, Bertini E (2001) Fatal infantile leukodystrophy, a severe variant of CACH/VWM syndrome, allelic to chromosome 3q27. Neurology 57:265–270 [DOI] [PubMed] [Google Scholar]
- Gomez E, Pavitt GD (2000) Identification of domains and residues within the ε subunit of eukaryotic translation initiation factor 2B (eIF2Bε) required for guanine nucleotide exchange reveals a novel activation function promoted by eIF2B complex formation. Mol Cell Biol 20:3965–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Chand AL, Winship IM, Gersak K, Aittomäki K, Shelling AN (2002) Identification of novel mutations in FOXL2 associated with premature ovarian failure. Mol Hum Reprod 8:729–733 [DOI] [PubMed] [Google Scholar]
- Kallio M, Chang Y, Manuel M, Alastalo TP, Rallu M, Gitton Y, Pirkkala L, Loones MT, Paslaru L, Larney S, Hiard S, Morange M, Sistonen L, Mezger V (2002) Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J 21:2591–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico AC, Anasti J, Arnhold IJ, Rapaport R, Mendonca BB, Bloise W, Castro M, Tsigos C, Chrousos GP (1996) Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone-receptor gene. N Engl J Med 334:507–512 [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Vermeulen G, Konst AA, Naidu S, Mulders J, Visser A, Kersbergen P, Mobach D, Fonds D, van Berkel CG, Lemmers RJ, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS (2001) Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet 29:383–388 [DOI] [PubMed] [Google Scholar]
- Liu Z, Gastard M, Verina T, Bora S, Mouton PR, Koliatsos VE (2001) Estrogens modulate experimentally induced apoptosis of granule cells in the adult hippocampus. J Comp Neurol 441:1–8 [DOI] [PubMed] [Google Scholar]
- Proud CG (2001) Regulation of eukaryotic initiation factor eIF2B. Prog Mol Subcell Biol 26:95–114 [DOI] [PubMed] [Google Scholar]
- Rodriguez D, Gelot A, della Gaspera B, Robain O, Ponsot G, Sarliève LL, Ghandour S, Pompidou A, Dautigny A, Aubourg P, Pham-Dinh D (1999) Increased density of oligodendrocytes in childhood ataxia with diffuse central hypomyelination (CACH) syndrome: neuropathological and biochemical study of two cases. Acta Neuropathol 97:469–480 [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Moller JR, Trapp BD, Shih HH, Farrer RG, Katz DA, Alger JR, et al (1994) Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol 35:331–340 [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Tedeschi G, Kinkel RP, Trapp BD, Frank JA, Kaneski CR, Brady RO, Barton NW, Nelson L, Yanovski JA (1997) Leukodystrophy in patients with ovarian dysgenesis. Ann Neurol 41:654–661 [DOI] [PubMed] [Google Scholar]
- Schlessinger D, Herrera L, Crisponi L, Mumm S, Percesepe A, Pellegrini M, Pilia G, Forabosco A (2002) Genes and translocations involved in POF. Am J Med Genet 111:328–333 [DOI] [PubMed] [Google Scholar]
- Shelling AN (2000) X chromosome defects and premature ovarian failure. Aust N Z J Med 30:5–7 [DOI] [PubMed] [Google Scholar]
- Shelling AN, Burton KA, Chand AL, van Ee CC, France JT, Farquhar CM, Milsom SR, Love DR, Gersak K, Aittomaki K, Winship IM (2000) Inhibin: a candidate gene for premature ovarian failure. Hum Reprod 15:2644–2649 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Barth PG, Gabreels FJ, Franzoni E, Begeer JH, Stroink H, Rotteveel JJ, Valk J (1997) A new leukoencephalopathy with vanishing white matter. Neurology 48:845–855 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Leegwater PA, Konst AA, Visser A, Naidu S, Oudejans CB, Schutgens RB, Pronk JC (2002) Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol 51:264–270 [DOI] [PubMed] [Google Scholar]
- Verghese J, Weidenheim K, Malik S, Rapin I (2002) Adult onset pigmentary orthochromatic leukodystrophy with ovarian dysgenesis. Eur J Neurol 9:663–670 [DOI] [PubMed] [Google Scholar]
- Wong K, Armstrong RC, Gyure KA, Morrison AL, Rodriguez D, Matalon R, Johnson AB, Wollmann R, Gilbert E, Le TQ, Bradley CA, Crutchfield K, Schiffmann R (2000) Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol 100:635–646 [DOI] [PubMed] [Google Scholar]