Abstract
Deletions of the long arm of chromosome 18 occur in ∼1 in 10,000 live births. Congenital aural atresia (CAA), or narrow external auditory canals, occurs in ∼66% of all patients who have a terminal deletion 18q. The present report describes a series of 20 patients with CAA, of whom 18 had microscopically visible 18q deletions. The extent and nature of the chromosome-18 deletions were studied in detail by array-based comparative genomic hybridization (arrayCGH). High-resolution chromosome-18 profiles were obtained for all patients, and a critical region of 5 Mb that was deleted in all patients with CAA could be defined on 18q22.3-18q23. Therefore, this region can be considered as a candidate region for aural atresia. The array-based high-resolution copy-number screening enabled a refined cytogenetic diagnosis in 12 patients. Our approach appeared to be applicable to the detection of genetic mosaicisms and, in particular, to a detailed delineation of ring chromosomes. This study clearly demonstrates the power of the arrayCGH technology in high-resolution molecular karyotyping. Deletion and amplification mapping can now be performed at the submicroscopic level and will allow high-throughput definition of genomic regions harboring disease genes.
Congenital aural atresia (CAA) is a rare anomaly, occurring in ∼1 in 10,000 live births (Melnick et al. 1979). CAA phenotypes may vary from mild symptoms (with narrowing of the external auditory canal and hypoplasia of the tympanic membrane and middle ear cavity) to severe symptoms (including the entire absence of the middle ear in combination with anotia, bony atresia of the external auditory canal, and hypoplasia of inner ear structures) (Cremers et al. 1988; Strathdee et al. 1995).
CAA has been reported frequently in patients with chromosomal anomalies, especially deletions on the long arm of chromosome 18 (18q) (de Grouchy et al. 1964; Kline et al. 1993; Keppler-Noreuil et al. 1998; Cody et al. 1999). The exact region on 18q that is involved in CAA is unclear, as most patients have terminal deletions starting at 18q23 (Schinzel 2001), but also patients with interstitial deletions of 18q21 or 18q22 have been reported (Wilson et al. 1979; Schinzel et al. 1991). In the present study, we employed arrayCGH to define a CAA-associated shortest region of deletion overlap on chromosome 18.
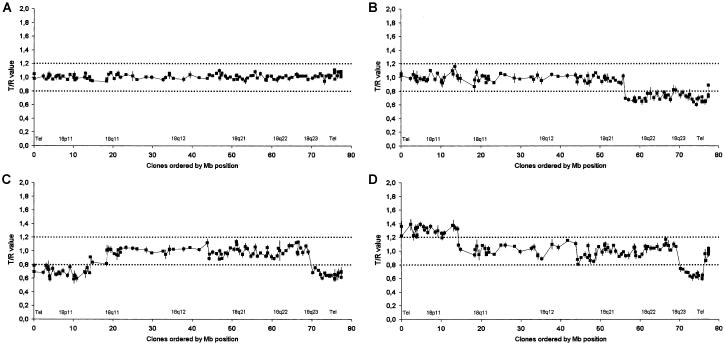
For this purpose, a high resolution chromosome-18 array was constructed using DOP-PCR (Telenius et al. 1992) products from 114 positionally selected and commercially obtained (BACPAC Resources) chromosome-18 BAC clones, covering the chromosome with an average spacing of one clone per 670 kb. The average clone-insert size is 175 kb, which results in a total chromosome-18 coverage of 26%. The chromosome-18 copy-number profile of 20 patients with CAA (18 with microscopically visible 18q deletions [Nuijten et al., in press]) was established through hybridization of the arrays with cy3-labeled patient-derived DNAs combined with cy5-labeled control-derived DNAs. After scanning, fluorescence ratios were determined for each clone. Ratios indicating copy-number gains or losses were compared with the original cytogenetic data on the basis of karyotyping (figs. 1 and 2; table 1). On the basis of the control experiments and work published elsewhere (Veltman et al. 2002; Wilhelm et al. 2002), thresholds for copy-number gain and loss were set at 1.2 and 0.8, respectively. As a representative example, figure 1B shows the arrayCGH profile of patient 5, with karyotype:46,XY,del(18)(q21.31). A normal intensity ratio was observed for all clones mapping from 18pter to 18q21.31, whereas ratios <0.8 were consistently observed from 18q21.31-18qter.
Figure 1.
Chromosome-18 profiling in patients with CAA by arrayCGH. Arrays contained 114 cloned chromosome-18 genomic DNA targets, ordered from pter to qter on the basis of physical mapping positions obtained from the June 2002 freeze of the UCSC genome browser. Dark squares represent normalized T/R ratios for a normal control subject (A). Patient 5 (46,XY,del[18][q21.31]) (B), patient 17 (46,XY,ring[18][p11q23]) (C), and patient 18 (46,XX,der[7]t[7;18][q36.1;q23],der[18][pter→q23::p11.2→pter]) (D) versus reference hybridization. Vertical lines represent SDs for each target clone in the control hybridizations. Dark dotted horizontal lines indicate the thresholds for copy-number loss (0.8) and gain (1.2).
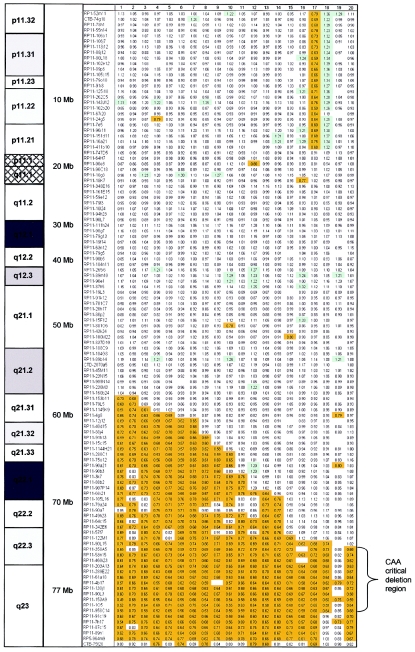
Figure 2.
Overview of all arrayCGH intensity ratios for the 20 patients with CAA. Arrays contained 114 cloned chromosome-18 genomic DNA targets, ordered from pter to qter on the basis of physical mapping positions obtained from the June 2002 freeze of the UCSC genome browser. Ratios above the threshold of copy-number gain (1.2) are color coded in light green; ratios below the threshold of copy-number loss (0.8) are color coded in orange. Note the deletion zone on the q arm of chromosome 18, with a minimal region of deletion present in 19 of the 20 patients in the region on 18q22.3-18q23. Mosaicism for the 18q deletion was present in patients 2 and 19. No intensity ratio is depicted if a clone did not pass the inclusion criteria in a particular experiment.
Table 1.
Patients with CAA, Karyotypes, and Results of Chromosome-18 Profiling by ArrayCGH
| Patient | CAAa | Conventional Karyotype | ArrayCGH-Based Karyotype |
| 1 | NAC | 46,XY,del(18)(q21.2) | del(18)(q21.31)b |
| 2 | NAC | 46,XY,del(18)(q21.3) in 75% of cells | del(18)(q21.31)b |
| 3 | NAC | 46,XX,del(18)(q21.31) | del(18)(q21.31) |
| 4 | NAC | 46,XX,del(18)(q21.31) | del(18)(q21.31) |
| 5 | NAC | 46,XY,del(18)(q21.31) | del(18)(q21.31) |
| 6 | MA | 46,XY,del(18)(q21.31) | del(18)(q21.31) |
| 7 | NAC | 46,XY,r(18)(p11q21.31) | del(18)(q21.31) |
| 8 | NAC | 46,XX,del(18)(q21.3) | del(18)(q21.32)b |
| 9 | MA | 46,XX,r(18)(pterq23) | del(18)(q21.32)b |
| 10 | NAC | 46,XY,del(18)(q21.3) | del(18)(q21.33)b |
| 11 | NAC | 46,XY,del(18)(q22) | del(18)(q22.1)b |
| 12 | MA | 46,XX,del(18)(q22.1) | del(18)(q22.1) |
| 13 | NAC | 46,XX,del(18)(q22.3) | del(18)(q22.1)b |
| 14 | NAC | 46,XX,del(18)(q22.1) | del(18)(q22.1) |
| 15 | NAC | 46,X,del(X)(q21.2), der(18)t(X :18)(q21.2;q22) | del(18)(q22.3)b |
| 16 | MA | 46,XY,del(18)(q22.2) | del(18)(q22.3)b |
| 17 | NAC | 46,XX,r(18)(p11q23) | del(18)(p11.21) + del(18)(q22.3)b |
| 18 | MA | 46,XY,der(7)t(7;18)(q36.1;q23),der(18)(pter→q23::p11.2→ pter) | gain(18)(p11.21) + del(18)(q22.3q23)b |
| 19 | MA | 46,XY,del(18)(q21.3) in 33% of cells | No copy-number change detected |
| 20 | MA | 46,XXc | del(18)(q22.3)b |
NAC = narrow external auditory canals; MA = meatus atresia.
Cases in which arrayCGH refined the initial cytogenetic diagnosis.
Julia et al. 2002.
In 19 of the 20 patients tested, copy-number losses involving part of the q arm were identified (mean fluorescence ratio of the deleted region 0.68; for overview of the results see table 1 and fig. 2). The size of the deletion varied considerably in these cases, with the largest deletion encompassing 44 clones mapping to 18q21.31-18qter (23-Mb deletion, patient 1) and the smallest deletion encompassing 13 clones mapping to 18q22.3-18q23 (6-Mb deletion, patient 18). In two patients, copy-number alterations were observed on the p arm as well, in agreement with the cytogenetic diagnosis; patient 17 carries a ring chromosome involving part of the p arm, and patient 18 carries a complex translocation involving a duplication of 18p11-pter.
The arrayCGH approach, in most cases, resulted in a more precise delineation of the deleted region, and, therefore, the cytogenetic diagnosis could be refined in 12 of the 20 patients with CAA (table 1): 7 patients with a terminal deletion, 2 patients with a ring chromosome, 2 patients with a translocation, and 1 patient with a previously unidentified submicroscopic terminal deletion. This study illustrates the added value of the arrayCGH technology for studying the genomic content of, especially, ring chromosomes and complex translocations.
In patient 18, an interstitial 18q22.3-18q23 deletion was identified by arrayCGH, which was not observed in the original karyotypic analysis. This subject with a complex translocation between chromosomes 7 and 18 was previously studied using routine karyotyping and FISH with probes for 7qter, 18pter, and 18qter, as well as whole chromosome arm paints for 18p and 18q. On the basis of these analyses, the following karyotype was established: 46,XY,der(7)t(7;18)(q36.1;q23),der(18)(pter→q23:p11.2→pter). However, since this patient had a meatus atresia, a deletion directly flanking the 18q breakpoint was suspected and, subsequently, proven in the present study by arrayCGH. Consequently, the karyotype has been adjusted to 46,XY,der(7)t(7;18)(q36.1;q23),der(18)(pter→q22.3::p11.2→pter), with a deletion of 18q22.3q23 and a duplication of 18p11.21pter. Similarly, in patient 20, a terminal deletion of 18q22.3 was identified by arrayCGH, whereas no cytogenetic abnormalities were observed in the original karyotypic analysis. Subject 20 was presented in 2002 as a patient with Rasmussen syndrome: a combination of external auditory canal atresia, club feet, and hypertelorism (Julia et al. 2002). This patient had many features in common with patients with a terminal deletion of 18q; besides CAA, hypertelorism, and club feet, she had a low implantation of the thumbs and a typical shape of the ear with prominent crus helix. The latter is very typical for patients with a terminal 18q deletion. Of note, the girl exhibited a normal mental development. She had the smallest terminal deletion of all cases in our series. Since the deletion was not seen by routine karyotyping, an unbalanced cryptic translocation should be excluded. At this writing, the parents are under investigation to test for the possible presence of a balanced cryptic translocation in either of them.
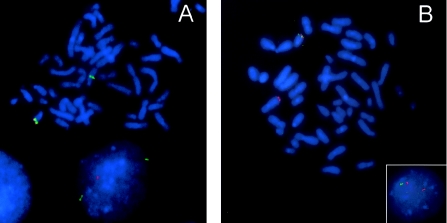
Patient 18 was the only case with an interstitial 18q deletion as observed by arrayCGH. All other cases showed terminal 18q deletions. The presence of an interstitial deletion and one normal copy of the terminal part of 18q in this patient was validated by FISH, by use of clones also present on the array (fig. 3). FISH validation was also performed for patient 15, with a translocation involving chromosomes 18 and X. In both cases, the arrayCGH findings were independently confirmed.
Figure 3.
FISH validation experiments. FISH validation experiments were performed for patients 15 (A) and 18 (B). The original karyotype of patient 15 is 46,X,del(X)(q21.2),der(18)t(X:18)(q21.1;q22), and that of patient 18 is 46,XY,der(7)t(7;18)(q36.1;q23),der(18)(pter-q23::p11.2-pter). A, The deleted 18q22.3 clone RP11-90L15 is labeled in red, the normal 18q21.33 clone RP11-75O12 in green. The normal chromosome 18 contains both clones in the correct order, whereas, on the derivative chromosome 18, only clone RP11-75O12 is present, thus confirming the unbalanced translocation as identified by both arrayCGH and karyotyping. B, The deleted 18q22.3 clone RP11-90L15 is labeled in green, the normal 18q23 clone RP11-91C19 in red. The normal chromosome 18 contains both clones in the correct order, whereas, in the derivative chromosome 7, only clone RP11-91C19 is present. The deletion is visible both in metaphase and in interphase (inset), thus confirming the unbalanced translocation as identified by arrayCGH but not by karyotyping.
In one case (patient 19), no deletion was identified by arrayCGH. Cytogenetically, however, an 18q21.3-qter deletion was observed in 33% of the cells. The mean ratio of the clones mapping to this deletion region was 0.86, and 5 clones mapping to this region showed ratios below the threshold of 0.8 (fig. 2). One other case (patient 2) with a genetic mosaicism was included in this study; in this patient, ∼75% of the cells carried the 18q deletion. This deletion could clearly be identified by arrayCGH, although the mean fluorescence ratios of the deleted clones were slightly higher than in cases with deletions in 100% of the cells (mean fluorescence ratio of deleted clones in this mosaic case was 0.72, compared with 0.68 for nonmosaic deletion cases; see fig. 2). This observation is in agreement with a study reported elsewhere dealing with the influence of normal genomic DNA contamination on the detection of single copy-number abnormalities in murine islet carcinomas by arrayCGH (Hodgson et al. 2001). In general, it can be stated that at least 50% of the cells should contain a single copy-number change to allow reliable detection by arrayCGH.
After integrating all data, one common genomic region could be defined that was deleted in all patients with CAA (fig. 2). The proximal deletion breakpoint maps between clone RP11-90L15 (70.6 Mb) and clone RP11-358A5 (71.3 Mb), whereas the distal deletion breakpoint resides between clone RP11-958C14 (76.2 Mb) and clone RP11-91C19 (77.0 Mb). This smallest region of deletion overlap has an approximate size of 5 Mb and is located on 18q22.3-18q23. This critical deletion region may be narrowed further on the basis of the work by Strathdee et al. (1997), showing a telomeric deletion with the proximal deletion breakpoint mapping between STSs D18S489 (72.6 Mb) and D18S1121 (72.9 Mb). A combination of our results with that study indicates that the minimal deletion region can be limited to <4 Mb. One or more genes related to aural atresia are likely to be located in this particular interval. There are well >40 transcribed sequences located in this 4-Mb region, including seven known protein coding genes. One of the candidate genes in this region is myelin basic protein, for which a role in the mental retardation phenotype associated with the 18q- syndrome has been postulated (Weiss et al. 1991). Other well-annotated genes include ZNF236, a glucose-regulated Kruppel-like zinc-finger gene potentially involved in diabetic nephropathy (Holmes et al. 1999); GALR1, galanin receptor-1, which belongs to a family of G protein–coupled receptors for the neuropeptide galanin (Lismaa et al. 1998); and SALL3, another zinc-finger gene of the human spaltlike gene family (Kohlhase et al. 1999).
The present study underscores the power of the arrayCGH technology, which is rapidly becoming the method of choice for high-resolution screening of genomic copy-number changes (Solinas-Toldo et al. 1997; Pinkel et al. 1998; Pollack and Iyer 2002; Veltman et al. 2002). High-resolution karyotyping identifies 19 cytogenetic bands for chromosome 18 (∼1 band every 4 Mb). In this particular chromosome-18 array, the average clone spacing was 670 kb, which resulted in a resolution that is a factor 6 higher, as compared with high-resolution karyotyping. Systematic, whole-genome deletion mapping can now be performed at the submicroscopic level, allowing the identification of microdeletions potentially harboring target disease genes, like the 5-Mb deletion on 18q22.3-18q23 described in the present study. To further narrow the critical region for CAA, we are currently screening patients with CAA without any apparent cytogenetic abnormalities on chromosome 18, as well as patients with balanced translocations affecting this region.
Acknowledgments
We thank Bert B. A. de Vries for useful discussions and reading of the manuscript. We acknowledge Lisenka E. L. M. Vissers, Saskia van der Velde-Visser, and Belinda van den Helm for expert technical assistance.
Electronic-Database Information
URLs for data presented herein are as follows:
- BACPAC Resources Center, http://www.chori.org/
- UCSC Genome Bioinformatics, http://genome.ucsc.edu/ (for the reference sequence for the human genome)
References
- Cody JD, Ghidoni PD, DuPont BR, Hale DE, Hilsenbeck SG, Stratton RF, Hoffman DS, Muller S, Schaub RL, Leach RJ, Kaye CI (1999) Congenital anomalies and anthropometry of 42 individuals with deletions of chromosome 18q. Am J Med Genet 85:455–462 [DOI] [PubMed] [Google Scholar]
- Cremers CWRJ, Teunissen E, Marres EHMA (1988) Classification of congenital aural atresia and results of reconstructive surgery. Adv Otorhinolaryngol 40:9–14 [DOI] [PubMed] [Google Scholar]
- de Grouchy J, Royer P, Salmon CH, Lamy M (1964) Délétion partielle des bras longs du chromosome 18. Pathol Biol 12:579–582 [PubMed] [Google Scholar]
- Hodgson G, Hager JH, Volik S, Hariono S, Wernick M, Moore D, Nowak N, Albertson DG, Pinkel D, Collins C, Hanahan D, Gray JW (2001) Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nat Genet 29:459–464 [DOI] [PubMed] [Google Scholar]
- Holmes DI, Wahab NA, Mason RM (1999) Cloning and characterization of ZNF236, a glucose-regulated Kruppel-like zinc-finger gene mapping to human chromosome 18q22-q23. Genomics 60:105–109 [DOI] [PubMed] [Google Scholar]
- Julia S, Pedespan JM, Boudard Ph, Barbier R, Gavilan-Cellie I, Chateil JF, Lacombe D (2002) Association of external auditory canal atresia, vertical talus, and hypertelorism: confirmation of Rasmussen syndrome. Am J Med Genet 110:179–181 [DOI] [PubMed] [Google Scholar]
- Keppler-Noreuil KM, Carroll AJ, Finley SC, Descartes M, Cody JD, DuPont BR, Gay CT Leach RJ (1998) Chromosome 18q paracentric inversion in a family with mental retardation and hearing loss. Am J Med Genet 76:372–378 [DOI] [PubMed] [Google Scholar]
- Kline AD, White ME, Wapner R, Rojas K, Biesecker LG, Kamholz J, Zackai EH, Muenke M, Scott CI Jr, Overhauser J (1993) Molecular analysis of the 18q- syndrome—and correlation with phenotype. Am J Hum Genet 52:895–906 [PMC free article] [PubMed] [Google Scholar]
- Kohlhase J, Hausmann S, Stojmenovic G, Dixkens C, Bink K, Schulz-Schaeffer W, Altmann M, Engel W (1999) SALL3, a new member of the human spalt-like gene family, maps to 18q23. Genomics 62:216–222 [DOI] [PubMed] [Google Scholar]
- Lismaa TP, Fathi Z, Hort YJ, Iben LG, Dutton JL, Baker E, Sutherland GR, Shine J (1998) Structural organization and chromosomal localization of three human galanin receptor genes. Ann NY Acad Sci 863:56–63 [DOI] [PubMed] [Google Scholar]
- Melnick M, Myranthopoulos NC, Paul NW (1979) External ear malformations: epidemiology, genetics, and natural history. Birth Defects Orig Artic Ser 15:1–140 [PubMed] [Google Scholar]
- Nuijten I, Admiraal R, van Buggenhout G, Cremers C, Frijns JP, Smeets D, van Ravenswaaij-Arts C. Congenital aural atresia in 18q deletion or de Grouchy syndrome. Otol Neurotol (in press) [DOI] [PubMed] [Google Scholar]
- Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, Collins C, Kuo WL, Chen C, Zhai Y, Dairkee SH, Ljung BM, Gray JW, Albertson DG (1998) High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet 20:207–211 [DOI] [PubMed] [Google Scholar]
- Pollack JR, Iyer VR (2002) Characterizing the physical genome. Nat Genet 32:S515–S521 [DOI] [PubMed] [Google Scholar]
- Schinzel A (2001) Chromosome 18. In: Schinzel A (ed) Catalogue of unbalanced chromosome aberrations in man. de Gruyter, Berlin and New York, pp 717–782 [Google Scholar]
- Schinzel A, Binkert F, Lillington DM, Sands M, Stocks RJ, Lindenbaum RH, Matthews H, Sheridan H (1991) Interstitial deletion of the long arm of chromosome 18, del(18)(q12.2q21.1): a report of three cases of an autosomal deletion with a mild phenotype. J Med Genet 28:352–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas-Toldo S, Lampel S, Stilgenbauer S, Nickolenko J, Benner A, Dohner H, Cremer T, Lichter P (1997) Matrix-based comparative genomic hybridization: biochips to screen for genomic imbalances. Genes Chrom Cancer 20:399–407 [PubMed] [Google Scholar]
- Strathdee G, Sutherland R, Jonsson JJ, Sataloff R, Kohonen-Corish M, Grady D, Overhauser J (1997) Molecular characterization of patients with 18q23 deletions. Am J Hum Genet 60:860–868 [PMC free article] [PubMed] [Google Scholar]
- Strathdee G, Zackai EH, Shapiro R, Kamholz J, Overhauser J (1995) Analysis of clinical variation seen in patients with 18q terminal deletions. Am J Med Genet 59:476–483 [DOI] [PubMed] [Google Scholar]
- Telenius H, Carter NP, Bebb CE, Nordenskjold M, Ponder BA, Tunnacliffe A (1992) Degenerate oligonucleotide-primed PCR: general amplification of target DNA by a single degenerate primer. Genomics 13:718–725 [DOI] [PubMed] [Google Scholar]
- Veltman JA, Schoenmakers EFPM, Eussen BH, Janssen I, Merkx G, van Cleef B, van Ravenswaaij CM, Brunner HG, Smeets D, Geurts van Kessel A (2002) High-throughput analysis of subtelomeric chromosome rearrangements by use of array-based comparative genomic hybridization. Am J Hum Genet 70:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss BJ, Kamholz J, Ritter A, Zackai EH, McDonald-McGinn DM, Emanuel B, Fischbeck KH (1991) Segmental spinal muscular atrophy and dermatological findings in a patient with chromosome 18q deletion. Ann Neurol 30:419–423 [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Veltman JA, Olshen A, Jain A, Moore DH, Kovacs G, Presti JC, Waldman FM (2002) Array based CGH for the differential diagnosis of renal cell cancer. Cancer Res 62:957–960 [PubMed] [Google Scholar]
- Wilson MG, Towner JW, Forsman I, Siris E (1979) Syndromes associated with deletion of the long arm of chromosome 18 [del(18q)]. Am J Med Genet 3:155–174 [DOI] [PubMed] [Google Scholar]