Abstract
Thirty-five mitochondrial (mt) DNAs from Spain that harbor the mutation A3243G in association with either MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes) syndrome or a wide array of disease phenotypes (ranging from diabetes and deafness to a mixture of chronic progressive external ophthalmoplegic symptoms and strokelike episodes) were studied by use of high-resolution restriction fragment length polymorphism analysis and control-region sequencing. A total of 34 different haplotypes were found, indicating that all instances of the A3243G mutation are probably due to independent mutational events. Haplotypes were distributed into 13 haplogroups whose frequencies were close to those of the general Spanish population. Moreover, there was no statistically significant difference in haplogroup distribution between patients with MELAS and those with disease phenotypes other than MELAS. Overall, these data indicate that the A3243G mutation harbors all the evolutionary features expected from a severely deleterious mtDNA mutation under strong negative selection, and they reveal that European mtDNA backgrounds do not play a substantial role in modulating the mutation's phenotypic expression.
Human mtDNA has a very high rate of sequence evolution (Miyata et al.1982; Wallace et al. 1987). Its mutations are random and have accumulated sequentially along radiating female lineages, giving rise to a wide variety of regional haplogroups and local haplotypes (Wallace 1995; Torroni et al. 1996; Macaulay et al. 1999; Richards et al. 2000; Herrnstadt et al. 2002). Analyses of these haplotypes and haplogroups have provided extensive information about the origin and relationships of modern populations, despite the fact that mtDNA evolution is not driven solely by drift. Indeed, selection acts on the fraction of newly occurring mutations that alter functionally important gene products and play a role in disease (Zeviani et al. 1998; Chinnery et al. 2000; Wallace 2001). Unfortunately, the demarcation line between neutral and pathogenic mtDNA mutations is very difficult to define and, for many mutations, could also vary depending the mtDNA and nuclear genotypes on which they happen to occur or with which they happen to segregate.
Deleterious mtDNA mutations should have very different evolutionary behaviors, depending on the severity of the disease phenotype, its age at onset (Torroni and Wallace 1994; De Benedictis et al. 1999), and, in some extreme cases, the function of the affected cell type or tissue (Ruiz-Pesini et al. 2000). Thus, we would expect severely deleterious mutations causing multisystem disorders to be acted on very strongly by selection and to be rapidly eliminated. Moderately deleterious mutations that affect mainly one tissue or organ (nonsyndromic) or mutations whose penetrance is much higher in male than in female individuals, such as the Leber hereditary optic neuropathy (LHON) mutations, could undergo weaker selection and be transmitted through a relatively large number of generations and, under particular demographic conditions, diffuse into the population. By contrast, mildly deleterious mutations, which may not be expressed until late in life, do not reduce the reproductive fitness of the mtDNAs on which they occur; thus, these mutations may become established in the population as polymorphisms.
If this overall scenario is correct, a mildly deleterious mutation may have arisen thousands of years ago because of a single mutational event, and such a mutation may be part of the sequence motif of a regional haplogroup. In contrast, moderately deleterious mutations are expected to occupy a somewhat intermediate position, with a majority of independent and recent occurrences and with few relatively old (on the order of tens of generations) founder events, as attested by the sharing of the same haplotype by families that are only apparently unrelated but belong to the same population or live in the same geographic area. At the other extreme, severely deleterious mutations causing multisystem disorders should be the result of very recent mutational events and are transmitted through very few generations (and only if heteroplasmic); thus, founder events are not expected.
The mtDNA mutation A3243G (Goto et al. 1990; Kobayashi et al. 1990, 1991) of the tRNALeu(UUR) gene causes a reduced association of mRNA with ribosomes, possibly the consequence of the tRNALeu(UUR) aminoacylation defect (Chomyn et al. 2000), and could be classified as either a severely or a moderately deleterious mutation. Indeed, it was initially associated with the MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes) syndrome (MIM 540000), whose clinical features are episodic vomiting, seizures, and recurrent cerebral insults that resemble strokes and cause hemiparesis, hemianopsia, or cortical blindness (Pavlakis et al. 1984; Montagna et al. 1988). However, it was also later found in patients who lacked the typical MELAS symptoms but had clinical manifestations ranging from maternally inherited diabetes and deafness (MIM 520000) (van den Ouweland et al. 1992) to a mixture of chronic progressive external ophthalmoplegia symptoms, strokelike episodes, cardiomyopathy, and progressive kidney disease (Moraes et al. 1993; Jean-Francois et al. 1994; van den Ouweland et al. 1999; Deschauer et al. 2001). Thus, the phenotypic expression of the A3243G mutation appears to be quite variable, ranging from mild to severe phenotypes.
Some studies have shown that most of the A3243G transitions are due to independent mutational events (Morten et al. 1995; Majamaa et al. 1998). However, these earlier studies did not completely rule out the possibility of some local founder events or, more importantly, the possibility that certain mtDNA haplogroups could affect the phenotypic expression of this mutation (Jacobs and Holt 2000). Moreover, a recent study of the A3243G mutation in Finland showed that the net reproduction rate, the average fertility, and the generation time of mutation carriers were similar to those of the general population. Thus, the genetic fitness of A3243G carriers does not appear to be reduced, and, apparently, there is no host-level selection in modern populations (Moilanen and Majamaa 2001).
In recent years, we have identified 35 unrelated Spanish subjects who either were affected by MELAS syndrome (N=24) or had phenotypes other than MELAS (N=11) but were positive for the presence of the A3243G mutation in the tRNALeu(UUR) gene when screened by use of the restriction enzyme HaeIII (Campos et al. 1995, 1996). This has prompted us to initiate a detailed analysis of their mtDNAs to determine whether, at the molecular level, the evolutionary features of the A3243G mutation were indeed those of a severely deleterious mutation and whether mtDNA backgrounds play a role in the variable phenotypic presentation of the mutation. The rationale of this study is the same as was previously applied to evaluate the common LHON mutations and which led to the conclusion that the milder mutations, ND4/11778 and ND6/14484, are preferentially associated with haplogroup J, whereas the biochemically more-severe ND1/3460 mutation is randomly distributed on all European haplogroups (Brown et al. 1997; Torroni et al. 1997).
All 35 individuals harboring the A3243G mutation were from families that, according to family member interviews, were maternally unrelated for at least four generations. Appropriate informed consent was obtained from patients. Genomic DNAs were extracted from blood and muscle tissue, using standard procedures. Details about the clinical and biochemical profiles of the 35 subjects are provided in tables 1 and 2.
Table 1.
Patients with MELAS[Note]
|
Patient |
||||||||||||||||||||||||
| PatientCharacteristic | 1 | 3 | 4 | 5 | 6 | 8 | 9 | 11 | 12 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 24 | 25 | 26 | 28 | 29 | 32 | 34 | 35 |
| Age at biopsy (years) | 14 | 10 | 26 | 15 | 39 | 24 | 22 | 16 | 20 | 26 | 27 | 33 | 46 | 33 | 6 | 57 | 41 | 15 | 42 | 56 | 30 | 50 | 32 | 9 |
| Age at onset (years) | 2 | 8 | 10 | 12 | 31 | 21 | 19 | 14 | 18 | 26 | 12 | 20 | 31 | 8 | 6 | 32 | 21 | 10 | 19 | 14 | 20 | 14 | 30 | 8 |
| Stroke | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Seizures | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Lactic acidosis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| RRF | + | + | + | + | + | + | NA | + | + | + | + | NA | + | NA | + | NA | NA | + | + | + | + | + | + | NA |
| Focal brain lesions (CT or MRI) | + | + | + | + | + | + | + | + | NA | + | + | NA | + | + | + | + | + | + | + | + | + | + | + | + |
| Additional signsa | + | + | + | + | + | + | + | + | − | − | − | + | + | + | − | − | − | + | + | + | − | + | − | + |
| BG calcifications | − | − | − | − | − | − | + | + | NA | − | − | NA | − | − | − | − | + | − | − | + | − | + | − | − |
| Hearing loss | − | + | + | − | + | + | − | + | − | − | + | + | + | − | − | + | + | − | + | + | + | + | − | − |
| Diabetes | − | − | − | − | − | − | − | + | − | − | − | + | + | + | − | + | − | − | − | + | − | − | − | − |
| Other symptoms | W | − | − | W | W | M | − | C | A,M | − | − | − | C | A | W | − | W | − | C | PR,C | − | − | − | − |
| Ophthalmoparesis | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − |
| Family history | + | + | + | NA | NA | NA | − | + | − | − | − | + | − | NA | + | + | − | + | − | + | + | + | − | + |
| Short stature | + | − | + | + | − | − | − | + | − | − | − | + | − | + | + | − | − | − | + | + | + | + | + | + |
| A3243G muscle (%) | 87 | 96 | 75 | 78 | 88 | 92 | NA | 73 | 56 | 82 | 81 | NA | 78 | NA | 80 | NA | NA | 76 | NA | 75 | 65 | 70 | 79 | NA |
| A3243G blood (%) | 37 | NA | NA | NA | NA | NA | 22 | NA | 28 | NA | 20 | 25 | 35 | 20 | NA | 30 | 37 | NA | 32 | NA | 22 | 33 | 38 | 49 |
| Respiratory-chain–complex defect | I | I,IV | I | I,III | I,IV | N | NA | I,III,IV | I | III,IV | IV | NA | IV | NA | N | NA | NA | I,IV | NA | I | I | N | IV | NA |
| mtDNA haplogroup | U8b | H | T | J | U6 | X | V | H | U2e | H | H | H | U2e | H | M8 | H | H | H | L2a | H | H | K | X | H |
Note.— A = ataxia; BG = basal ganglia; C = cardiomyopathy; CT = computed tomography; M = myoclonus; MRI = magnetic resonance image; PR = pigmentary retinopathy; NA = not available; RRF = ragged-red fibers; and W = weakness.
Either recurrent headache and vomiting or dementia or both.
Table 2.
Patients with Phenotypes other than MELAS[Note]
|
Patient |
|||||||||||
| PatientCharacteristic | 2 | 7 | 10 | 13 | 14 | 15 | 23 | 27 | 30 | 31 | 33 |
| Age at biopsy (years) | 27 | 10 | 38 | 66 | 51 | 34 | 29 | 60 | 20 | 39 | 42 |
| Age at onset (years) | 12 | 4 | 12 | 30 | 32 | 20 | 10 | 50 | 12 | 24 | 10 |
| Stroke | − | − | − | − | − | − | − | − | − | − | − |
| Seizures | − | − | − | − | − | − | − | − | − | − | − |
| Lactic acidosis | + | − | + | − | − | − | + | − | − | − | + |
| RRF | + | + | + | + | + | NA | + | + | NA | + | NA |
| Focal brain lesions (CT or MRI) | − | − | − | NA | − | NA | − | − | NA | NA | + |
| Additional signsa | − | − | − | − | − | − | − | − | − | − | − |
| BG calcifications | − | − | + | NA | − | NA | − | − | NA | NA | + |
| Hearing loss | − | − | + | + | + | + | + | + | − | + | + |
| Diabetes | − | − | + | + | + | + | − | + | − | + | + |
| Other symptoms | W | W | C | − | − | − | C | PR | − | W | C |
| Ophthalmoparesis | − | + | − | + | + | − | − | + | + | + | − |
| Family history | − | − | + | + | + | + | + | + | − | + | + |
| Short stature | + | − | + | − | − | − | + | − | + | + | − |
| A3243G muscle (%) | 96 | 58 | 47 | 50 | 52 | NA | 32 | 53 | NA | 57 | NA |
| A3243G blood (%) | 83 | NA | NA | 10 | NA | 50 | NA | NA | 34 | NA | 22 |
| Respiratory chain complex defect | I | N | III | NA | N | NA | I | N | NA | I | NA |
| mtDNA haplogroup | J | H | W | H | H | U5 | H | U5 | T | H | T |
Note.— A = ataxia; BG = basal ganglia; C = cardiomyopathy; CT = computed tomography; M = myoclonus; MRI = magnetic resonance image; NA = not available; PR = pigmentary retinopathy; RRF = ragged−red fibers; and W = weakness.
Either recurrent headache and vomiting or dementia or both.
To determine high-resolution RFLP haplotypes, the entire mtDNA was amplified using PCR in nine overlapping fragments utilizing primer pairs described elsewhere (Torroni et al. 1997). Each of the nine PCR segments was then digested with 14 restriction endonucleases (AluI, AvaII, BamHI, DdeI, HaeII, HaeIII, HhaI, HincII, HinfI, HpaI, MspI, MboI, RsaI, and TaqI). In addition, all mtDNAs were screened for the presence/absence of the BstOI site at nucleotide position (np) 13704, the AccI sites at nps 14465 and 15254, the BfaI site at np 4914, the NlaIII sites at nps 4216 and 4577, the XbaI site at np 7440, the MseI sites at nps 14766 and 16297, and the MnlI site at np 10871. The polymorphisms at nps 12308 and 11719 were also tested; the first by using a mismatched primer that generates a HinfI site when the A12308G mutation is present (Torroni et al. 1996) and the second by using a mismatched primer that generates a HaeIII site when the A11719G mutation is present (Saillard et al. 2000). The sequencing of the mtDNA control-region was performed as described elsewhere (Torroni et al. 2001) and encompasses 537–825 nt, beginning from np 16000. Thus, in all cases, it included much more than the entire HVS-I (nps 16024–16383).
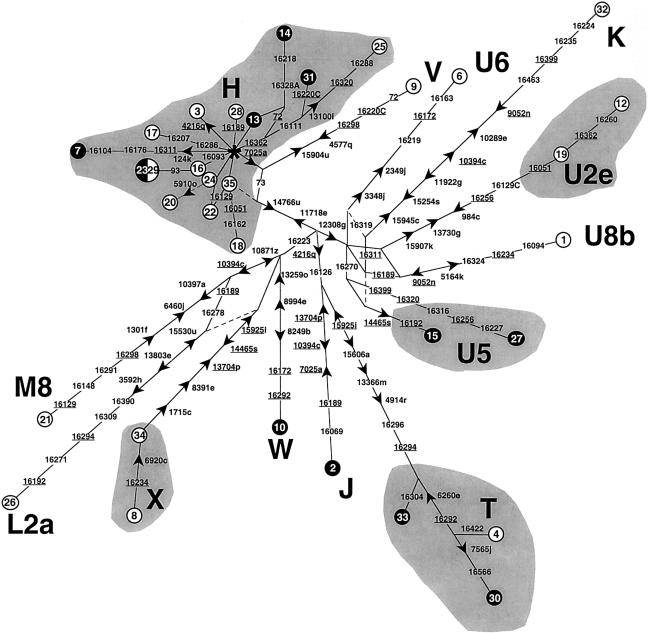
High-resolution RFLP analysis and control-region sequencing revealed that the Spanish mtDNAs carrying A3243G fell into 34 different haplotypes (table 3; fig. 1), strongly suggesting 34 independent occurrences of the mutation. Indeed, even those mtDNAs that were members of the same haplogroup—and thus could theoretically harbor the A3243G mutation by descent—often differed by several mutations in both the coding and the control regions. Moreover, some of their different control-region motifs have been described in the general Spanish population (Richards et al. 2000), further supporting the independence of the mutational events. Actually, the only two identical mtDNAs (23 and 29) also harbored a haplotype that is not uncommon in the general Spanish population, thus raising the possibility that the mutation could have occurred independently in these two cases too.
Table 3.
RFLP and Control-Region Variation of Spanish mtDNAs Harboring the A3243G Mutation
|
Haplotypea |
||||
| Sample | Haplogroup | RFLPb | Control Regionc | RegionSequenced |
| 35 | H | −7025a, +11718e, −14766u | 73 | 16000–00225 |
| 16 | H | −7025a, +11718e, −14766u, +16517e | 16093 (70%), 16519 | 16000–00207 |
| 23 | H | −7025a, +11718e, −14766u, +16517e | 16093, 16519, 93 | 16000–00225 |
| 29 | H | −7025a, +11718e, −14766u, +16517e | 16093, 16519, 93 | 16000–00225 |
| 31 | H | −7025a, +11718e, −14766u, +16517e | 16111, 16220C, 16362, 16519 | 16000–00105 |
| 25 | H | −7025a, +11718e, +13100i, −14766u | 16111, 16288, 16320, 16362, 146, 195 | 16000–00225 |
| 20 | H | +5910o, −7025a, +11718e, −14766u, +16517e | 16129, 16519 | 16000–00120 |
| 24 | H | −7025a, +11718e, −14766u, +16517e | 16129, 16519 | 16002–00225 |
| 22 | H | −7025a, +11718e, −14766u, +16517e | 16129, 16519, 73, 197 | 16000–00225 |
| 28 | H | −7025a, +11718e, −14766u, +16517e | 16189, 16519, 152 | 16000–00215 |
| 17 | H | −7025a, +11718e, −14766u, +16517e | 16207, 16286, 16519, 152 | 16000–00207 |
| 13 | H | −7025a, +11718e, −14766u, +16517e | 16519, 72 (70%), 150 | 16000–00207 |
| 3 | H | +4216q, −7025a, +11718e, −14766u, +16517e | 16519, 189 | 16000–00207 |
| 18 | H | −7025a, +11718e, −14766u, −16049k, +16517e | 16051, 16162, 16519, 73 | 16000–00207 |
| 7 | H | +124k, −7025a, +11718e, −14766u, −16310k, +16517e | 16104, 16176, 16311, 16519, 127 | 16000–00207 |
| 14 | H | −7025a, +11718e, −14766u, +16217u | 16218, 16328A, 16362, 72 (40%) | 16000–00207 |
| 11d | H | ND | 16217, 16311, 16519 | 16000–16537 |
| 9 | V | −4577q, +11718e, −14766u, +15904u, −16297u | 16220C, 16298, 72, 200 | 16000–00207 |
| 2e | J | +4216q, −7025a, +10394c, −13704p, −16065g | 16069, 16126, 16189, 73, 185 | 16000–00190 |
| 5 | J | ND | 16069, 16126, 16519, 73, 185 | 16000–00207 |
| 33 | T | +4216q, +4914r, +13366m/−13367b/+13367j, +15606a, −15925i, −16303k, +16517e | 16126, 16294, 16296, 16304, 16519, 73 | 16000–00225 |
| 4 | T | +4216q, +4914r, −6260e, +13366m/−13367b/+13367j, +15606a, −15925i, +16517e | 16126, 16292, 16294, 16296, 16422 (50%), 16519, 73, 146 | 16000–00180 |
| 30 | T | +4216q, +4914r, −6260e, +7565j, +13366m/−13367b/+13367j, +15606a, −15925i, +16517e | 16126, 16292, 16294, 16296, 16519, 16566, 73, 146 | 16000–00225 |
| 32 | K | −9052n/−9053f, +10289e, +10394c, −11922j, +12308g, −15254s, +15945c, −16310k, +16398e, +16517e | 16224, 16235 (70%), 16311, 16319, 16399, 16463, 16519, 73, 152 | 16000–00255 |
| 12 | U2e | −984c, +12308g, +13730g, +15907k, −16049k | 16051, 16129C, 16183del, 16189, 16256, 16260, 16362, 73, 152 | 16000–00212 |
| 19 | U2e | −984c, +12308g, +13730g, +15907k, −16049k, +16517e | 16051, 16129C, 16189, 16256, 16519, 73, 152 | 16000–00215 |
| 27 | U5 | +12308g, +16223c/+16226a, +16398e | 16227, 16256, 16270, 16316, 16320, 16399, 73, 152 | 16000–00225 |
| 15 | U5 | +12308g, +14465s | 16192, 16270, 16319, 73, 150 | 16000–00207 |
| 6 | U6 | +2349j, +3348j, +12308g, +16217l, −16310k, +16517e | 16163, 16172, 16219, 16311, 16519, 73 | 16000–00207 |
| 1 | U8b | +5164k, −9052n/−9053f, +12308g, +16517e | 16094 (60%), 16182del, 16183del, 16189, 16234, 16319, 16324, 16519, 73, 152 | 16000–00212 |
| 10 | W | +8249b/−8250e, −8994e, −13259o/+13262a, 16517e | 16172, 16223, 16292, 16519, 73, 189, 194, 195, 204, 207 | 16000–00207 |
| 34 | X | −1715c, −8391e/+8391b, −13704p, +14465s, −15925i, +16517e | 16182del, 16183del, 16189, 16223, 16278, 16519, 73 | 16000–00109 |
| 8 | X | −1715c, −6920c, −8391e/+8391b, −13704p, +14465s, −15925i, +16517e | 16189, 16223, 16234, 16278, 16519, 73 | 16000–00109 |
| 26 | L2a | +3592h, +10394c, −10871z, +13803e, −15530u, +16389g/−16390b | 16189, 16192, 16223, 16271, 16278, 16294, 16309, 16390, 73, 143, 146, 152, 195 | 16000–00225 |
| 21 | M8 | −1301f, −6460j/+6460b, +10394c, +10397a, −10871z, −16297u, +16517e | 16129, 16148, 16223, 16291, 16298, 16519, 73 | 16000–00225 |
Motifs diagnostic of each haplogroup are shown in boldface italic type.
Sites are numbered from the first nucleotide of the recognition sequence. A “+” indicates the presence of a restriction site, a “−” the absence. The explicit indication of the presence/absence of a site implies the absence/presence in haplotypes not so designated. The restriction enzymes used in the analysis are designated by the following single-letter codes: a, AluI; b, AvaII; c, DdeI; e, HaeIII; f, HhaI; g, HinfI; h, HpaI; i, MspI; j, MboI; k, RsaI; l, TaqI; m, BamHI; n, HaeII; o, HincII; p, BstOI; q, NlaIII; r, BfaI; s, AccI; t, XbaI; u, MseI; z, MnlI. A slash (/) separating states indicates the simultaneous presence or absence of restriction sites that can be correlated with a single-nucleotide substitution. ND = not determined.
Only those nps (<16000) that differ from the Cambridge reference sequence (Andrews et al. 1999) are shown. Mutations are transitions unless the base change is specified explicitly. The percentage value in parentheses is associated to nps that have been found to be heteroplasmic and refers to the mutant form.
This sample was RFLP screened to determine the 7025 AluI status, and it was found to lack that site.
The region encompassing −7025a was sequenced, revealing that the absence of the site was due to the transition at np 7028.
Figure 1.
Reduced median-joining network (Bandelt et al. 1995) of the haplotypes observed among the Spanish mtDNAs harboring the A3243G mutation. The data were processed by the RM algorithm (r=2). A double weight was assigned to RFLP sites 4216q, 7025a, 11718e, and 12308g relative to all other sites. The circles represent combined high-resolution RFLP and control-region haplotypes, their areas being proportional to the frequency. Subjects 5 and 11 were not included, because they lacked complete RFLP data (table 3). Unblackened circles indicate mtDNAs from subjects with MELAS, and blackened circles indicate mtDNAs from patients with a phenotype other than MELAS. RFLP mutations are indicated next to the branches, with the arrow pointing in the direction of a site gain. The numbers indicate the nucleotide at the beginning of the recognition sequence (according to the numbering of the reference sequence by Anderson et al. [1981] and Andrews et al. [1999]); the letter suffix indicates the enzyme (see table 3). The asterisk indicates the node of haplogroup H, which corresponds to the reference sequence. Mutations observed in the portion of the control region sequenced in all subjects (16000–00105) are shown on the branches; they are transitions unless the base change is explicitly indicated. Underlining indicates resolved recurrent mutations, and dashed lines show links considered implausible on the basis of data published elsewhere. The hypervariable RFLP site 16517e was not considered, nor were indel events.
Furthermore, all mtDNAs were found to harbor diagnostic sequence motifs that allowed their classification into haplogroups. The range of observed haplogroups was extremely wide and included the typically European haplogroups H, V, J, T, K, U2e, U5, U8b, X, and W, plus one representative each of the Asian haplogroup M8 (Yao et al. 2002) and the African haplogroups U6 and L2a (Macaulay et al. 1999; Torroni et al. 2001). The presence of the last two haplogroups is not unexpected, since African mtDNAs are not uncommon in Iberia, and members of the Asian haplogroup M are sporadically observed in Europe (Richards et al. 2000). Haplogroup H mtDNAs were the most represented, and they encompassed 48.6% of the A3243G subjects. Haplogroup H is the most common in all European populations (except the Saami) and occurs at a particularly high frequency in the general Spanish population (45.1%) (Torroni et al. 1999). The other haplogroups were represented by either one (haplogroups V, K, U6, U8b, W, M8, and L2a), two (haplogroups J, U2e, U5, and X), or three (haplogroup T) samples, and their frequencies are very similar to those previously observed in the Spanish population (Torroni et al. 1999; Richards et al. 2000). An analogous result was obtained when the different subsets of the superhaplogroup U (K, U2, U5, U6, and U8b) were aggregated. The global frequency was 20.0%, very close to the 17.6% previously reported in Spanish controls (Torroni et al. 1999), and thus did not support the possibility of an excess of U mtDNAs with the A3243G mutation, as has been observed in Finland (Majamaa et al. 1998).
The comparison of haplogroup distributions in the two cohorts of patients, those affected by MELAS (N=24) and those affected by other phenotypes (N=11), did not provide evidence of clustering on specific haplogroups. Indeed, the frequencies of haplogroup H were virtually identical (50.0% vs. 45.5%), and those of the less frequent haplogroups were not different either from each other or from those of the general Spanish population.
In conclusion, the present molecular survey, performed on Spanish mtDNAs harboring the A3243G mutation, points to two general conclusions that can be extended to wider population contexts. First, virtually all instances of the A3243G mutation, in families that are maternally unrelated for at least a few generations, are indeed due to independent mutational events. This finding indicates that, even though the genetic fitness of the A3243G carriers in some modern populations might not be reduced (Moilanen and Majamaa 2001), the survival time of each A3243G mutation is extremely short. This result is in complete agreement with what would be expected for a mutation that causes a severe disease phenotype and is under strong negative selection. Second, there is no clustering of this mutation on specific haplogroups, indicating that European haplogroups do not increase or reduce the risk of expressing MELAS or the other associated disease phenotypes. Again, this perfectly fits the expected features of a severely deleterious mtDNA mutation whose penetrance is very unlikely to be modulated by mtDNA backgrounds. Therefore, it appears that the variable phenotypic presentation of the A3243G mutation can be attributed to only two factors: its level of heteroplasmy in the different tissues and the influence of still-unidentified nuclear genes.
Acknowledgments
This research received support from Telethon-Italy E.0890 (to A.T.); the Italian Ministry of the University (Fondo Investimenti Ricerca di Base 2001 [to A.T.], and Progetti Ricerca Interesse Nazionale 2002 [to A.T. and R.S.]; Progetto MIUR-CNR Genomica Funzionale [to A.T.]; and Fondo d’Ateneo per la Ricerca 2002 dell’Università di Pavia [to A.T.]); the “Istituto Pasteur Fondazione Cenci Bolognetti,” Università di Roma “La Sapienza” (to R.S.); Fondo de Investigación Sanitaria, Ministerio de Sanidad, Spain, grants 00/370 (to Y.C.) and 01/1426 (to J.A.); Programa Nacional de Biomedicina, Ministerio de Ciencia y Tecnología, Spain (01/122 to J.A.); and Instituto Carlos III “contrato de investigación” (to Y.C.).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Mitomap, http://www.mitomap.org
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MELAS syndrome and diabetes-deafness syndrome)
References
- Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organisation of the human mitochondrial genome. Nature 290:457–465 [DOI] [PubMed] [Google Scholar]
- Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23:147 [DOI] [PubMed] [Google Scholar]
- Bandelt H-J, Forster P, Sykes BC, Richards MB (1995) Mitochondrial portraits of human populations using median networks. Genetics 141:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Sun F, Wallace DC (1997) Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 or 14484 mutations on an mtDNA lineage. Am J Hum Genet 60:381–387 [PMC free article] [PubMed] [Google Scholar]
- Campos Y, Bautista J, Gutierrez-Rivas E, Chinchon D, Cabello A, Segura D, Arenas J (1995) Clinical heterogeneity in two pedigrees with the 3243 bp tRNALeu(UUR) mutation of mitochondrial DNA. Acta Neurol Scand 91:62–65 [DOI] [PubMed] [Google Scholar]
- Campos Y, Martin MA, Lorenzo G, Aparicio M, Cabello A, Arenas J (1996) Sporadic MERRF/MELAS overlap syndrome associated with the 3243 tRNALeu(UUR) mutation of mitochondrial DNA. Muscle Nerve 19:187–190 [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Thorburn DR, Samuels DC, White SL, Dahl HM, Turnbull DM, Lightowlers RN, Howell N (2000) The inheritance of mitochondrial DNA heteroplasmy: random drift, selection or both? Trends Genet 16:500–505 [DOI] [PubMed] [Google Scholar]
- Chomyn A, Enriquez JA, Micol V, Fernandez-Silva P, Attardi G (2000) The mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode syndrome-associated human mitochondrial tRNALeu(UUR) mutation causes aminoacylation deficiency and concomitant reduced association of mRNA with ribosomes. J Biol Chem 275:19198–19209 [DOI] [PubMed] [Google Scholar]
- De Benedictis G, Rose G, Carrieri G, De Luca M, Falcone E, Passarino G, Bonafe M, Monti D, Baggio G, Bertolini S, Mari D, Mattace R, Franceschi C (1999) Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB J 13:1532–1536 [DOI] [PubMed] [Google Scholar]
- Deschauer M, Muller T, Wieser T, Schulte-Mattler W, Kornhuber M, Zierz S (2001) Hearing impairment is common in various phenotypes of the mitochondrial DNA A3243G mutation. Arch Neurol 58:1885–1888 [DOI] [PubMed] [Google Scholar]
- Goto Y, Nonaka I, Horai S (1990) A mutation in the tRNA-LeuUUR gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348:651–653 [DOI] [PubMed] [Google Scholar]
- Herrnstadt C, Elson JL, Fahy E, Preston G, Turnbull DM, Anderson C, Ghosh SS, Olefsky JM, Beal MF, Davis RE, Howell N (2002) Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am J Hum Genet 70:1152–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HT, Holt IJ (2000) The np 3243 MELAS mutation: damned if you aminoacylate, damned if you don’t. Hum Mol Genet 9:463–465 [DOI] [PubMed] [Google Scholar]
- Jean-Francois MJ, Lertrit P, Berkovic SF, Crimmins D, Morris J, Marzuki S, Byrne E (1994) Heterogeneity in the phenotypic expression of the mutation in the mitochondrial tRNA(LeuUUR) gene generally associated with the MELAS subset of mitochondrial encephalomyopathies. Aust N Z J Med 24:188–193 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Momoi MY, Tominaga K, Momoi T, Nihei K, Yanagisawa M, Kagawa Y, Ohta S (1990) A point mutation in the mitochondrial tRNA-LeuUUR gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun 173:816–822 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Momoi MY, Tominaga K, Shimoizumi H, Nihei K, Yanagisawa M, Kagawa Y, Ohta S (1991) Respiration-deficient cells are caused by a single point mutation in the mitochondrial tRNA-Leu (UUR) gene in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS). Am J Hum Genet 49:590–599 [PMC free article] [PubMed] [Google Scholar]
- Macaulay V, Richards M, Hickey E, Vega E, Cruciani F, Guida V, Scozzari R, Bonné-Tamir B, Sykes B, Torroni A (1999) The emerging tree of West Eurasian mtDNAs: a synthesis of control-region sequences and RFLPs. Am J Hum Genet 64:232–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majamaa K, Moilanen JS, Uimonen S, Remes AM, Salmela PI, Karppa M, Majamaa-Voltti KAM, Rusanen H, Sorri M, Peuhkurinen KJ, Hassinen IE (1998) Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Hayashida H, Kikuno R, Hasegawa M, Kobayashi M, Koike K (1982) Molecular clock of silent substitution: at least six-fold preponderance of silent changes in mitochondrial genes over those in nuclear genes. J Mol Evol 19:28–35 [DOI] [PubMed] [Google Scholar]
- Moilanen JS, Majamaa, K (2001) Relative fitness of carriers of the mitochondrial DNA mutation 3243A→G. Eur J Hum Genet 9:59–62 [DOI] [PubMed] [Google Scholar]
- Montagna P, Gallassi R, Medori R, Govoni E, Zeviani M, Di Mauro S, Lugaresi E, Andermann F (1988) MELAS syndrome: characteristic migrainous and epileptic features and maternal transmission. Neurology 38:751–754 [DOI] [PubMed] [Google Scholar]
- Moraes CT, Ciacci F, Silvestri G, Shanske S, Sciacco M, Hirano M, Schon EA, Bonilla E, DiMauro S (1993) Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul Disord 3:43–50 [DOI] [PubMed] [Google Scholar]
- Morten KJ, Poulton J, Sykes B (1995) Multiple independent occurrence of the 3243 mutation in mitochondrial tRNA-LeuUUR in patients with the MELAS phenotype. Hum Mol Genet 4:1689–1691 [DOI] [PubMed] [Google Scholar]
- Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP (1984) Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 16:481–488 [DOI] [PubMed] [Google Scholar]
- Richards M, Macaulay V, Hickey E, Vega E, Sykes B, Guida V, Rengo C, et al (2000) Tracing European founder lineages in the Near Eastern mtDNA pool. Am J Hum Genet 67:1251–1276 [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lapena AC, Diez-Sanchez C, Perez-Martos A, Montoya J, Alvarez E, Diaz M, Urries A, Montoro L, Lopez-Perez MJ, Enriquez JA (2000) Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am J Hum Genet 67:682–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillard J, Magalhaes PJ, Schwartz M, Rosenberg T, Nørby S (2000) Mitochondrial DNA variant 11719G is a marker for the mtDNA haplogroup cluster HV. Hum Biol 72:1065–1068 [PubMed] [Google Scholar]
- Torroni A, Cruciani F, Rengo C, Sellitto D, López-Bigas N, Rabionet R, Govea N, López de Munain A, Sarduy M, Romero L, Villamar M, del Castillo I, Moreno F, Estivill X, Scozzari R (1999) The A1555G mutation in the 12S rRNA gene of human mtDNA: recurrent origins and founder events in families affected by sensorineural deafness. Am J Hum Genet 65:1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, Obinu D, Savontaus ML, Wallace DC (1996) Classification of European mtDNAs from an analysis of three European populations. Genetics 144:1835–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Petrozzi M, D’Urbano L, Sellitto D, Zeviani M, Carrara F, Carducci C, Leuzzi V, Carelli V, Barboni P, De Negri A, Scozzari R (1997) Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet 60:1107–1121 [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Rengo C, Guida V, Cruciani F, Sellitto D, Coppa A, Luna Calderon F, Simionati B, Valle G, Richards M, Macaulay V, Scozzari R (2001) Do the four clades of the mtDNA haplogroup L2 evolve at different rates? Am J Hum Genet 69:1348–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Wallace DC (1994) Mitochondrial DNA variation in human populations and implications for detection of mitochondrial DNA mutations of pathological significance. J Bioenerg Biomembr 26:261–271 [DOI] [PubMed] [Google Scholar]
- van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA (1992) Mutation in mitochondrial tRNA(LeuUUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 1:368–371 [DOI] [PubMed] [Google Scholar]
- van den Ouweland JM, Maechler P, Wollheim CB, Attardi G, Maassen JA (1999) Functional and morphological abnormalities of mitochondria harbouring the tRNA(LeuUUR) mutation in mitochondrial DNA derived from patients with maternally inherited diabetes and deafness (MIDD) and progressive kidney disease. Diabetologia 42:485–492 [DOI] [PubMed] [Google Scholar]
- Yao YG, Kong QP, Bandelt HJ, Kivisild T, Zhang YP (2002) Phylogeographic differentiation of mitochondrial DNA in Han Chinese. Am J Hum Genet 70:635–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC (1995) Mitochondrial DNA variation in human evolution, degenerative disease, and aging. Am J Hum Genet 57:201–223 [PMC free article] [PubMed] [Google Scholar]
- ——— (2001) Mitochondrial defects in neurodegenerative disease. Ment Retard Dev Disabil Res Rev 7:158–166 [DOI] [PubMed] [Google Scholar]
- Wallace DC, Ye JH, Neckelmann SN, Singh G, Webster KA, Greenberg BD (1987) Sequence analysis of cDNAs for the human and bovine ATP synthase beta subunit: mitochondrial DNA genes sustain seventeen times more mutations. Curr Genet 12:81–90 [DOI] [PubMed] [Google Scholar]
- Zeviani M, Tiranti V, Piantadosi C (1998) Mitochondrial disorders. Medicine (Baltimore) 77:59–72 [DOI] [PubMed] [Google Scholar]