Abstract
We present a series of 26 patients, all >50 years of age, who are carriers of the fragile X premutation and are affected by a multisystem, progressive neurological disorder. The two main clinical features of this new syndrome are cerebellar ataxia and/or intention tremor, which were chosen as clinical inclusion criteria for this series. Other documented symptoms were short-term memory loss, executive function deficits, cognitive decline, parkinsonism, peripheral neuropathy, lower limb proximal muscle weakness, and autonomic dysfunction. Symmetrical regions of increased T2 signal intensity in the middle cerebellar peduncles and adjacent cerebellar white matter are thought to be highly sensitive for this neurologic condition, and their presence is the radiological inclusion criterion for this series. Molecular findings include elevated mRNA and low-normal or mildly decreased levels of fragile X mental retardation 1 protein. The clinical presentation of these patients, coupled with a specific lesion visible on magnetic resonance imaging and with neuropathological findings, affords a more complete delineation of this fragile X premutation–associated tremor/ataxia syndrome and distinguishes it from other movement disorders.
Introduction
Fragile X syndrome (MIM 309550) is the most common inherited form of mental retardation. The disorder is caused by an expansion >200 repeats (full mutation) of a trinucleotide element, (CGG)n, located in the 5′ UTR of the fragile X mental retardation 1 (FMR1) gene (Verkerk et al. 1991). Full mutation expansions are generally accompanied by complete or partial silencing of the FMR1 gene, with subsequent deficiency or absence of FMR1 protein (FMRP), which leads to fragile X syndrome.
In contrast, carriers of premutations (55–200 CGG repeats) typically have normal intelligence, although emotional difficulties and subtle physical features occur in ∼25% of these carriers (Dorn et al. 1994; Franke et al. 1996, 1998; Sobesky et al. 1996; Riddle et al. 1998). The frequency of the premutation in the general population is ∼1 in 259 females and 1 in 813 males (Rousseau et al. 1995; Dombrowski et al. 2002). A clinical finding unique to women with the premutation is premature ovarian failure, which is present in ∼20% of women who carry premutation expansions (Schwartz et al. 1994; Allingham-Hawkins 1999).
We recently reported a unique form of clinical involvement in five male carriers of the fragile X premutation (Hagerman et al. 2001). These men, all >50 years of age, developed progressive intention tremor and cerebellar ataxia. Their symptoms were accompanied by progressive cognitive and behavioral difficulties, including memory loss, executive function deficits, anxiety, and reclusive behavior. Significant dementia has been seen in a limited number of patients (Hagerman et al. 2001; Greco et al. 2002). In addition, more variable features include parkinsonism, peripheral neuropathy, lower limb proximal muscle weakness, and autonomic dysfunction (urinary and bowel incontinence and impotence).
We have also described a hyperintensity on the T2 weighted magnetic resonance (MR) images in the cerebellar white matter and the middle cerebellar peduncles (MCP) (Brunberg et al. 2002). This remarkable radiological finding, visible in nearly all the premutation carriers affected with tremor/ataxia, is not seen in control individuals. It serves as a major diagnostic criterion for the fragile X–associated tremor/ataxia syndrome (FXTAS).
In five affected individuals examined to date, neuropathological examination has revealed eosinophilic, intranuclear inclusions in neurons and astrocytes (Greco et al. 2002; Hagerman et al. 2002). These inclusions are found throughout the cortex and brain stem, with the greatest density in the hippocampus and the frontal cortical regions. Purkinje cells did not possess the inclusions, although there was evidence of axonal degeneration in the cerebellum (Greco et al. 2002; Hagerman et al. 2002). These inclusions are polyglutamine negative, which makes them distinctly different from those found in the CAG repeat disorders. Results of immunostaining for tau and synuclein were also negative.
For the present report, we have analyzed clinical, radiological, and molecular data on 20 new patients who met our inclusion criteria (both clinical and radiological; radiological features previously described by Brunberg et al. [2002] in 7 patients) and on an additional 6 patients who met the clinical but not the radiological criteria. This is the only large series published to date. The consistent clinical presentation of these patients, associated with a specific lesion visible on MR images and with neuropathological findings, may distinguish FXTAS from other movement disorders. We propose provisional diagnostic criteria to help clinicians recognize this new entity. We also discuss several elements regarding the progression of the disorder. The presence of elevated FMR1 mRNA in all patients suggests that this syndrome may represent a gain-of-function effect from the elevated message levels (Hagerman et al. 2001; Greco et al. 2002) as proposed previously for myotonic dystrophy (Mankodi et al. 2000).
Methods
Ascertainment and Inclusion Criteria
To obtain a homogeneous representation of this neurological disorder in the current series, all patients were carriers of the premutation and were selected on the basis of presentation with at least one clinical criterion (ataxic gait or intention tremor) and one radiological criterion (presence of symmetric white matter lesions in the MCPs). Thirty-five men were recruited, either through families with children affected by fragile X syndrome or through referring physicians. These patients all presented with late onset (>50 years of age) of neurological symptoms (specifically, ataxia affecting ambulation and/or tremor) Twenty of these individuals met the inclusion criteria for the main series. The remaining 15 men were excluded from the main series because they did not meet the radiological criteria (n=1), did not have results of magnetic resonance imaging (MRI) available (n=5), or did not meet the clinical criteria on examination (n=9). Six of the excluded patients who did not meet radiological criteria are described separately.
Patients were usually examined at one of three sites: University of Colorado Health Sciences Center (n=4), University of California, Davis, Medical Center (n=4), and Rush-Presbyterian–St. Luke’s Medical Center (n=2). Patients not seen in those centers were evaluated on videotape (made during home visits [n=3] or during examination at other neurology departments [n=7]). Of the 20 men in the main series, 4 were deceased; 2 of these 4 men were examined and followed before death, and 2 were studied retrospectively, with the use of complete medical records and MRI films. The clinical examination focused on movement disorders. A videotaped neurological examination was performed in 15 of the 20 patients, using three standardized scales—the Clinical Rating Scale for Tremors (CRST) (Fahn et al. 1998), the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn et al. 1987), and the International Cooperative Ataxia Rating Scale (ICARS) (Trouillas et al. 1997)—to evaluate tremor, parkinsonism, and cerebellar dysfunction, respectively. The same rating scales were performed on a group of 20 age-matched male control individuals who did not carry a premutation FMR1 allele. Male control subjects were obtained from a population-based epidemiology study focusing on families with fragile X in the state of California.
MRI
All 20 patients in the main series underwent an MRI, which was ordered for clinical purposes in 18/20 patients and for research purposes in 2/20 patients (performed under a specific research protocol after obtaining signed consent). The indications for examination included cerebellar and cognitive symptoms (n=16), acute vertigo (n=1), multiple-system atrophy (MSA) (n=1), and unexplained gait difficulties (n=2). All MRIs included T1 and T2 weighted images.
Analysis of Genomic DNA and mRNA
Genomic DNA was isolated from peripheral blood leukocytes, using standard procedures (Puregene; Gentra). Southern blots and PCR analyses were performed on each sample, as described by Taylor et al. (1994). For Southern blot analysis, the enzymes EcoRI and NruI were used for DNA digestion, and StB12.3 was used for the FMR1-specific probe (Oberle et al. 1991). The number of CGG repeats was determined using primers 1 and 3 in the PCR analysis (Brown et al. 1993). For the analysis of FMR1 mRNA levels, total RNA was isolated from peripheral blood leukocytes by a single-step method (Purescript; Gentra). Quantitative (fluorescence) RT-PCR measurements were performed in 13 patients, as described by Tassone et al. (2000b).
Immunocytochemical Detection of FMRP
FMRP immunocytochemistry was performed for 13 patients. Blood smears were made from 20 μl of peripheral blood, following the method described by Willemsen et al. (1995, 1997). Immunoincubation employed monoclonal antibodies from hybridoma clone 1C3-1a (Devys et al. 1993). For each slide, 200 lymphocytes were counted, and the percent of lymphocytes expressing detectable FMRP was determined, as described by Tassone et al. (1999).
Results
The 20 men who constitute the inclusion group were all carriers of premutation (CGG)n expansions (55–200), with a mean age (± SD) of 71 (± 6) years (table 1). An additional six men, who had clinical symptoms but did not satisfy radiological criteria for inclusion, are presented separately. All patients had a family history of fragile X syndrome. Before presenting a detailed view of the clinical, radiological, and molecular data, we will summarize two case reports that are representative of the main clinical presentations.
Table 1.
Patient Demographics (n = 20)
| Characteristic | Measure |
| Mean age ± SD (years) | 71 ± 6 |
| White | 85% |
| Hispanic | 15% |
| College educated | 60% |
| Grandchild with fragile X syndrome | 100% |
Patient 1 is a 77-year-old left-handed former engineer with the Air National Guard and the National Aeronautics and Space Administration who carries fragile X premutation (85 CGG repeats). He first noticed tremor in his left hand, in his early 40s, as a very transient phenomenon after flying a jet aircraft. At age 60 years, he noticed worsening of his tremor related to job stress, and he switched to writing with his right hand. However, in his mid-60s, the tremor extended to the right hand.
At age 64 years, neurological examination showed dystonic tremor of the upper extremities, evidenced by marked intention and postural tremor (without resting tremor), positional exacerbation of tremor, and dramatic improvement of tremor with intravenous scopolamine. Toes were up-going bilaterally, and reflexes were brisk. Throughout his 70s, gait instability became increasingly prominent. At age 72 years, examination showed continuing tremor, ataxia in the heel-to-shin test, loss of vibration sense in the feet, and loss of pinprick discrimination below the knees. Deep tendon reflexes were absent in the ankles and were 1+ in the knees. The patient also complained of muscle weakness in his right leg, and muscle testing revealed a deficit in his hip flexors (3/5 on the right) and quadriceps (4/5 on the right). Electrical studies revealed normal conduction, but electromyelographic examination found evidence of denervation, suggesting an L3-L4 radiculopathy, although the appearance of the spinal cord was normal on MRI. He wore a knee brace for a year because his knee gave out frequently. MRIs performed at ages 68 and 77 years showed generalized atrophy, as well as a bilateral increased T2 signal intensity in the MCPs. By age 77 years, atrophy became severe, particularly in the parietal lobe. Other medical problems included diabetes, which was diagnosed at age 76 and was treated by diet. The patient had no history of excessive alcohol consumption. At 77 years old, he underwent angioplasty. Recovery from the procedure was protracted. After anesthesia, 48 h were necessary to regain consciousness and orientation. Two months later, the patient’s motor function was still not back to baseline, with marked lower limb proximal weakness. There was no evidence of stroke or focal neurological anomalies, and heart function was normal.
Patient 2 is a 73-year-old premutation carrier (70 CGG repeats), who retired from the U.S. Air Force at 50 years of age. In his mid 40s, he noted fatigue and aching in his legs. In his early 50s, he started walking with a cane because of an unstable drunkenlike gait and numbness and weakness of the legs. At age 62 years, one knee frequently gave out, precipitating falls.
The patient’s bilateral leg numbness (more pronounced on the left than on the right) has progressed over the past 20 years, ascending to a point just below the knees. He also noted a deterioration of fine motor coordination over the past several years, causing difficulty with writing and manipulating small buttons. A gradual decline in hearing has been accompanied by tinnitus. He has had episodic bladder and bowel incontinence since his early 60s.
Neurological examination at age 73 years revealed gaze-evoked nystagmus, mild dysarthria, mild muscle weakness in the arms and weakness in the legs that was more pronounce proximally than distally. Gait was very unsteady and wide based. Reflexes were 2+ throughout, except for absent ankle jerks. Babinski response was flexor. Sense of vibration was minimally reduced in the feet. DNA testing was negative for hereditary neuropathy with liability to pressure palsies (HNPP) and spinocerebellar atrophy (SCA1, -2, -3, -6, and -7). Electrical studies (including electromyography [EMG] and nerve conduction) at age 73 years suggested a mild distal axonal polyneuropathy or distal motor neuron process.
Electrical studies at age 76 years showed mild-to-moderate, distal, symmetric, sensory-motor polyneuropathy with axonal demyelination features. Results of muscle biopsy were normal. Brain MRI showed a marked increase in T2 signal intensity in the white matter of the anterior portions of the cerebellar hemispheres, extending toward and including the MCPs bilaterally. There were ∼30 scattered high signals in the corona radiata. Tremor was absent during all clinical examinations.
Clinical Findings
The clinical findings for the 20 men who met the inclusion criteria and the additional 6 men are summarized in table 2. The most prominent complaints were directly related to the inclusion criteria: gait difficulties (95% of the patients) and impaired fine motor skill (90% of the patients), with writing impairments in 90% of the patients (related to tremor in 71%). Other complaints, unrelated to the inclusion criteria, were fluctuating muscle weakness (50%) and numbness and/or pain (35%) in the lower extremities. Forty-five percent of the patients could not walk more than one block. Impotence (82%) and urinary (53%) and bowel (30%) incontinence were also frequent. In four men, pain was a major complaint, which required treatment (gabapentin being effective in three patients). Pain was described as burning or cramping or as paresthesias. In two patients, it was associated with effort, and arteritis was excluded by Doppler ultrasonography.
Table 2.
Clinical Summary
|
Affected Subjects (%) |
||
| Condition | MainGroupa(n = 20) | AdditionalGroupb(n = 6) |
| Reported symptom: | ||
| Gait difficulties | 95 | 83 |
| Leg weakness | 55 | 55 |
| Leg pain | 35 | 50 |
| Leg numbness | 35 | 33 |
| Deterioration of fine motor skills | 90 | 83 |
| Significant writing impairment | 95 | 83 |
| Clinical findingc: | ||
| Cerebellar ataxia: | ||
| Dysarthria | 78 | … |
| Dysmetria | 93 | … |
| Postural and gait abnormalitiesc | 86 | … |
| Tremor: | ||
| Intention | 70 | 83 |
| Resting alone | 10 | 17 |
| Combined (frequency 3–5 Hz) | 30 | 17 |
| Parkinsonian symptomd: | ||
| Mild bradykinesia | 57 | … |
| Mild rigidity | ||
| Upper extremity | 71 | … |
| Lower extremity | 35 | |
| Cogwheeling | 5 | … |
| Neuropathy below the kneee | 60 | 30 |
| Muscle testing: | ||
| Proximal lower limb <5 | 30 | … |
| Distal lower limb <5 | 10 | … |
| Other medical problems (n = 20): | ||
| Urinary incontinence | 55 | 17 |
| Bowel incontinence | 30 | 0 |
| Impotence | 80 | 50 |
| Heart diseasef | 55 | 50 |
| Hypertension | 50 | 50 |
Patients meeting both the radiological criteria and at least one clinical criterion.
Patients not having MRIs (n=5) or not meeting radiological criteria (n=1).
Patients were required to have a score of at least one on the items of the ICARS referred to for each symptom. Results were obtained on 14 videotaped patients.
Patients were required to have a score of at least one on the items of the UPDRS referred to for each symptom. Results were obtained on 14 videotaped patients.
Neuropathy was identified clinically by impaired toe position or absence of pinprick or vibration sensation.
History of ischemia related to congestive heart failure.
On the clinical examination, four symptoms were present in the majority of patients: gait ataxia, intention tremor, parkinsonism, and lower extremity neuropathy. We performed logistic regression analyses on the summary scores of the CRST, ICARS, and UPDRS, comparing 15 male carriers with 20 age- and sex-matched control individuals. Male carriers of the premutation demonstrated significant impairment on the three standardized scales which measured tremor (CRST, P=.006), cerebellar dysfunction (ICARS, P=.004), and parkinsonian symptoms (UPDRS, P=.001) (table 3).
Table 3.
P Values and Odds Ratios Differentiating Male Carriers of FMR1 Premutation (n = 15) from Age- and Sex-Matched Control Individuals (n = 20) on Three Standardized Neurological Scales
| Scale and Item | P | OddsRatio |
| ICARS: | ||
| Dysarthria fluency of speech | .048 | .17 |
| Dysmetria, right arm | .07 | .08 |
| Dysmetria, left arm | .010 | .05 |
| Intention tremor of finger: | .12 | |
| Right | .013 | |
| Left | .009 | .10 |
| Standing capacitiesa | .028 | .11 |
| Body sway with feet togethera | .019 | .12 |
| Walking capacities | .001 | .02 |
| Gait speed | .019 | .15 |
| UPDRS: | ||
| Facial expression | .003 | .07 |
| Speech | .015 | .12 |
| Finger taps, left | .016 | .13 |
| Leg agility: | .006 | .08 |
| Right | ||
| Left | .013 | .11 |
| Postural stability | .038 | .08 |
| Gait | .004 | .07 |
| CRST: | ||
| Speaking | .038 | .08 |
| Arm intention tremor | .022 | .17 |
| Handwriting | .001 | .04 |
| First drawing:b | ||
| Right hand | .035 | .18 |
| Left hand | .011 | .12 |
| Second drawing:c | ||
| Right hand | .001 | .03 |
| Left hand | .060 | .22 |
| Third drawing:d | ||
| Right hand | .002 | .06 |
| Left hand | .033 | .18 |
Eyes open.
Small spiral.
Large spiral.
Straight line.
Cerebellar symptoms
Such symptoms as postural and gait disturbance, dysmetria, and dysarthria were found in 78%–93% of the patients, depending on the type of symptom (table 2). Dysarthria (ICARS section 15–16), dysmetria (ICARS sections 15 and 16), and gait and postural symptoms (ICARS sections 1, 2, and 6) were scored in the mild-to-moderate range of problems.
Tremor
Tremor was intentional (typically 3–5 Hz) in 70% of patients, including intentional and mild resting in 30%, and was resting alone in 10%. Tremor was completely absent in 20% of patients (e.g., patient 2). Intention tremor always started in the dominant hand and progressed to the contralateral hand in the following years. The severity of the intention tremor was always discordant with the milder cerebellar symptoms (dysmetria and postural symptoms). The intention tremor was most frequently activated by writing. In four patients, moderate-to-severe impairment of fine motor skill was present in patients without clinically recognizable tremor.
Lower distal neuropathy
Neuropathies, such as abolished reflexes, loss of vibration sense, and abnormal pinprick discrimination and proprioception response, were present in 61% of patients. The levels of neuropathy were below the knee in all patients except one, in whom the symptoms extended to the thigh. Peripheral electrophysiological studies were performed in four patients, and nerve conduction was mildly but clearly reduced in all four. Thirty-six percent of patients also reported leg pain. In four patients, lower extremity arteritis was excluded by Doppler ultrasonography.
Parkinsonism
Parkinsonian symptoms were present in 57% of the patients. Mild bradykinesia (UPDRS 31) was present in 57% of the patients. Rigidity, which was rated as slight or was detected only when activated by mirror movements (UPDRS 22), was found in the upper limbs in 71% of the patients and in the lower limbs in 35%. Rigidity was scored as moderate in only three patients. The mean score of the UPDRS motor domain was 34/108. Slight resting tremor was present in 40% of patients. None of the patients presented with severe parkinsonian features or typical Parkinson disease.
Clinical muscle testing
Lower limb muscle weakness was present in 30% of the patients. Abnormal results of muscle testing occurred predominantly in the proximal muscle groups, with some muscle groups (psoas-gluteus) being as weak as 3/5. Toes were up-going or equivocal in 23% of the patients. However, there were no clear pyramidal symptoms, except for one patient who presented with a positive Babinski reflex and spasticity (initially diagnosed as amyotrophic lateral sclerosis). EMG of the lower extremities revealed borderline spontaneous activity in two patients (in proximal muscle groups). In the patient with spastic paraparesis, transcranial stimulation showed a significant blockage of the corticomotor neuron connection. Spine MRIs, performed in six patients, revealed no evidence of compression or radiculopathy. Results of a muscle biopsy performed on one patient were normal.
Cardiovascular symptoms
A history of ischemia and/or congestive heart failure was present in 55% of patients. Hypertension was present in 50%.
Additional patients
An additional group of six patients was not included in the main series because the MRI was not available (n=5) or did not demonstrate the main neuroimaging inclusion criterion (n=1). These patients had similar clinical presentations (table 2), with similar proportions of gait instability (83%), leg weakness (55%), intention tremor (83%), and resting tremor (17%). Urinary (17%) and bowel (0%) incontinence were less frequent. Results of standardized rating scales were not obtained for these patients.
Cognitive Symptoms
A relatively consistent pattern of cognitive impairment was observed among those individuals (n=11) who underwent a cognitive neuropsychological examination. Those who had been affected longest had lower IQ scores on the Wechsler Adult Intelligence Scale–III (Wechsler 1997). This was noteworthy on the performance (nonverbal) scales, in which scores ranged from 70 to 114 (mean 89.5). Difficulty on nonverbal tasks reflected both the effects of the movement disorder (since many of the tests involve manipulation of the materials and timing of performance) and impairment of working memory. Verbal IQ scores ranged from 77 to 128 (mean 97.3).
Deficits in executive cognitive functioning were especially noteworthy, and these were consistent with a behavioral pattern that included disinhibition, distractibility, and inappropriate jocularity. On the Behavioral Dyscontrol Scale (BDS) (Grigsby et al. 1998), only 3 of 11 individuals demonstrated a capacity for behavioral self-regulation that was in the normal range for age. Similarly, performance on tests of verbal fluency was significantly impaired, with only 3 of 11 scoring in the average range on the Animal Naming Test of the Boston Diagnostic Aphasia Examination (Goodglass and Kaplan 1983); 4 of 10 were in the average range on a word-generation test involving phonemic cues (Controlled Oral Word Association Test [Spreen and Benton 1977]). Apart from impaired executive functioning, the most commonly observed cognitive deficits were impairment in the speed and capacity of information processing.
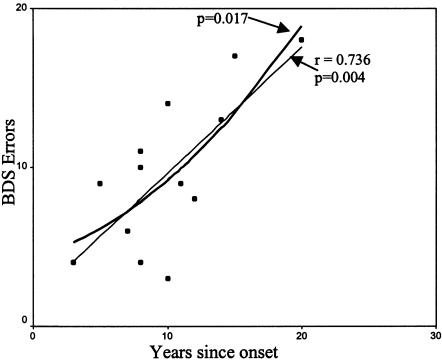
When compared with a series of 90 control individuals—matched to patients for sex, age, and education—who were given the BDS in another study, the decline of the BDS score with age is significantly greater in the premutation group (slope of the regression line for the premutation group, 0.390; slope of the regression line for the control group, 0.189). Using a linear regression to correlate the increase of mistakes on the BDS with the duration of the disease, we found a significant correlation (r=0.736, P=.004) (fig. 1).
Figure 1.
Executive function decline correlated to disease duration: linear and quadratic regression.
MRI
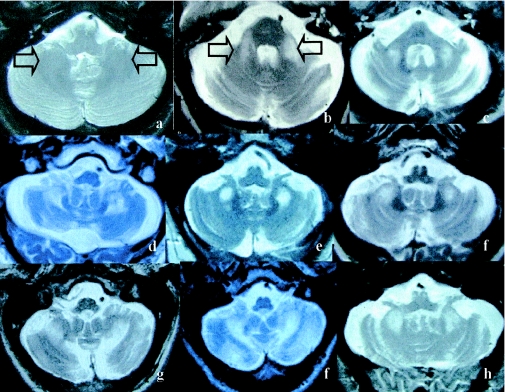
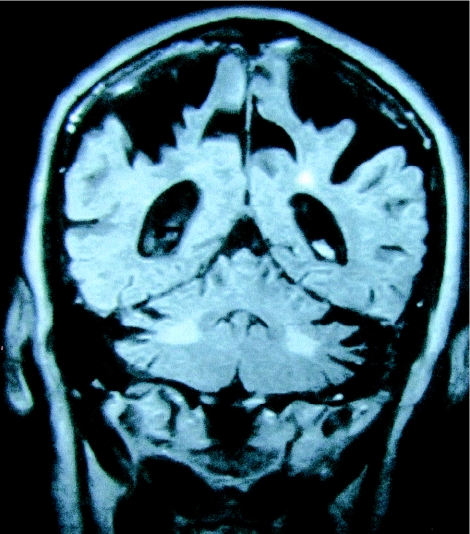
The presence of symmetric white matter lesions of the MCP was a mandatory inclusion criterion for the 20 patients. These lesions were strikingly similar (fig. 2), demonstrating decreased T1 and increased T2 signal intensity in deep white matter of the cerebellar hemispheres and the MCPs, sparing the dentate nuclei (fig. 2). Mild-to-moderate loss of cerebellar hemisphere cortical volume was present in 85% of the patients and was severe in one patient. Mild-to-moderate loss of cerebral cortical volume was present in 75% of the patients (fig. 3). The volume loss was severe in 20% of the patients. Areas of increased T2 signal intensity in the subependymal and deep white matter, disproportionate for age, were present in the frontal and parietal lobe in 75% of the patients. Three patients demonstrated more-diffuse white matter T2 hyperintensities, extending from the periventricular areas to the entire adjacent white matter. In two patients, the neuroradiologists diagnosed Binswanger disease, on the basis of the great extent of white matter disease on T2 and MRIs of fluid-attenuated inversion recovery.
Figure 2.
MRI findings, T2-weighted axial images of the cerebellum. a, Normal T2 weighted axial image of the cerebellum (arrows indicate cerebellar white matter). b and c, Symmetrical regions of increased T2 signal intensity in the MCPs. d–h, Adjacent cerebellar white matter.
Figure 3.
Coronal inversion recovery image demonstrating severe parietal volume loss and increased signal intensity in the cerebellar white matter.
Progression Of Clinical Involvement
Patients experienced onset of intention tremor at a mean (± SD) age of 63 (± 8) years, gait instability at 63 (± 6) years, memory difficulties at 63 (± 6) years, urinary incontinence at 64 (± 6) years. Half (55%) of our patients required a cane, beginning at a mean age of 70 (± 7) years; 35% of the patients required a walker at 71 (± 6) years; 30% of the patients required a wheel chair at 72 (± 5) years. The onset of the first ambulation impairment (use of a cane) was not significantly correlated (P=.174) with the onset of the disorder. Three patients were bedridden at ages 55, 65, and 80 years. Additional data from five deceased patients previously reported by us (Hagerman et al. 2001; Greco et al. 2002) were also added. The mean (± SD) age at death was 74 (± 6) years. In 9 patients, the age at death was correlated with the age at onset of the disorder (P=.039). The cause of death was reported as congestive heart failure in 8/9 patients and as pneumonia in 1/9 patients.
Molecular Findings
Molecular findings are summarized in table 4. Our patients were all in a very narrow CGG count range (mean 84; range 69–99). The FMRP levels (mean [± SD] 75% [± 8]) were borderline or slightly lower than those reported in nonaffected premutation carriers (Tassone et al. 1999; Willemsen et al. 1997), and two patients revealed levels just below 70%. Correlations between CGG repeat count, mRNA levels, FMRP levels, and age at disease onset were not statistically significant.
Table 4.
Molecular Findings
| Mean ± SD | |
| CGG repeat number | 84 ± 10 |
| FMR1 RNA levelsa | 4 ± 2 |
| FMRP levels (%) | 75 ± 8 |
RNA levels are reported as fold elevated over those in normal control individuals.
Discussion
The patients were selected to obtain a conservative representation of FXTAS. They were required to meet one clinical criterion (intention tremor or ataxia) in addition to a specific radiological criterion (symmetric white matter lesions of the MCP) to be included in this series. This group of patients allows us to delineate FXTAS and to describe the progression and the frequency of other symptoms that are not among the inclusion criteria. We have also presented clinical data on six additional patients who met the clinical, but not the radiological criterion. For the latter group, the clinical presentations were similar to those of the inclusion group.
Elsewhere, we described a cerebellar white matter lesion involving the MCP and deep cerebellar white matter (fig. 2), and we demonstrated its association with the neurological findings (Brunberg et al., 2002). This MCP finding is the most specific marker of FXTAS, other than the neuropathologic feature of intranuclear inclusions (Greco et al. 2002). We have not found these radiological signs in six asymptomatic male carriers of the premutation (>50 years old) (authors' unpublished data), suggesting that the MRI findings are closely related to the clinical manifestations. However, two patients have presented with moderate parkinsonism, and one patient has a combination of intention tremor and cerebellar ataxia, in the absence of MCP lesions (unpublished data), so the association is not absolute. Recently, the MCP lesion has been described in as many as 42% of a group of patients with MSA type C (Bhattacharya et al. 2002; Naka et al. 2002).
In the early stage of the disorder, patients may present with only one clinical symptom (essential tremor being the most common), which can suggest many diagnoses (Leehey et al. 2003). The breadth of early clinical diagnoses is suggestive of great clinical heterogeneity; however, as the disorder progresses, these patients generally develop gait ataxia, intention tremor, parkinsonism, peripheral neuropathy, and cognitive decline. In our group, 55% of the patients combined three of these symptoms. Thus, in the later stages of the disorder, the clinical and radiological picture can resemble multiple-system atrophy–cerebellar type (MSA-C).
Four factors appear to play a role in the walking difficulties: cerebellar ataxia, peripheral neuropathy, parkinsonian symptoms, and lower extremity muscle weakness. The cerebellar symptoms appear to be responsible for part of the instability and abnormal gait reported by the patients. Parkinsonian symptoms are mild and do not appear to contribute to the loss of ambulation (posture, postural stability, gait, and bradykinesia were scored in the mild range on the UPDRS for the majority of patients). Impaired proprioception is also common and could contribute to instability.
In the course of disease progression, precipitating factors seem to play an important role. Four of our patients experienced dramatic clinical decline after medical procedures that required general anesthesia. In two of these patients, computed axial tomography excluded strokes. None of the four patients ever returned to baseline levels of cognitive and motor function. The duration of the anesthesia seemed to correlate with the extent of the motor and cognitive declines. The significant correlation (P=.039) of the age at death and at onset of the disorder in a small sample (n=9) suggests that FXTAS is a significant factor in the death of these patients. Congestive heart failure was reported as the cause of death in 8/9 patients, suggesting that FXTAS is a risk factor for heart failure. This finding needs to be confirmed in a larger sample with age-matched controls. It should be noted that our sample is probably biased with regard to the progression and severity of disease, because many of these patients were reported to us as a result of the severity of their clinical presentation.
To aid in the diagnosis of FXTAS, we propose three diagnostic categories: “definite,” “probable,” and “possible.” Each category reflects combinations of symptom types (see table table 5 for definitions of symptom types). The diagnostic categories are as follows:
-
1.
“Definite” indicates the presence of one major radiological sign plus one major clinical symptom.
-
2.
“Probable” indicates the presence of either one major radiology sign plus one minor clinical symptom or two major clinical symptoms.
-
3.
“Possible” indicates the presence of one minor radiology sign plus one major clinical symptom.
Table 5.
Symptom Types[Note]
| Examination and Degree | Observation |
| Radiological: | |
| Major | MRI white matter lesions in MCPs and or brain stem |
| Minor | MRI white matter lesions in cerebral white matter |
| Minor | Moderate-to-severe generalized atrophy |
| Clinical: | |
| Major | Intention tremor |
| Major | Gait ataxia |
| Minor | Parkinsonism |
| Minor | Moderate-to-severe short-term memory deficiency |
| Minor | Executive function deficit |
Note.— Inclusion criterion: CGG repeat 55–200.
The criteria for definite diagnosis were used for the 20 patients of the main series in the present article. The six additional patients, who did not meet the radiological criterion, fell into the categories of probable diagnosis (n=4) or possible diagnosis (n=2). In this series, the CGG repeat number is in the mid-to-low premutation range, and the sample could be biased by the fact that all of the included individuals have a grandchild affected with fragile X. We do not know the limits of repeat size responsible for this disorder; it is likely that as we identify patients who attend neurology clinics and do not have a family history of fragile X, we will see lower numbers of CGG repeats. Furthermore, the range of CGG repeats (55–200) defining the premutation could be different from the range of CGG repeats responsible for this new disorder, which may start at <54 and end at >200 (135 repeats being the highest repeat observed to date [Greco et al. 2002]). Further studies and different means of ascertainment are needed to determine the lower and upper limits of the CGG repeat associated with this disorder, and further clinicopathological/radiological correlates will help refine the diagnostic criteria for this syndrome.
As presently defined, FXTAS appears to be limited to older adult male carriers of the premutation. This striking sex ratio bias is consistent with an X-linked disorder. However, we do not know why the bias is so much greater than for fragile X syndrome. The progressive neurological phenotype associated with the premutation allele places the FMR1 gene in the same category as most of the other trinucleotide repeat disorders, including those with gain of function (e.g., CAG repeats in SCA1, -2, -3, -6, and -7; Huntington disease; spino-bulbar-muscular atrophy; and dentatorubropallidoluysian atrophy) or a loss of function (e.g., SCA8 or -12 or Friedreich ataxia).
Only 40% of the patients had a positive family history in first- or second-degree relatives for Parkinson disease, dementia, or unspecified progressive neurological disorders. Therefore, the penetrance of this disorder among male premutation carriers will be incomplete and, in most of the families, will manifest itself as a sporadic condition. We are currently conducting an epidemiological study in California, which suggests that the penetrance is age related (authors' unpublished data).
Conclusion
We now believe that the FMR1 gene is responsible for two distinct clinical entities: a neurodevelopmental disorder (fragile X syndrome) and a neurodegenerative disorder (FXTAS). The full expansion (>200 CGG repeats) is responsible for the silencing of the FMR1 gene through methylation (Pieretti et al. 1991). In fragile X syndrome, the lack of FMRP causes mental retardation and other behavioral symptoms. However, in the patients described herein, there is no evidence for a neurodevelopmental disorder in childhood; 60% of the patients had a college education, and FMRP levels were normal or only mildly decreased. We suggest that this disorder is allelic to the fragile X syndrome and should be considered as a distinct neurodegenerative disorder. The molecular mechanism leading to FXTAS is likely to be quite distinct from the silencing mechanism operating in fragile X syndrome and, as suggested previously (Hagerman et al. 2001; Greco et al. 2002), may be related to a gain-of-function toxic effect of the elevated FMR1 mRNA levels (and, specifically, elevated CGG repeat levels) previously reported (Tassone et al. 2000a, 2000b; Kenneson et al. 2001). The mechanism of an mRNA gain of function has been proposed for myotonic dystrophy, on the basis of the demonstration, in transgenic mice, that the myotonia phenotype was obtained by expressing untranslated CUG repeats alone (Mankodi et al. 2000). Finally, we suggest that DNA testing for the FMR1 premutation may reveal additional carriers among patients presenting clinically with MSA, essential tremor associated with late-onset cerebellar ataxia, or atypical Parkinson disease.
Acknowledgments
This work was supported by National Institute of Child Health and Human Development grant HD 36071 (to R.J.H.), National Institute of Neurological Disorder and Stroke grant NS 43532 (to P.J.H.), and by the Lavoisier fund (support to S.J.) and the M.I.N.D. Institute (support to S.J.).
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FMR1) [PubMed]
References
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, et al. (1999) Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 83:322–325 [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya K, Saadia D, Eisenkraft B, Yahr M, Olanow W, Drayer B, Kaufmann H (2002) Brain magnetic resonance imaging in multiple-system atrophy and Parkinson disease: a diagnostic algorithm. Arch Neurol 59:835–842 [DOI] [PubMed] [Google Scholar]
- Brown WT, Houck GE Jr, Jeziorowska A, Levinson FN, Ding X, Dobkin C, Zhong N, Henderson J, Brooks SS, Jenkins EC (1993) Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA 270:1569–1575 [PubMed] [Google Scholar]
- Brunberg J, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ (2002) Fragile X premutation carriers: characteristic MR imaging findings in adult male patients with progressive cerebellar and cognitive dysfunction. Am J Neurol Radiol 23:1757–1766 [PMC free article] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL (1993) The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet 4:335–340 [DOI] [PubMed] [Google Scholar]
- Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F (2002) Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet 11:371–378 [DOI] [PubMed] [Google Scholar]
- Dorn MB, Mazzocco MM, Hagerman RJ (1994) Behavioral and psychiatric disorders in adult male carriers of fragile X. J Am Acad Child Adolesc Psychiatry 33:256–264 [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, UPDRS Development Committee (1987) Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Godstein M (eds) Recent developments in Parkinson’s disease. Macmillan Health Care Information, Florham Park, NJ, pp 153–164 [Google Scholar]
- Fahn S, Tolosa E, Marin C (1998) Clinical rating scale for tremor. In: Jankovic J, Tolosa E (eds) Parkinson’s disease and movement disorders. Urban & Schwarzenberg, Baltimore, pp 225–234 [Google Scholar]
- Franke P, Leboyer M, Gansicke M, Weiffenbach O, Biancalana V, Cornillet-Lefebre P, Croquette MF, Froster U, Schwab SG, Poustka F, Hautzinger M, Maier W (1998) Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychr Res 80:113–127 [DOI] [PubMed] [Google Scholar]
- Franke P, Maier W, Hautzinger M, Weiffenbach O, Gansicke M, Iwers B, Poustka F, Schwab SG, Froster U (1996) Fragile-X carrier females: evidence for a distinct psychopathological phenotype. Am J Med Genet 64:334–339 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E (1983) The assessment of aphasia and related disorders, 2nd ed. Lea & Febiger, Philadelphia [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ (2002) Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125:1760–1771 [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF (1998) Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc 46:590–596 [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Greco C, Hagerman RJ, Tassone T, Chudley AE, Del Bbigio MR, Jacquemont S, Gane L, Leehey M (2002) Neuronal intranuclear inclusions in a tremor/ataxia syndrome among fragile X premutation carriers. Am J Hum Genet Suppl 71:A508 [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ (2001) Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57:127–130 [DOI] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST (2001) Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 10:1449–1454 [DOI] [PubMed] [Google Scholar]
- Leehey M, Munhoz RP, Lang AE, Brunberg J, Grigsby J, Greco C, Jacquemont S, Tassone F, Lozano AM, Hagerman PJ, Hagerman R (2003) The fragile X premutation presenting as essential tremor. Arch Neurol 60:117–121 [DOI] [PubMed] [Google Scholar]
- Mankodi A, Logigian E, Callahan L, McClain C, White R, Henderson D, Krym M, Thornton CA (2000) Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289:1769–1773 [DOI] [PubMed] [Google Scholar]
- Naka H, Ohshita T, Murata Y, Imon Y, Mimori Y, Nakamura S (2002) Characteristic MRI findings in multiple system atrophy: comparison of the three subtypes. Neuroradiology 44:204–209 [DOI] [PubMed] [Google Scholar]
- Oberle L, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL (1991) Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252:1097–1102 [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL (1991) Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66:817–822 [DOI] [PubMed] [Google Scholar]
- Riddle JE, Cheema A, Sobesky WE, Gardner SC, Taylor AK, Pennington BF, Hagerman RJ (1998) Phenotypic involvement in females with the FMR1 gene mutation. Am J Ment Retard 102:590–601 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K (1995) Prevalence of carriers of premutation-size alleles of the FMRI gene and implications for the population genetics of the fragile X syndrome. Am J Hum Genet 57:1006–1018 [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Dean J, Howard Peebles PN, Bugge M, Mikkelsen M, Tommerup N, Hull C, Hagerman R, Holden JJ, Stevenson RE (1994) Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet 51:400–402 [DOI] [PubMed] [Google Scholar]
- Sobesky WE (1996) The treatment of emotional and behavioral problems in fragile X syndrome. In: Hagerman RJ, Cronister A (eds) Fragile X syndrome: diagnosis, treatment, and research, 2nd ed. The Johns Hopkins University Press, Baltimore, pp 332–348 [Google Scholar]
- Spreen O, Benton AL (ed) (1977) Neurosensory Center Comprehensive Examination for Aphasia (NCCEA)—Revised. University of Victoria Neuropsychology Laboratory, Victoria, Canada [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain W, Hagerman P (2000a) Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet 97:195–203 [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Iklé DN, Dyer PN, Lampe M, Willemsen R, Oostra B, Tayor AK (1999) FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet 84:250–261 [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ (2000b) Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in fragile X syndrome. Am J Hum Genet 66:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AK, Safanda JF, Fall MZ, Quince C, Lang KA, Hull CE, Carpenter I, Staley LW, Hagerman RJ (1994) Molecular predictors of cognitive involvement in female carriers of fragile X syndrome. JAMA 271:507–514 [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B (1997) International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145:205–211 [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, Van Ommen JB, Blonden LA, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 3165:905–914 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997) WAIS-III: Wechsler Adult Intelligence Scale–Third Edition: administration and scoring manual. The Psychological Corporation, San Antonio, TX [Google Scholar]
- Willemsen R, Mohkamsing S, de Vries B, Devys D, van den Ouweland A, Mandel JL, Galjaard H, Oostra B (1995) Rapid antibody test for fragile X syndrome. Lancet 345:1147–1148 [DOI] [PubMed] [Google Scholar]
- Willemsen R, Smits A, Mohkamsing S, van Beerendonk H, de Haan A, de Vries B, van den Ouweland A, Sistermans E, Galjaard H, Oostra BA (1997) Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Hum Genet 99:308–311 [DOI] [PubMed] [Google Scholar]