Abstract
Although the molecular basis of pseudohypoparathyroidism type 1b (PHP type 1b) remains unknown, a defect in imprinting at the GNAS1 locus has been suggested by the consistent finding of paternal-specific patterns of DNA methylation on maternally inherited GNAS1 alleles. To characterize the relationship between the genetic and epigenetic defects in PHP type 1b, we analyzed allelic expression and methylation of CpG islands within exon 1A of GNAS1 in patients with sporadic PHP type 1b and in affected and unaffected individuals from five multigenerational kindreds with PHP type 1b. All subjects with resistance to parathyroid hormone (PTH) showed loss of methylation of the exon 1A region on the maternal GNAS1 allele and/or biallelic expression of exon 1A–containing transcripts, consistent with an imprinting defect. Paternal transmission of the disease-associated haplotype was associated with normal patterns of GNAS1 methylation and PTH responsiveness. We found that affected and unaffected siblings in one kindred had inherited the same GNAS1 allele from their affected mother, evidence for dissociation between the genetic and epigenetic GNAS1 defects. The absence of the epigenetic defect in subjects who have inherited a defective maternal GNAS1 allele suggests that the genetic mutation may be incompletely penetrant, and it indicates that the epigenetic defect, not the genetic mutation, leads to renal resistance to PTH in PHP type 1b.
Introduction
Pseudohypoparathyroidism (PHP), the first human disorder of hormone resistance to be identified (Albright et al 1942), is characterized by biochemical hypoparathyroidism (i.e., hypocalcemia and hyperphosphatemia) due to parathyroid hormone (PTH) resistance rather than PTH deficiency. Two genetically distinct forms of PHP type 1 have been described (Levine et al. 1980). The more common variant, termed “PHP type 1a” (MIM 30080 and MIM 103580), is an autosomal dominant, pleiotropic disorder with resistance to multiple hormones (PTH, thyroid-stimulating hormone [TSH], luteinizing hormone [LH], growth hormone–releasing hormone [GHRH]) and a constellation of developmental defects termed “Albright’s hereditary osteodystrophy” (AHO) (Albright et al. 1942). Patients with PHP type 1a have mutations in maternal GNAS1 alleles that abrogate expression or activity of Gs, the heterotrimeric G protein (Farfel et al. 1980a, 1980b, 1982; Levine et al. 1980, 1983b; Downs et al. 1983) that couples receptors to activation of adenylyl cyclase (Levine et al. 1983a; Namnoum et al. 1998; Kaartinen et al 1994). By contrast, identical mutations in paternal alleles are associated with AHO and normal hormone responsiveness, a variant termed “pseudopseudohypoparathyroidism” (PPHP), which results from tissue-specific genomic imprinting at this locus.
PHP type 1b (MIM #603233) is a clinically distinct variant of PHP that is also linked to the GNAS1 locus (Juppner et al. 1998; Bastepe et al. 2001b; Jan de Beur et al. 2003). Subjects with PHP type 1b lack features of AHO, show renal resistance to PTH as the only manifestation of hormone resistance, and have normal Gsα activity in accessible tissues (Jan de Beur and Levine 2001). Subjects with PHP type 1b have paternal-specific patterns of cytosine methylation within differentially methylated regions (DMR) of maternally inherited GNAS1 alleles (Liu et al. 2000a; Bastepe et al. 2001b), suggesting that an imprinting defect that affects expression of GNAS1 in the proximal renal tubule is the basis of this disorder; however, the specific genetic mutation(s) that accounts for this epigenetic defect is unknown. In the present study, we show that loss of imprinting of exon 1A on maternal GNAS1 alleles is a consistent finding in DNA from peripheral blood leukocytes and transformed lymphoblast cell lines of subjects with sporadic or inherited PHP type 1b. Remarkably, some subjects who inherit a defective maternal GNAS1 allele do not manifest an imprinting defect, which implies that the genetic mutation may be incompletely penetrant. The presence of normal PTH responsiveness in these subjects indicates that the epigenetic defect, not the genetic mutation, is predictive of the development of renal resistance to PTH in PHP type 1b
Methods
Patients
The five kindreds with PHP type 1b studied in this project have been described elsewhere (Jan de Beur et al. 2003). The clinical and biochemical characteristics of affected members, as well as six additional subjects with sporadic PHP type 1b, met the criteria for PHP type 1b as described elsewhere (Levine et al. 1980, 1983a; Jan de Beur and Levine 2001, Jan de Beur et al. 2003). The diagnosis of PHP type 1b was established in family R by evidence of PTH resistance (hypocalcemia or normocalcemia with elevated serum levels of intact PTH), absence of features of AHO, normal responsiveness to TSH, absence of evidence of vitamin D deficiency or hypomagnesemia, and normal sequence of 13 coding exons and exon/intron boundaries of the GNAS1 gene. The Joint Committee on Clinical Investigation of The Johns Hopkins University School of Medicine approved the study protocol, and written informed consent was obtained from all subjects or their parents.
DNA Analyses
For GNAS1 gene analysis, DNA was extracted from peripheral blood leukocytes by standard methods (Sambrook et al. 1989), and the nucleotide sequence of exons 1–13, the flanking exon/intron boundaries, the exon 1 promoter region, and exon 1A were analyzed as described elsewhere (Jan de Beur et al. 1998, 2003).
For restriction analysis, 10-μg aliquots of genomic DNA were digested with the indicated restriction enzymes (New England Biolabs). Products were separated on 1.5% agarose gels (SeaKem, FMC Bioproducts), transferred to nylon membranes, and hybridized to a 32P-radiolabeled genomic fragment of GNAS1 (nucleotides 29078–29993 of chromosome 20q [GenBank accession number AL121917]). Hybridization signals were detected with a BioRad PhosphorImager or on Kodak XAR film.
Bisulfite-Modified Methylation Analysis of GNAS1
DNA (1 μg) was digested with 20 U EcoRI (at 37°C for 2 h) and denatured (in 3M NaOH at 37°C for 15 min, then at 95°C for 3 min); 400 μl of sodium bisulfite solution (Nagane et al. 2000) was added and incubated at 55°C for 20 h. The DNA was desalted using the Wizard DNA Clean-Up system (Promega). Bisulfite-treated DNA was subjected to methylation-specific nested PCR, to amplify GNAS1 sequences, using primers and PCR conditions as described in table A (online only). PCR products were gel purified and sequenced using the USB Radiolabeled Terminator Cycle Sequencing kit (USB).
Table A.
Conditions Used for PCR
|
Primer |
||||
| Transcript | Forward | Reverse | PCR Conditions | Product size(bp) |
| NESP55 | 5′-GTCACTAATGGAGGACGCCGT-3′ | 5′-TTCGTAGCAGGCACGCACTCCTTCAACCTC-3 | 94°C × 3 min; 39 cycles of 94°C × 40 s, 62°C × 35 s, 72°C × 1 min; then 72°C × 5 min | 400 |
| XLαs | 5′-GGATGCCTCCGCTGGTTTGAGCATCGGG-3′ | 5′-TTCGTAGCAGGCACGCACTCCTTCAACCTC-3′ | 94°C × 4 min; 39 cycles of 94°C × 40 s, 64°C × 30 s, 72°C × 1 min; then 72°C × 5 min | 818 |
| Exon 1A | 5′-GGACACTCAGTCGCGTCGGCAC-3′ | 5′-TTCGTAGCAGGCACGCACTCCTTCAACCTC-3′ | 94°C × 3 min; 30 cycles of 94°C × 30 s, 72°C × 3 min; then 72°C × 5 min | 510 |
| Gsα | 5′-ATGGGCTGCCTCGGGAACAGT-3′ | 5′-TTCGTAGCAGGCACGCACTCCTTCAACCTC-3′ | 94°C × 3 min; 35 cycles of 94°C × 1 min, 67°C × 35 s, 72°C × 40 sec; then 72°C × 5 min | 482 |
| Exon 1Abisulfite sequencing: | ||||
| 1st round | 5′-TAGGGGTYGTTGTTATGGGT-3′ | 5′-AATAACAAAAATCTATTTACCCTCAAAC-3′ | 94°C × 4 min; 39 cycles of 94°C × 45 s, 62°C × 45 s; then 72°C × 6 min | …a |
| 2nd round | 5′-GGTTGYGTTAGGTGGTTGG-3′ | 5′-CTCCTTAATTTAACTCTTAAACAC-3′ | 94°C× 4 min; 39 cycles of 94°C × 45 s, 62°C × 45 s; then 72°C × 6 min | 340 |
Use PCR product as template in second round.
RT-PCR Analysis of GNAS1 Expression
First, strand cDNA was synthesized from total RNA from fresh lymphocytes or cultured Epstein-Barr virus–transformed lymphoblasts with Superscript II (Invitrogen). PCR was performed with specific forward primers that anneal to nucleotide sequences in alternative first exons corresponding to NESP55, XLαs, exon 1A, and Gsα transcripts, plus a reverse primer that corresponds to exon 6 sequences, to include a common exon 5 (Ala 131) polymorphism. (PCR primer pairs and conditions are listed in table A [online only].) PCR products were gel purified and sequenced using the USB Radiolabeled Terminator Cycle Sequencing kit (USB).
Results
Imprinting of Alternative First Exons of GNAS1
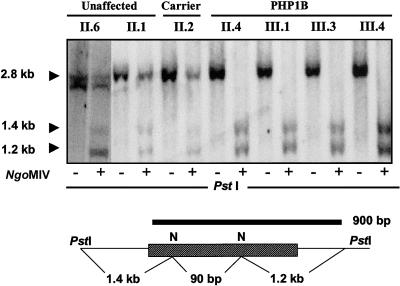
Differential methylation of cytosines within specific sequences of the GNAS1 locus has been implicated as the basis of monoallelic expression of NESP55, XLαs, and exon 1A transcripts (Hayward et al. 1998a,1998b; Liu et al. 2000a). We used restriction endonuclease digestion to determine the methylation status of the exon 1A DMR region in subjects with sporadic and familial PHP type 1b. A probe spanning the exon 1A DMR was hybridized to genomic DNA digested with PstI alone or with PstI plus the methylation-sensitive restriction enzyme NgoMIV (fig. 1). Digestion of DNA samples from unaffected family members, PHP1b carriers, unaffected subjects, or patients with PHP type 1a with PstI produced a single 2.8-kb band; and digestion with both PstI and NgoMIV generated a 2.8-kb band of ∼50% intensity, plus three smaller bands, two of which (1.4 and 1.2 kb) were visible on the autoradiograms, representing digestion at unmethylated NgoMIV sites in the exon 1A region. This pattern is consistent with methylation of exon 1A on the maternal GNAS1 allele and lack of methylation on the paternal GNAS1 allele. By contrast, digestion of DNA samples from patients with familial or sporadic PHP type 1b with both NgoMIV and PstI resulted in complete loss of the 2.8-kb band, indicating that the NgoMIV sites on both alleles were unmethylated and susceptible to cleavage (fig. 1). These results are consistent with a loss of methylation of the exon 1A region of the maternal GNAS1 allele.
Figure 1.
Exon 1A methylation status revealed by restriction digestion of genomic DNA. Genomic DNA was digested with PstI alone or PstI plus NgoMIV, which cuts only unmethylated sequences. Digests were transferred to nylon membranes and hybridized to a radiolabeled genomic DNA probe corresponding to exon 1A of GNAS1. A representative autoradiogram (top) indicates results of individuals from family R labeled with the pedigree number corresponding to figure 4. Bottom, positions of the recognition sites for the two restriction enzymes with respect to exon 1A (striped box), along with the corresponding position of the 900-bp probe. Digestion of DNA from unaffected individuals with PstI generates a single, 2.8-kb fragment from both alleles, whereas digestion with both PstI and NgoMIV yields a 2.8-kb fragment from the maternal (methylated) allele and fragments of 1.4 kb, 1.2 kb, and 90 bp (not shown) from the paternal (unmethylated) allele. Digestion of DNA from subjects with PHP type 1b with PstI yields a single fragment of 2.8-kb, but after digestion with PstI plus NgoM IV there is complete loss of the 2.8-kb fragment, indicating that both alleles are unmethylated within the DMR of exon 1A (see text).
Bisulfite Sequencing of GNAS1
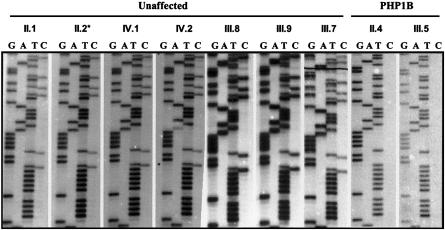
Exon 1A sequences were analyzed after treatment of DNA with sodium bisulfite, to examine cytosine methylation more comprehensively. We performed methylation-sensitive PCR, using oligonucleotide primers that were designed to anneal to sequences containing cytosine residues that were certain to be converted to uracil by sodium bisulfite treatment because they do not occur in CpG dinucleotides. Bisulfite-treated DNA from control subjects revealed a 50% reduction in the intensity of cytosine bands within CpG dinucleotides of exon 1A, consistent with methylation of only one allele (fig. 2). By contrast, bisulfite-treated DNA from subjects with PHP type 1b showed conversion of all C bands to T bands, indicating that both GNAS1 alleles were unmethylated at CpG positions within exon 1A (fig. 2).
Figure 2.
Bisulfite sequencing of exon 1A of GNAS1. Autoradiograms showing the nucleotide sequence of a portion of GNAS1 exon 1A after bisulfite treatment of genomic DNA in unaffected subjects, a PHP1b carrier (*), and subjects with PHP1b from family R, identified with pedigree numbers corresponding to figure 4. PCR products were directly sequenced, to avoid quantitative errors that might arise because of different cloning efficiencies (see text), and thus represent sequences derived from maternal and paternal alleles. The sequence ladder from the unaffected and carrier subject shows both Cs and Ts at multiple positions, corresponding to cytosines within CpGs that were methylated (maternal allele) or unmethylated (paternal allele), respectively. By contrast, the sequence ladder from the subjects with PHP type 1b shows only Ts, indicating that cytosines on both maternal and paternal alleles were unmethylated.
Expression of GNAS1 Alleles by RT-PCR Analysis
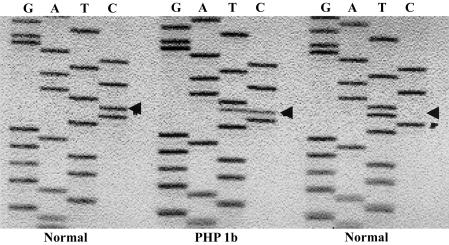
To evaluate allelic expression of GNAS1 transcripts generated from the promoter regions for NESP55, XLαs, exon 1A, and Gsα, we performed RT-PCR using RNA from individuals who were heterozygous for a polymorphism (T/C) within GNAS1 exon 5. Unaffected subjects and patients with PHP 1a showed uniallelic expression for transcripts corresponding to NESP55, XLαs, and exon 1A, and biallelic expression of Gsα transcripts (fig. 3; table 1). Similar RT-PCR analyses in subjects with PHP type 1b who were heterozygous for polymorphisms in either GNAS1 exon 5 or exon 1A (Liu et al. 2000a; Jan de Beur et al. 2003) showed biallelic expression in exon 1A in all subjects with familial or sporadic PHP type 1b. Expression of NESP55 was uniallelic in all subjects, and expression of XLαs was uniallelic in five of six subjects. Expression of Gsα was biallelic in all tested individuals. The biallelic expression of exon 1A was a heritable epigenetic trait and was completely concordant with the presence of PTH resistance in subjects with PHP type 1b (fig. 4; table 1).
Figure 3.
RT-PCR expression analysis of GNAS1 exon 1A. Total RNA from fresh peripheral blood leukocytes or transformed lymphoblasts was reverse transcribed and amplified using an exon 1A–specific upstream primer and an exon 6 downstream primer. Direct sequencing of the RT-PCR products is shown for subjects who are heterozygous for a common T/C polymorphism in exon 5. The normal individuals show uniallelic expression of exon 1A transcripts, as indicated by either the C or T (arrow in the sequence ladder) at the polymorphic nucleotide site, indicating expression from only the paternal allele. By contrast, the subject with PHP type 1b shows both the C and T nucleotides, indicating biallelic expression of exon 1A.
Table 1.
Expression and Methylation Status of Lymphoblast GNAS1Gene[Note]
|
Expression ata |
|||||
| Group and Subject(Disorder) | NESP55 | XLαs | Exon 1A | Exon 1 | Methylationat Exon 1Ab |
| Family R: | |||||
| II.2 (Carrier) | NP | NP | NP | NP | +/− B,S |
| II.4 (PHP1B) | NP | NP | Biallelic | NP | −/− B,S |
| III.1 (PHP1B) | NP | NP | NP | NP | −/−S |
| III.3 (PHP1B) | NP | NP | Biallelic | NP | −/− S |
| III.4 PHP1B) | NP | NP | Biallelic | NP | −/− S |
| II.1 (Unaffected) | NP | NP | NP | NP | +/− B,S |
| II.6 (Unaffected) | NP | NP | Uniallelic | NP | +/− S |
| III.5 (Unaffected) | ND | ND | Uniallelic | ND | +/− B |
| III.6 (Unaffected) | ND | ND | Uniallelic | Biallelic | ND |
| III.7 (Unaffected) | NP | NP | NP | NP | +/− B |
| III.8 (Unaffected) | ND | ND | ND | ND | +/− B |
| III.9 (Unaffected) | ND | ND | ND | ND | +/− B |
| IV.1 (Unaffected) | ND | ND | Uniallelic | ND | +/− B |
| IV.2 (Unaffected) | ND | ND | Uniallelic | ND | +/− B |
| Familial PHP1b (N = 17) | Uniallelic (4/4) | Uniallelic (4/4) | Biallelic (5/5) | Biallelic (4/4) | −/− (14/14) |
| Sporadic PHP1b (N = 6) | Uniallelic (2/2) | Biallelic (1/2) | Biallelic (4/4) | Biallelic (3/3) | −/− (6/6) |
| PHP1b carrier (N = 2) | Uniallelic (1/1) | Uniallelic (1/1) | Uniallelic (1/1) | Biallelic (1/1) | +/− (2/2) |
| PHP1a (N = 9) | ND | ND | Uniallelic (5/5) | Biallelic (1/1) | +/− (7/7) |
| Unaffected (N = 20) | Uniallelic (1/1) | Uniallelic (3/3) | Uniallelic (11/11) | Biallelic (5/5) | +/− (12/12) |
Note.— Numbers in parentheses are no. of subjects with expression or no. of subjects with methylation/total no. of subjects.
Biallelic = expressed from maternal and paternal allele; ND = not done; NP = noninformative for exon 5 polymorphism; uniallelic = expressed from only one parental allele.
B = bisulfite sequencing; S = Southern blot analysis; −/−= absence of methylation demonstrated by sequencing and/or Southern analysis; +/ = heterozygous methylation.
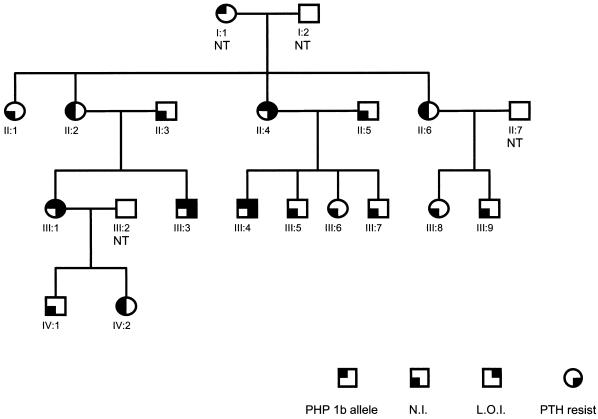
Figure 4.
Family R pedigree. Unaffected individuals are indicated by an open circle (female) or square (male). An individual carrying the defective PHP type 1b allele is indicated by a blackened upper left quadrant of the symbol; PTH resistance is indicated by a blackened lower right quadrant; normal imprinting is indicated by a blackened lower left quadrant; loss of maternal imprinting is indicated by a blackened upper right quadrant. NT = not tested. Imprinting of exon 1A was determined by restriction endonuclease digestion using methylation sensitive enzymes or by bisulfite sequencing (see text).
Inheritance and Penetrance of the GNAS1 Epigenotype
In an earlier study, we used linkage analysis to map the genetic defect causing autosomal dominant PHP type 1b in these five kindreds to a region of chromosome 20q13 that includes part of GNAS1 (Jan de Beur et al. 2003). All subjects with PTH resistance in these five kindreds showed evidence of the PHP type 1b epigenetic abnormality in GNAS1, as documented by loss of methylation of exon 1A on the maternal GNAS1 allele and/or biallelic expression of exon 1A–containing transcripts (fig. 4; table 1). Moreover, affected subjects had a maternally inherited disease-associated haplotype, whereas unaffected subjects who were obligate gene carriers had inherited the same haplotype paternally, consistent with a model of maternal-specific transmission of PTH resistance due to presumed tissue-specific imprinting of the Gsα promoter (Juppner et al. 1998; Liu et al. 2000a; Bastepe et al. 2001b; Germain-Lee et al. 2002; Mantovani et al. 2002). These results are consistent with complete concordance between the epigenetic defect and PTH resistance.
By contrast, analysis of pedigree R (fig. 4) provides evidence for dissociation between maternal transmission of the disease-associated locus and the epigenetic GNAS1 defect. Specifically, members of the same sibship show either PTH resistance (subject II.4) or PTH responsiveness (subjects II.2 and II.6), despite having inherited the same GNAS1 allele from their mother (fig. 4) (Jan de Beur et al. 2003). That this is not a result of an undetected genetic rearrangement or conversion in the maternal allele inherited by subjects II.2 and II.6 is demonstrated by the subsequent transmission of this allele to subject II.2’s two children, subjects III.1 and III.3, both of whom exhibit PTH resistance and the epigenetic GNAS1 methylation. Remarkably, subject IV.2, who inherited the disease-associated GNAS1 allele from his affected mother (subject III.1), also lacks the epigenetic methylation defect. As expected from his normal epigenotype, subject IV.2 has normal PTH responsiveness at age 4 years. However, in some cases, hormone resistance in PHP 1a may manifest after age 5 years. Thus, the genetic mutation that is presumed to alter imprinting at the GNAS1 locus exhibits incomplete penetrance and results in inconsistent acquisition of a paternal epigenotype on a maternal GNAS1 allele.
Discussion
Genomic imprinting results from an epigenetic modification of a gene or the chromosome on which it resides that leads to the preferential expression of a specific allele in somatic tissues according to parental origin (Reik and Walter 2001). In most cases, imprinting is a reversible process, because the previous parental imprint is required to switch in the germline of progeny of the opposite sex. Thus, establishment and maintenance of genomic imprints are critical, and abnormalities in imprinting can result in genetic disorders that do not conform to traditional modes of Mendelian inheritance.
GNAS1 produces a variety of sense and antisense transcripts that exhibit reciprocal imprinting (Weinstein et al. 2001). Four alternative first exons splice onto a common set of 12 downstream exons to generate distinct products. Transcripts that contain exon 1 encode Gsα, whereas transcripts containing alternative, upstream first exons encode XLαs and NESP55 (Hayward et al. 1998a, 1998b; Hayward and Bonthron 2000). Transcripts that contain a third alternative first exon, termed “1A,” are apparently untranslated (Swaroop et al. 1991). NESP55 is expressed exclusively from the maternal allele, whereas XLαs (Hayward et al. 1998a, 1998b) and exon 1A (Liu et al. 2000b) transcripts are expressed only from the paternal allele. Expression of Gsα is biallelic in most tissues (Campbell et al. 1994; Hayward et al. 1998a, 1998b; Zheng et al. 2001), but recent studies show that preferential expression of the maternal allele occurs in human pituitary (Hayward et al. 2001), human thyroid (Germain-Lee et al. 2002; Mantovani et al. 2002), and human ovary (Mantovani et al. 2002), and in proximal tubule cells from mice in which one Gnas allele is disrupted (Schwindinger et al. 1998; Yu et al. 1998; Weinstein et al. 2000).
Our present studies confirm and extend recent reports that demonstrated defective genomic imprinting of GNAS1 as an epigenetic abnormality in patients with PHP type 1b. An apparent loss of maternal-specific GNAS1 epigenotype could be caused by paternal uniparental disomy of GNAS1, which has been described in one patient with a PHP type 1b–like syndrome (Bastepe et al. 2001a). Uniparental disomy, as well as deletion of maternal GNAS1 sequences, was excluded in our patients by the demonstration that affected subjects inherited maternally- and paternally-derived haplotypes that span the GNAS1 locus (Jan de Beur et al. 2003), including, in many cases, a 5-bp polymorphism present within exon 1A, and methylation patterns at the NESP55 and (in nearly all subjects) XLαs promoter regions.
A more likely explanation for our findings is a genetic defect that disturbs the proper establishment of the maternal-specific methylation imprint at exon 1A and, in one subject, also at XLαs. Linkage analysis suggests that this defect is tightly linked to the GNAS1 locus on chromosome 20q13 (Juppner et al. 1998; Bastepe et al. 2001b; Jan de Beur et al. 2003), but sequence analysis of the GNAS1 gene, including the exon 1A region, in one affected subject from each kindred and in each subject with sporadic PHP type 1b failed to disclose a heterozygous mutation. Thus, it is likely that a cis-acting mutation is outside the surveyed regions of the imprinting center and is located at some distance from GNAS1 (Bastepe et al. 2001b; Jan de Beur et al. 2003).
Taken in the context of other studies of familial PHP type 1b (Bastepe et al. 2001b), our observations indicate that PHP type 1b is caused by mutations that interfere with the required switch in epigenotype in the germline. Similar cis-acting mutations that disrupt imprinting at specific loci have been described in several other human genetic disorders, including Prader-Willi/Angelman (Horsthemke et al. 1997; Buiting et al. 1998, 2000; Ohta et al. 1999; Glenn et al. 2000) and Beckwith-Wiedemann (Lee et al. 1999) syndromes. However, we are unaware of previous reports of incomplete penetrance of an imprinting mutation similar to that identified in kindred R. The presence of both normal and abnormal epigenotypes following maternal transmission of the disease-associated locus (fig. 4) provides evidence for a unique disturbance of the process of imprint erasure and establishment. This apparent discordance could arise from segregation of a genetic modifier or be due to some intrinsic variable expressivity of the gene defect. We believe that the former explanation is unlikely, because inheritance of a modifier is inconsistent with observations in the extended pedigree. Rather, it is more likely that the genetic defect leads to an inconsistent failure to erase and properly reset the imprint at the exon 1A DMR in the germline, thereby leading to variable inheritance of an inappropriate epigenotype. Variable expressivity and incomplete parental imprinting have been described in mice carrying an imprinted transgene (Kearns et al. 2000) as well as at an endogenous locus (agouti viable yellow) that has been modified by insertion of a retroviral element (Wolff et al. 1978; Duhl et al. 1994).
Despite growing knowledge about the genetics of PHP type 1b, the basis of renal resistance to PTH remains uncertain. The loss of methylation at exon 1A on the maternal chromosome is associated with biallelic expression of exon 1A in lymphoblasts and leukocytes. Although this defect did not alter expression of Gsα in these cells, in which Gsα transcription remained biallelic, we assume that the maternally inherited imprinting defect would silence transcription of the Gsα promoter in paternally imprinted tissues. Under this model, there should be little or no expression of Gsα in these tissues, because the presence of paternal-specific imprinting on both alleles should inhibit transcription from both Gsα promoters. Support for this proposal derives from the recent demonstration of reduced Gsα expression in platelets from a single patient with PHP type 1b (Freson et al. 2002), as well as from the description of renal resistance to PTH in a patient with paternal uniparental isodisomy (Bastepe et al. 2001a). Relaxed paternal imprinting of Gsα, with continued production of sufficient Gsα to meet signaling needs, could explain why patients with PHP type 1b do not generally manifest hormone resistance in other tissues in which Gsα expression appears to be paternally imprinted. By contrast, transcription of Gsα from both maternal and paternal GNAS1 alleles in other tissues provides an explanation for normal levels of Gsα in erythrocytes from patients with PHP type 1b and for a similar 50% reduction in Gsα in patients with PHP type 1a or PPHP, who have mutations on maternal or paternal alleles, respectively (Farfel et al. 1980b; Levine et al. 1980, 1986).
In conclusion, our data indicate that the epigenetic defect, not the putative genetic mutation, accounts for PTH resistance in subjects with PHP type 1b. Moreover, to our knowledge, kindred R represents the first demonstration of incomplete expression of a reprogramming defect that affects imprinting, and the observations presented here suggest that accurate genetic diagnosis of PHP type 1b should be based on methylation analysis rather than haplotype analysis. The identification of the genetic defect(s) in subjects with PHP type 1b, particularly the affected members of kindred R, will be important to our understanding of the genetics of PHP type 1b, as well as the mechanism of genomic imprinting.
Acknowledgments
We are grateful to Professor D. T. Bonthron, of Leeds, for providing a genomic GNAS1. This work was supported in part by United States Public Health Service Grants R01-DK34281, R01-DK46720, and T32-DK07751 (all to M.A.L.), and by the Johns Hopkins University School of Medicine General Clinical Research Center, National Institutes of Health/National Center for Research Resources grant M01 RR00052. S.J.d.B. was supported in part by the Pearl M. Stettler Award for Women Physicians and by a Clinician Scientist Award from the Johns Hopkins University School of Medicine. We thank Z. Deng for expert technical assistance.
Electronic-Database Information
The accession number and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for GNAS1 [accession number AL121917])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PHP 1a and PHP 1b)
References
- Albright F, Burnett CH, Smith PH (1942) Pseudohypoparathyroidism: an example of “Seabright-Bantam syndrome.” Endocrinology 30:922–932 [Google Scholar]
- Bastepe M, Lane AH, Juppner H (2001a) Paternal uniparental isodisomy of chromosome 20q—and the resulting changes in GNAS1 methylation—as a plausible cause of pseudohypoparathyroidism. Am J Hum Genet 68:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Pincus JE, Sugimoto T, Tojo K, Kanatani M, Azuma Y, Kruse K, Rosenbloom AL, Koshiyama H, Juppner H (2001b) Positional dissociation between the genetic mutation responsible for pseudohypoparathyroidism type Ib and the associated methylation defect at exon A/B: evidence for a long-range regulatory element within the imprinted GNAS1 locus. Hum Mol Genet 10:1231–1241 [DOI] [PubMed] [Google Scholar]
- Buiting K, Dittrich B, Gross S, Lich C, Farber C, Buchholz T, Smith E, et al (1998) Sporadic imprinting defects in Prader-Willi syndrome and Angelman syndrome: implications for imprint-switch models, genetic counseling, and prenatal diagnosis. Am J Hum Genet 63:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Farber C, Kroisel P, Wagner K, Brueton L, Robertson ME, Lich C, Horsthemke B (2000) Imprinting center deletions in two PWS families: implications for diagnostic testing and genetic counseling. Clin Genet 58:284–290 [DOI] [PubMed] [Google Scholar]
- Campbell R, Gosden CM, Bonthron DT (1994) Parental origin of transcription from the human GNAS1 gene. J Med Genet 31:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs RW Jr, Levine MA, Drezner MK, Burch WM Jr, Spiegel AM (1983) Deficient adenylate cyclase regulatory protein in renal membranes from a patient with pseudohypoparathyroidism. J Clin Invest 71:231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS (1994) Neomorphic agouti mutations in obese yellow mice. Nat Genet 8:59–65 [DOI] [PubMed] [Google Scholar]
- Farfel Z, Abood ME, Brickman AS, Bourne HR (1982) Deficient activity of receptor-cyclase coupling protein in transformed lymphoblasts of patients with pseudohypoparathyroidism, type I. J Clin Endocrinol Metab 55:113–117 [DOI] [PubMed] [Google Scholar]
- Farfel Z, Bourne HR (1980a) Deficient activity of receptor-cyclase coupling protein in platelets of patients with pseudohypoparathyroidism. J Clin Endocrinol Metab 51:1202–1204 [DOI] [PubMed] [Google Scholar]
- Farfel Z, Brickman AS, Kaslow HR, Brothers VM, Bourne HR (1980b) Defect of receptor-cyclase coupling protein in pseudohypoparathyroidism. N Engl J Med 303:237–242 [DOI] [PubMed] [Google Scholar]
- Freson K, Thys C, Wittevrongel C, Proesmans W, Hoylaerts MF, Vermylen J, Van Geet C (2002) Pseudohypoparathyroidism type Ib with disturbed imprinting in the GNAS1 cluster and Gsα deficiency in platelets. Hum Mol Genet 11:2741–2750 [DOI] [PubMed] [Google Scholar]
- Germain-Lee EL, Ding CL, Deng Z, Crane JK, Saji M, Ringel MD, Levine MA (2002) Paternal imprinting of Gαs in the human thyroid as the basis of TSH resistance in pseudohypoparathyroidism type 1a. Biochem Biophys Res Commun 296:62–72 [DOI] [PubMed] [Google Scholar]
- Glenn CC, Deng G, Michaelis RC, Tarleton J, Phelan MC, Surh L, Yang TP, Driscoll DJ (2000) DNA methylation analysis with respect to prenatal diagnosis of the Angelman and Prader-Willi syndromes and imprinting. Prenat Diagn 20:300–306 [PubMed] [Google Scholar]
- Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, Enjalbert A, Bonthron DT (2001) Imprinting of the Gsα gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest 107:R31-R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward BE, Bonthron DT (2000) An imprinted antisense transcript at the human GNAS1 locus. Hum Mol Genet 9:835–841 [DOI] [PubMed] [Google Scholar]
- Hayward BE, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, Bonthron DT (1998a) The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc Natl Acad Sci USA 95:10038–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward BE, Moran V, Strain L, Bonthron DT (1998b) Bidirectional imprinting of a single gene: GNAS1 encodes maternally, paternally, and biallelically derived proteins. Proc Natl Acad Sci USA 95:15475–15480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsthemke B, Dittrich B, Buiting K (1997) Imprinting mutations on human chromosome 15. Hum Mutat 10:329–337 [DOI] [PubMed] [Google Scholar]
- Jan de Beur SM, Deng Z, Ding CL, Levine MA (1998) Amplification of the GC-rich exon 1 of GNAS1 and identification of three novel nonsense mutations in Albright’s hereditary osteodystrophy. Proceedings of the 80th Annual Meeting of Endocrine Society, New Orleans, June 24–27, p 62 [Google Scholar]
- Jan de Beur SM, Levine MA (2001) Pseudohypoparathyroidism: clinical, biochemical, and molecular features. In: Bilezikian JP, Marcus R, Levine MA (eds) The parathyroids: basic and clinical concepts, 2nd ed. Academic Press, San Diego, pp 807–825 [Google Scholar]
- Jan de Beur SM, O’Connell JR, Peila R, Deng Z, Kam S, Levine MA (2003) Refinement of the pseudohypoparathyroidism type 1b locus to a region including GNAS1 at 20q13.3. J Bone Miner Res 18:424–433 [DOI] [PubMed] [Google Scholar]
- Juppner H, Schipani E, Bastepe M, Cole DE, Lawson ML, Mannstadt M, Hendy GN, Plotkin H, Koshiyama H, Koh T, Crawford JD, Olsen BR, Vikkula M (1998) The gene responsible for pseudohypoparathyroidism type Ib is paternally imprinted and maps in four unrelated kindreds to chromosome 20q13.3. Proc Natl Acad Sci USA 95:11798–11803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen JM, Kaar ML, Ohisalo JJ (1994) Defective stimulation of adipocyte adenylate cyclase, blunted lipolysis, and obesity in pseudohypoparathyroidism 1a. Pediatr Res 35:594–597 [PubMed] [Google Scholar]
- Kearns M, Preis J, McDonald M, Morris C, Whitelaw E (2000) Complex patterns of inheritance of an imprinted murine transgene suggest incomplete germline erasure. Nucleic Acids Res 28:3301–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MP, DeBaun MR, Mitsuya K, Galonek HL, Brandenburg S, Oshimura M, Feinberg AP (1999) Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc Natl Acad Sci USA 96:5203–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MA, Downs RW Jr, Moses AM, Breslau NA, Marx SJ, Lasker RD, Rizzoli RE, Aurbach GD, Spiegel AM (1983a) Resistance to multiple hormones in patients with pseudohypoparathyroidism. Association with deficient activity of guanine nucleotide regulatory protein. Am J Med 74:545–556 [DOI] [PubMed] [Google Scholar]
- Levine MA, Downs RW Jr, Singer MJ Jr, Marx SJ, Aurbach GD, Spiegel AM (1980) Deficient activity of guanine nucleotide regulatory protein in erythrocytes from patients with pseudohypoparathyroidism. Biochem Biophys Res Commun 94:1319–1324 [DOI] [PubMed] [Google Scholar]
- Levine MA, Eil C, Downs RW Jr, Spiegel AM (1983b) Deficient guanine nucleotide regulatory unit activity in cultured fibroblast membranes from patients with pseudohypoparathyroidism type I. a cause of impaired synthesis of 3′,5′-cyclic AMP by intact and broken cells. J Clin Invest 72:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MA, Jap TS, Mauseth RS, Downs RW, Spiegel AM (1986) Activity of the stimulatory guanine nucleotide-binding protein is reduced in erythrocytes from patients with pseudohypoparathyroidism and pseudopseudohypoparathyroidism: biochemical, endocrine, and genetic analysis of Albright’s hereditary osteodystrophy in six kindreds. J Clin Endocrinol Metab 62:497–502 [DOI] [PubMed] [Google Scholar]
- Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, Weinstein LS (2000a) A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest 106:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu S, Litman D, Chen W, Weinstein LS (2000b) Identification of a methylation imprint mark within the mouse Gnas locus. Mol Cell Biol 20:5808–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A (2002) The Gsα gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab 87:4736–4740 [DOI] [PubMed] [Google Scholar]
- Nagane Y, Utsugisawa K, Tohgi H (2000) PCR amplification in bisulfite methylcytosine mapping in the GC-rich promoter region of amyloid precursor protein gene in autopsy human brain. Brain Res Brain Res Protoc 5:167–171 [DOI] [PubMed] [Google Scholar]
- Namnoum AB, Merriam GR, Moses AM, Levine MA (1998) Reproductive dysfunction in women with Albright’s hereditary osteodystrophy. J Clin Endocrinol Metab 83:824–829 [DOI] [PubMed] [Google Scholar]
- Reik W, Walter J (2001) Genomic imprinting: parental influence on the genome. Nat Rev Genet 2:21–32 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. 1st ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Schwindinger WF, Lawler AM, Gearhart JD, Levine MA (1998) A murine model of Albright hereditary osteodystrophy. Proceedings of the 80th Annual Meeting of the Endocrine Society, New Orleans, June 24–27, p 480 [Google Scholar]
- Swaroop A, Agarwal N, Gruen JR, Bick D, Weissman SM (1991) Differential expression of novel Gsα signal transduction protein cDNA species. Nucleic Acids Res 19:4725–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LS, Yu S, Ecelbarger CA (2000) Variable imprinting of the heterotrimeric G protein G(s) alpha-subunit within different segments of the nephron. Am J Physiol Renal Physiol 278:F507-F514 [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Yu S, Warner DR, Liu J (2001) Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev 22:675–705 [DOI] [PubMed] [Google Scholar]
- Wolff GL (1978) Influence of maternal phenotype on metabolic differentiation of agouti locus mutants in the mouse. Genetics 88:529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein LS (1998) Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc Natl Acad Sci USA 95:8715–8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Radeva G, McCann JA, Hendy GN, Goodyer CG (2001) Gαs transcripts are biallelically expressed in the human kidney cortex: implications for pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab 86:4627–4629 [DOI] [PubMed] [Google Scholar]