Abstract
The von Hippel-Lindau (pVHL) protein plays an important role in hypoxia sensing. It binds to the hydroxylated hypoxia-inducible factor 1α (HIF-1α) and serves as a recognition component of an E3-ubiquitin ligase complex. In hypoxia or secondary to a mutated VHL gene, the nondegraded HIF-1α forms a heterodimer with HIF-β and leads to increased transcription of hypoxia-inducible genes, including erythropoietin (EPO). The autosomal dominant cancer-predisposition von Hippel-Lindau (VHL) syndrome is due to inheritance of a single mutated allele of VHL. In contrast, we recently showed that homozygous germline 598C→T VHL mutation leads to Chuvash polycythemia (CP). We subsequently found VHL mutations in three unrelated individuals unaffected with CP, one of whom was compound heterozygous for the 598C→T mutation and another VHL mutation. We now report seven additional polycythemic patients with VHL mutations in both alleles. Two Danish siblings and another American boy were homozygous for the VHL 598C→T mutation. Three unrelated white Americans were compound heterozygotes for 598C→T and another VHL mutation, 562C→G in two and 574C→T in the third. Additionally, a Croatian boy was homozygous for a 571C→G VHL mutation, the first example of homozygous VHL germline mutation causing polycythemia, other than the VHL 598C→T mutation. We have not observed VHL syndrome–associated tumors in polycythemic subjects or their heterozygous relatives; however, this will need to be evaluated by longitudinal studies. Over all, we found that up to half of the consecutive patients with apparent congenital polycythemia and increased serum Epo we have examined have mutations of both VHL alleles. Those findings, along with reports of CP, underscore that VHL mutations are the most frequent cause of congenital polycythemia and define a new class of polycythemic disorder, polycythemias due to augmented hypoxia sensing.
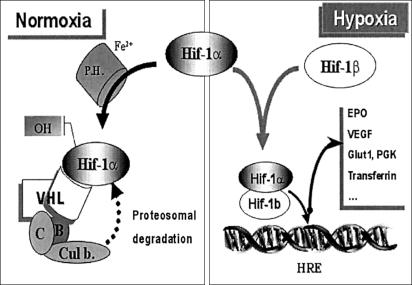
The von Hippel-Lindau protein (pVHL) plays a crucial role in hypoxia sensing (Semenza 2001). pVHL binds to the hydroxylated form of the hypoxia-inducible factor 1α (HIF-1α) and serves as the recognition component of an E3-ubiquitin ligase complex that comprises elongins B and C, cullin 2, and ring-box 1 (Epstein et al. 2001; Ivan et al. 2001; Jaakkola et al. 2001). In hypoxia, or as a result of a mutated VHL, the accumulation of the nondegraded HIF-1α leads to the formation of a heterodimer with HIF-β and the activation of an array of hypoxia-inducible genes, including erythropoietin (EPO) (see fig. 1; Semenza 2001). The cancer-predisposition von Hippel-Lindau syndrome (MIM 193300) is an autosomal dominant disorder due to inheritance of a mutation in a single VHL allele. Faithful to Knudson’s two-hit hypothesis (Knudson et al. 1975), tumors develop upon somatic mutation or inactivation of the other, normal VHL allele. However, until the recent molecular elucidation of the cause of Chuvash polycythemia (CP) or autosomal recessive benign congenital polycythemia (MIM 263400), true inheritance of germline mutations in both VHL alleles, to our knowledge, had never been reported.
Figure 1.
Hypoxia-sensing pathway. The hypoxia-inducible factor 1α (HIF-1α) is a master regulator of hypoxia. In normoxia, HIF-1α is hydroxylated by proline hydroxylase; pVHL binds to the hydroxylated form of HIF-1α and serves as the recognition component of an E3-ubiquitin ligase complex that comprises elongins B (B) and C (C), Cullin 2, and ring-box 1; this includes a proteosomal degradation of HIF-1α. In hypoxia, HIF-1α accumulates and forms a heterodimer with HIF-1β that will bind to hypoxia-responsive elements (HRE) and leads to an increased synthesis of the related hypoxia-responsive genes, such as EPO, vascular endothelial growth factor (VEGF), glucose transporter 1 (Glut1), phospho-glycerate kinase (PGK), transferrin and its receptor, and other genes.
There are numerous causes for an increased red-blood-cell mass, or “polycythemia.” Polycythemia can be either acquired or congenital. Primary polycythemias have low serum Epo and are due to somatic or germline mutations expressed within the erythroid progenitors. In contrast, increased circulating level of erythropoiesis-stimulating substance, typically Epo, leads to secondary polycythemias (Prchal 2003). CP is a unique polycythemic disorder, the only recessively inherited disorder, and endemic in the Chuvash Autonomous Republic of Russia (Sergeyeva et al. 1997; Ang et al. 2002b). We recently demonstrated that CP was caused by homozygosity for the 598C→T (R200W) VHL mutation (Ang et al. 2002a, 2002b). Subsequently, we have found VHL mutations in three unrelated non-Chuvash children; of those three children, one was a compound heterozygote for the VHL 598C→T mutation and a VHL 388G→C (V130L) mutation in the other allele (Pastore et al. 2003). Homozygosity for the VHL 598C→T mutation was also recently observed in three Bangladesh families living in the United Kingdom (Percy et al. 2002).
We pursued the hypothesis that inheritance of the VHL mutation is an important cause of congenital polycythemia. Patients were referred to our laboratory by pediatric hematologists and other clinical collaborators. Thirteen patients were selected and evaluated because of their history of sporadic, apparently congenital polycythemia and “a normal” (considered as an inappropriate level for the elevated hemoglobin level) or elevated serum Epo; all patients except two siblings were unrelated. Patients were of various ethnic backgrounds; none of them were of Chuvash origin. All patients signed an informed consent for the study that had been reviewed and approved by the institutional review board of Baylor College of Medicine. One stored specimen was previously collected with approval of the internal review board of University of Alabama at Birmingham. Peripheral blood from patients, and, in most instances, also from their available relatives were collected in acid-citrate-dextrose, and genomic DNA samples were isolated by use of a QIAamp DNA blood mini kit (QIAGEN).
To search for VHL mutations, we proceeded to a systematic sequencing of all three VHL exons and intron-exon boundaries. PCR reactions were performed in 50-μl volume that contained 20 mM Tris-HCl pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 100 μM dNTP, 300 nM primers, and 2.5 U/reaction Taq DNA polymerase (Life Technologies). The following sets of primers were used: VHL1F 5′-CGAAGACTATGGAGGTCGAC-3′, VHL1R 5′-GGCTTCAGACCGTGCTATCG-3′ for exon 1; VHL2F 5′-GTGTGGCTCTTTAACAACC-3′, VHL2R 5′-CTGTACTTACCACAACAACC-3′ for exon 2; and VHL3F 5′-CCTTGTACTGAGACCCTAG-3′, VHL3R 5′-GCTGAGATGAAACAGTGTA-3′ for exon 3. DMSO (10%) was added for amplification of exon 1. PCR products were then purified with a QIAquick Gel Extraction Kit (QIAGEN). Ten to 20 ng of purified PCR product was used as the template for sequencing with a DNA Sequencing Kit (BigDye Terminator Cycle Sequencing Ready Reaction, Applied Biosystems), with forward or reverse primers (identical to those used for PCR reaction). The sequencing reaction was performed in the Peltier Model P200 thermocycler. The products were then analyzed on the ABI Prism 377 DNA Sequencer (Applied Biosystems) according to the manufacturer’s protocol.
Of the 13 patients analyzed, 7 were found to have VHL mutations; all 7 had both VHL alleles mutated (see table 1). Eleven of 13 tested subjects had the EPOR gene analyzed for mutation (Kralovics et al. 2001); none was found.
Table 1.
Patient Characteristics
| Patient | Sex | Age Pa(years) | Age Stb(years) | Hgc | Hctd | Epoe | Ethnic Origin | VHL Mutations | Remarks |
| 1 | M | 1 | 14 | 20.8 | 64 | N-Hf | Danish | R200W/R200W | Sibling of patient 2 |
| 2 | M | 5 | 12 | 19.5 | 56 | N-Hf | Danish | R200W/R200W | Sibling of patient 1 |
| 3 | M | 3 | 19 | 16.4 | 53.2 | N | White American | R200W/R200W | Thrombosis |
| 4 | M | 9 | 10 | 23.5 | 70.2 | H | White American | R200W/P192A | … |
| 5 | F | 5 | 13 | 16.3 | 51.6 | N | White American | R200W/L188V | … |
| 6 | M | 5 | 15 | 21 | 58.7 | H | White American | R200W/L188V | Lost of follow-up |
| 7 | M | 1 | 17 | 18 | 61 | H | Croatian | H191D/H191D | … |
Age P = age at presentation.
Age St = age at which we studied the patient.
Hg = hemoglobin.
Hct = hematocrit.
H = high; N = normal (since several independent laboratories at local institutions performed serum Epo measurement and had different normal ranges).
Patient 1 and patient 2 had their serum Epo measured several times; the serum Epo was high once and at normal-to-high–normal range on other occasions.
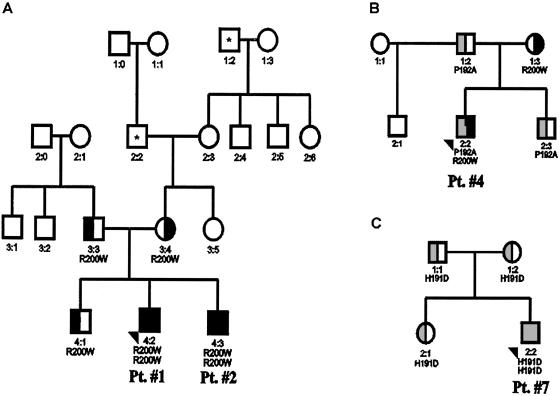
Patient 1 and patient 2, two healthy Danish siblings, and another, unrelated white boy from the United States (patient 3) were all homozygous for the 598C→T VHL mutation. Both Danish siblings had been diagnosed in early childhood (patient 1 was incidentally found polycythemic while hospitalized because of wheezing at age 8 mo). Evaluation of family members revealed polycythemia in his otherwise-asymptomatic brother (patient 2); no other family members were found to be polycythemic (see pedigree [fig. 2A]; table 1).
Figure 2.
Pedigree of families available for DNA studies and with more extended family history. A, Pedigree of patient 1 and patient 2. Asterisks (*) denote that family members of patient 1 (Pt. #1) and patient 2 (Pt. #2) had developed the following complications: 1:2 had history of brain tumor, whereas 2:2 had history of ocular thrombosis. B, Pedigree of patient 4. C, Pedigree of patient 7.
Patient 3 had incidentally been found to be polycythemic on day 3 of life and had been treated by occasional phlebotomies since then. At age 15 years, he was hospitalized for severe abdominal pain and was found to have infarction of the great omentum secondary to a mesenteric thrombosis. He later had deep venous thrombosis complicated by pulmonary embolism at age 18 years. He has been on anticoagulation therapy with warfarin and remains polycythemic (age 19 years). No evidence for intra-abdominal tumors or cystic lesions was found by abdominal CT scan or during abdominal surgery.
Patient 4, patient 5, and patient 6 (two white American males and one white American female) were found to be compound heterozygous for the 598C→T mutation and another VHL mutation. Patient 4 had been diagnosed with polycythemia at age 9 years; he is a compound heterozygote for the VHL 598C→T mutation and a previously undescribed 574C→T (P192A) VHL mutation. Analysis of family members revealed that the VHL 598C→T mutation had been inherited from his mother, whereas the 574C→T mutation was inherited from his father and also found in his brother; they were not polycythemic (see fig. 2B).
Patient 5 is a 13-year-old girl incidentally found to have polycythemia at age 5 years, the first time her hemoglobin concentration was tested; she has a mild clubbing without any other symptoms or stigmata of polycythemia and has been treated with periodic phlebotomies. This girl is a compound heterozygote for the 598C→T and 562C→G (L188V) VHL mutations. The 598C→T mutation had been inherited from her mother. The inheritance of the 562C→G mutation could not be determined, as we were unable to obtain consent from other family members and her undisclosed father.
Patient 6 was diagnosed with polycythemia at age 5 years; he had been previously reported as having secondary polycythemia because of a markedly increased serum Epo (Mankad et al. 1987). The patient was subsequently evaluated in our laboratory 15 years ago: (a) in vitro evaluation of erythroid progenitors responses to Epo after careful removal of serum from erythroid progenitors could not confirm the reported Epo independence, (b) sequencing of EPO cDNAs revealed no mutations, and (c) the etiology of his polycythemic disorder remained unclear at that time (J. T. Prchal, unpublished data). Recent re-examination of his stored sample for the VHL mutation revealed the same mutations as in patient 5: a 598C→T mutation and a 562C→G VHL mutation; it is unfortunate that we have not been able to locate him. Subject 5 and subject 6 were not related and originate from central Tennessee and southern Alabama, respectively.
Patient 7 is a 17-year-old Croatian male who was diagnosed with polycythemia and elevated serum Epo in early childhood. He was found to be homozygous for a previously undescribed 571C→G (H191D) VHL mutation. The 571C→G mutation can be distinguished from other alleles, as it abolishes the MslI restriction site. The parents of patient 7 were unrelated. They and his sister were heterozygous for the 571C→G (H191D) VHL mutation (see fig. 2C). We then screened 179 normal control subjects (DNA kindly provided by the Baylor College of Medicine Polymorphism Resource Core, comprising 48 whites, 43 African American, 40 Asians, and 48 Hispanics), and all had normal MslI restriction pattern. Thus, the 571C→G mutation likely represents a disease-causing mutation, and it is not a common polymorphism. Our discovery of homozygosity for the 571C→G (H191D) mutation is the first example, to our knowledge, of a homozygous germline VHL mutation other than the VHL 598C→T mutation causing polycythemia. The exact phenotype of the 571C→G VHL mutation will need to be defined by longitudinal studies.
In the six remaining patients with a similar polycythemic phenotype, no VHL mutations were found. With the exception of subject 3, all polycythemic patients had no medical problem; some, but not all, polycythemic subjects gave a history of headaches and difficulty in concentrating when their hematocrit (Hct) was high, and, in some, these symptoms were immediately relieved by phlebotomy. On the basis of our observation, it appears that mutation of the VHL gene is an important cause for congenital polycythemia. Combined with our previous data (Pastore et al. 2003), up to 50% of patients with apparent congenital polycythemia and elevated serum Epo (11 of 21 patients analyzed so far) appear to have mutation in the VHL gene. The phenotype of this polycythemic disorder may comprise an increased risk for thrombosis. Although this complication was observed in only one of our patients (patient 3), preliminary report of an epidemiological study in the Chuvash population had reported an increased risk for thrombosis possibly associated with homozygosity for the 598C→T VHL mutation (Gordeuk et al. 2001).
Inheritance of a mutation in the VHL tumor suppressor is known to predispose to the high risk for various tumors (Richards et al. 1998; Friedrich 2001). Through studies of families with VHL syndrome, genotype-phenotype correlation has emerged. Families with VHL are usually differentiated on the basis of their risk for pheochromocytomas: low risk for families with type 1 VHL and high risk for families with type 2 VHL. Type 2 VHL is further divided into a low (type 2A) or high (type 2B) risk for renal cell carcinoma, whereas families with type 2C present only with familial pheochromocytomas. Type 2 VHL is generally associated with missense VHL mutations, whereas type 1 VHL mutations tend to have loss-of-function mutations of the VHL gene, which are frequently deletions or truncated mutations (Friedrich 2001). In our families, we would therefore expect to observe not only a high incidence for pheochromocytomas and/or renal cell carcinoma, but possibly other VHL-associated tumors, as well. To evaluate this, all patients with identified VHL mutations except patient 6 were subject to evaluation on the basis of recommendations, developed by the National Institutes of Health, that comprise abdominal and cerebral imaging and ophthalmologic examination (Choyke 1995). To our surprise, no VHL tumors were observed, although MRI of spinal cord was not performed on every child. We then reviewed history and medical records of 11 subjects heterozygous for the VHL mutations described here (three heterozygotes for the 571C→G mutation, seven for the 598C→T mutation, and two for the 574C→T VHL mutation), three heterozygotes for the previously reported 388G→C mutation (Pastore et al. 2003), as well as 27 Chuvash subjects heterozygous for the VHL 598C→T mutation (Ang et al. 2002a, 2002b). None of these studied heterozygote carriers had any VHL syndrome–associated tumors, though we could not exclude a possible VHL-associated tumor in the Danish family in which one man had a history of otherwise-uncharacterized brain tumor and another had a history of ocular thrombosis. However, both these two ancestors of patient 1 and patient 2 were older than age 70 years (see fig. 2A), their DNA was not available for testing, and these medical disorders likely represent sporadic tumors unrelated to VHL syndrome. One VHL mutation, 562C→G, observed in our polycythemic subjects has been reported to have a high risk for pheochromocytoma development and to be associated with families with type 2C VHL syndrome (Ritter et al. 1996). We have been unable to locate heterozygous relatives of our polycythemic subjects who carry this VHL mutation. The 388G→C mutation, another VHL mutation previously reported in compound heterozygosity with the 598C→T mutation (Pastore et al. 2003), has also been reported as a germline mutation in two patients with VHL syndrome (Universal VHL-Mutation Database). Yet, the consequences of the association of the VHL 562C→G mutation or the 388G→C mutation with the VHL 598C→T mutation in the compound heterozygous propositus will need to be evaluated prospectively, and the low penetrance of these tumors and the young age of the patients we studied cannot exclude a possible VHL tumor association at this point.
The absence of tumors in our families is in keeping with the results of an ongoing epidemiological study in the Chuvash population that failed to demonstrate an increased risk of the VHL 598C→T mutation for VHL syndrome–associated tumors (Gordeuk et al. 2001). VHL syndrome is known to have variable penetrance, and tumor development may also be influenced by other genetic or environmental factors, such as carcinogen exposure (Bruning et al. 1997; Webster et al. 1998). Furthermore, certain mutations, such as the VHL 505T→C mutation, may even confer an advanced-age penetrance of tumor development (Bender et al. 2001). Therefore, longitudinal studies will be necessary to determine the exact risk of tumor development in our patients. Although as yet unconfirmed, this apparent low penetrance for tumor development contrasts with a highly penetrant polycythemic phenotype in all patients with homozygosity or compound heterozygosity for those VHL mutations we discuss here.
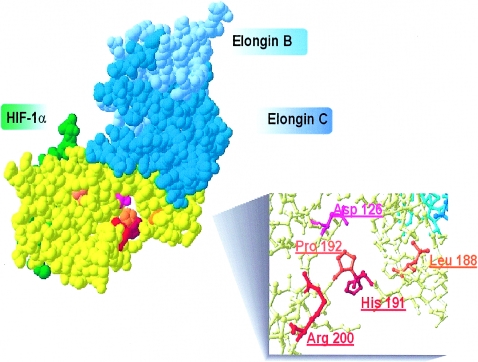
It is of interest that patients with the mutations in the VHL gene reported here do not appear at risk of developing tumors; however, this will need to be confirmed by longitudinal studies as well as studies including adults who have these VHL mutations. Our findings emphasize a distinction between VHL “polycythemia-causing” mutations and other VHL mutations associated with the tumor-predisposition syndrome. The 571C→G, 562C→G, and 574C→T VHL mutations found in compound heterozygosity with the VHL 598C→T mutation could be considered “polycythemia-associated” mutations. Whether homozygosity for those mutations would also lead to a polycythemic phenotype remains to be determined. Both VHL-polycythemia–causing” mutations (571C→G and 562C→G) and most of the “polycythemia-associated” mutations (i.e., 388G→C, 562→G, and 574C→T) are missense mutations localized in the C-terminal portion of VHL. Moreover, both “polycythemia-causing” mutations and “polycythemia-associated” mutations are located within the same structural region (see fig. 3). Further, the 376G→T (D126Y) VHL mutation, recently reported as a possible autosomal dominant polycythemia-causing mutation found in two siblings (Pastore et al. 2003), is localized within the same domain (see fig. 3). This domain is distinct from the region directly interacting with elongins B and C (Ohh et al. 1999) and from the VHL beta domain known to interact with HIF-1α (Ohh et al. 2000). The ability of the 562C→G VHL mutant to interact with elongin B and C, as well as to capture and ubiquinate HIF-1α, was shown to be similar to the wild-type VHL (Clifford et al. 2001). Similarly, the VHL 598C→T mutation was shown to lead to only a mild decrease in its ability to capture HIF-1α (Ang et al. 2002b). It is therefore conceivable that those mutations cause only a modest impairment of HIF-1α degradation. Alternatively, it is possible that those mutations alter the interaction of VHL with other proteins, such as fibronectin, which was observed with the L188V mutation (Hoffman et al. 2001), perhaps resulting in the tissue-specific effect that may be more expressed in Epo-producing tissues and the erythroid cells. It is interesting that the 376G→T, 388G→C, 562C→G, 574C→T, and 598C→T VHL mutations discussed here are encoding for amino acids that are conserved between mouse and human; however, the 571C→G mutation is not (Gao et al. 1995). Only two mutations (the 388G→C and 562C→G VHL mutations) affect highly conserved amino acids, when comparing human and Drosophila VHL protein (Adryan et al. 2000). In conclusion, we show here that inheritance of mutations of both alleles of the VHL gene represents an important cause of congenital polycythemia. We report that about one-half of patients with apparent congenital polycythemia and increased or inappropriate serum Epo (11 of 21) have VHL mutations. In our experience, mutations of both alleles of the VHL gene are the most common of all congenital polycythemias with the defined molecular defect so far (Prchal 2003) and are compatible with long-term survival. In contrast to VHL syndrome, this congenital disorder appears to be associated with a low penetrance for cancer development. The VHL mutations define a new category of polycythemic disorder: polycythemias due to inherited augmentation of hypoxia sensing.
Figure 3.
VHL-polycythemia–causing and polycythemia-associated mutations. Illustration of VHL protein structure (yellow) and interactions with elongin B (pale blue), elongin C (dark blue), and HIF-1α (green) was produced by use of Swiss PDB-viewer software and was based on the PDB-1LM8 crystallized structure (Min 2002). Red, Homozygous VHL mutations found at position R200 and H191. Orange, VHL mutations found in compound heterozygosity with the 598C→T (R200W) mutation. Pink, Mutation at Y126, previously proposed as a dominant negative VHL mutation found in two siblings with apparent congenital polycythemia (Pastore et al. 2003).
Acknowledgments
This work is supported by grants R01HL66333-01 and R01HL5007-08 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. We are indebted to our clinical collaborators who referred patients and provided the clinical material: Dr. Thomas Thelle, James Whitlock, Deborah Smith, and Helmuth Gardner.
Electronic-Database Information
URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Chuvash polycythemia and VHL syndrome)
- Protein Data Bank, http://www.rcsb.org/pdb/ (for the structure of Hif-1α-Pvhl-Elonginb-Elonginc Complex: PDB-1LM8)
- Swiss PDB viewer, http://us.expasy.org/spdbv/
- Universal VHL-Mutation Database, http://www.umd.necker.fr:2005/)
References
- Adryan B, Decker HJ, Papas TS, Hsu T (2000) Tracheal development and the von Hippel-Lindau tumor suppressor homolog in Drosophila. Oncogene 19:2803–2811 [DOI] [PubMed] [Google Scholar]
- Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, Kralovics R, Stockton DW, Prchal JT (2002a) Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis 28:57–62 [DOI] [PubMed] [Google Scholar]
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT (2002b) Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32:614–621 [DOI] [PubMed] [Google Scholar]
- Bender BU, Eng C, Olschewski M, Berger DP, Laubenberger J, Altehofer C, Kirste G, Orszagh M, van Velthoven V, Miosczka H, Schmidt D, Neumann HP (2001) VHL c.505 T→C mutation confers a high age related penetrance but no increased overall mortality. J Med Genet 38:508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning T, Weirich G, Hornauer MA, Hofler H, Brauch H (1997) Renal cell carcinomas in trichloroethene (TRI) exposed persons are associated with somatic mutations in the von Hippel-Lindau (VHL) tumour suppressor gene. Arch Toxicol 71:332–335 [DOI] [PubMed] [Google Scholar]
- Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B (1995) von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology 194:629–642 [DOI] [PubMed] [Google Scholar]
- Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER (2001) Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet 10:1029–1038 [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54 [DOI] [PubMed] [Google Scholar]
- Friedrich CA (2001) Genotype-phenotype correlation in von Hippel-Lindau syndrome. Hum Mol Genet 10:763–737 [DOI] [PubMed] [Google Scholar]
- Gao J, Naglich JG, Laidlaw J, Whaley JM, Seizinger BR, Kley N (1995) Cloning and characterization of a mouse gene with homology to the human von Hippel-Lindau disease tumor suppressor gene: implications for the potential organization of the human von Hippel-Lindau disease gene. Cancer Res 55:743–747 [PubMed] [Google Scholar]
- Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin DJ, Prchal JT, Polyakova LA (2001) High mortality due to thrombosis and cerebral hemorrhage in Chuvash polycythemia. Blood 98:22411418484 [Google Scholar]
- Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG Jr (2001) von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet 10:1019–1027 [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468 [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472 [DOI] [PubMed] [Google Scholar]
- Knudson AG Jr, Hethcote HW, Brown BW (1975) Mutation and childhood cancer: a probabilistic model for the incidence of retinoblastoma. Proc Natl Acad Sci USA 72:5116–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovics R, Josef T, Prchal JT (2001) Genetic heterogeneity of primary familial and congenital polycythemia. Am J Hemat 68:115–121 [DOI] [PubMed] [Google Scholar]
- Mankad VN, Moore RB, McRoyan D, Zuckerman K (1987) Erythrocytosis associated with spontaneous erythroid colony formation and idiopathic hypererythropoietinemia. J Pediatr 111:743–745 [DOI] [PubMed] [Google Scholar]
- Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP (2002) Structure of an HIF-1α-pVHL complex: hydroxyproline recognition in signaling. Science 296:1886–1889 [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nat Cell Biol 2:423–427 [DOI] [PubMed] [Google Scholar]
- Ohh M, Takagi Y, Aso T, Stebbins CE, Pavletich NP, Zbar B, Conaway RC, Conaway JW, Kaelin WG Jr (1999) Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein. J Clin Invest 104:1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore YD, Jelinek J, Ang S, Guan Y, Liu E, Jedlickova K, Krishnamurti L, Prchal JT (2003) Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood 101:1591–1595 [DOI] [PubMed] [Google Scholar]
- Percy MJ, McMullin MF, Treacy M, Potter M, Watson WH, Jowitt SN, Lappin TRJ (2002) Identification of the Chuvash-type congenital polycythemia in patients of Asian and Western European ancestry. Blood 100:660 [DOI] [PubMed] [Google Scholar]
- Prchal JT. Classification and molecular biology of polycythemias (erythrocytoses) and thrombocytosis. Med Clin North Am (in press) [DOI] [PubMed] [Google Scholar]
- Richards FM, Webster AR, McMahon R, Woodward ER, Rose S, Maher ER (1998) Molecular genetic analysis of von Hippel-Lindau disease. J Intern Med 243:527–533 [DOI] [PubMed] [Google Scholar]
- Ritter MM, Frilling A, Crossey PA, Hoppner W, Maher ER, Mulligan L, Ponder BA, D Engelhardt (1996) Isolated familial pheochromocytoma as a variant of von Hippel-Lindau disease. J Clin Endocrinol Metab 81:1035–1037 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2001) HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13:167–171 [DOI] [PubMed] [Google Scholar]
- Sergeyeva A, Gordeuk VR, Tokarev YN, Sokol L, Prchal JF, Prchal JT (1997) Congenital polycythemia in Chuvashia. Blood 89:2148–2154 [PubMed] [Google Scholar]
- Webster AR, Richards FM, MacRonald FE, Moore AT, Maher ER (1998) An analysis of phenotypic variation in the familial cancer syndrome von Hippel-Lindau disease: evidence for modifier effects. Am J Hum Genet 63:1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]