Abstract
Mutations in the gene coding for the renal tight junction protein claudin 16 cause familial hypomagnesemia with hypercalciuria and nephrocalcinosis, an autosomal recessive disorder of renal Ca2+ and Mg2+ handling that progressively leads to chronic renal failure, with nephrolithiasis having been reported in heterozygous carriers. Screening a cohort of 11 families with idiopathic hypercalciuria identified a novel homozygous mutation in the claudin 16 gene in two families. In contrast to classical symptoms of familial hypomagnesemia with hypercalciuria and nephrocalcinosis, the patients displayed serious but self-limiting childhood hypercalciuria with preserved glomerular filtration rate. The mutation results in inactivation of a PDZ-domain binding motif, thereby disabling the association of the tight junction scaffolding protein ZO-1 with claudin 16. In contrast to wild-type claudin 16, the mutant no longer localizes to tight junctions in kidney epithelial cells but instead accumulates in lysosomes. Thus, mutations at different intragenic sites in the claudin 16 gene may lead to particular clinical phenotypes with a distinct prognosis. Mutations in claudin 16 that affect interaction with ZO-1 lead to lysosomal mistargeting, providing—for the first time, to our knowledge—insight into the molecular mechanism of a disease-associated mutation in the claudin 16 gene.
Introduction
Hypercalciuria (HC) is a common cause of nonglomerular hematuria, nocturnal enuresis, and frequency dysuria in children (Stapleton et al. 1984; Alon et al. 1990). In children and adults, it is one of the major determinants of calcium-related kidney-stone diseases and nephrocalcinosis (NC) (Asplin 1996). However, HC represents a common symptom of various underlying disorders, meaning that the etiology of HC is heterogeneous. One such disorder, familial hypomagnesemia with HC and NC (FHHNC [MIM 248250]), a progressive disorder of renal Ca2+ and Mg2+ wasting leading to chronic renal failure, is caused by mutations in the claudin 16 (CLDN16) gene (also known as “paracellin-1” gene) (Simon et al. 1999; Weber et al. 2001b). CLDN16 is a member of a family of membrane-bound proteins that constitute the intercellular tight-junction (TJ) barrier in various epithelia (Morita et al. 1999). Claudins span the lipid bilayer four times, with their two luminal loops mediating cell-cell adhesion via homo- or heterotypic interaction with claudins on a neighboring cell (Tsukita et al. 2001). In addition, claudins form paracellular ion channels, the characteristic permeability of an epithelium thought to arise from the particular claudin molecules expressed (Heiskala et al. 2001). Cytosolic scaffold proteins of the ZO family (i.e., ZO-1, ZO-2, and ZO-3) link TJ transmembrane proteins to the actin cytoskeleton and recruit signaling molecules to regions of cell-cell contact (Gonzalez-Mariscal et al. 2000). CLDN16 is exclusively expressed in the thick ascending part of Henle’s loop, where it is believed to form paracellular channels that allow reabsorption of Ca2+ and Mg2+ and are basically driven by an electrochemical gradient (Simon et al. 1999). Consequently, patients with FHHNC experience severe renal Ca2+ and Mg2+ wasting, which leads to chronic renal failure.
Because a recent study of patients with FHHNC suggested that heterozygously affected family members might be at an increased risk of developing renal-stone disease, we investigated a cohort of families with HC for mutations in the CLDN16 gene (Weber et al. 2001b). It was surprising that we identified a novel homozygous mutation in the CLDN16 gene in the affected members of two families in this cohort. In contrast to the clinical phenotype thus far reported to be associated with mutations in the CLDN16 gene, the phenotype of this mutation is characterized by serious but self-limiting symptoms of HC during childhood with conserved kidney function. Biochemical and cell biological characterization of the wild-type and mutant CLDN16 provide a molecular mechanism for this rare phenotype. These findings indicate that the clinical appearance and long-term prognosis of mutations in CLDN16 is determined by the location of the mutation within the gene.
Methods
Mutation Analysis of the CLDN16 Gene
Genomic DNA of available members of 11 families with HC was isolated from peripheral leukocytes, and all exons of the CLDN16 gene were amplified by using oligonucleotides that anneal to noncoding (intronic) sites ∼30 bp from the intron-exon boundaries (GenBank accession number NT005883) as described elsewhere (Meij et al. 2003). This family cohort also included nine families from an earlier study (Muller et al. 2002a). PCR products were purified, sequenced (Perkin Elmer), and compared with the published human CLDN16 sequence (GenBank accession number AF152101) (Simon et al. 1999). Consent for the studies was obtained from patients or a parent, in accordance with the local guidelines at each participating institution. DNA for sequencing the CLDN16 exons on control chromosomes was obtained from blood donated by healthy volunteers of European descent.
Antibodies and Plasmids
The antibodies used were rabbit anti-human ZO-1 (Zymed), rat monoclonal anti-HA (Roche), mouse monoclonal anti–dog-lamp-2 (AC17) (Nabi et al. 1991), and mouse monoclonal anti–rat GM130 (Nakamura et al. 1997). HRP-labeled secondary antibodies were from Jackson Laboratories and Pierce; fluorescently labeled (Alexa 488 and 594) secondary antibodies were from Molecular Probes. The full-length human CLDN16 cDNA was obtained by PCR from a kidney cDNA library (Clontech), using suitable primers covering the 5′ and 3′ coding region of the cDNA. The primers were designed to introduce a 5′ EcoRI site, an N-terminal HA-epitope tag, and a 3′ XbaI restriction site. The epitope tag was fused in frame after the second translation-initiation site, which, according to sequence comparison and mutation analysis, is used in vivo (Ohba et al. 2000; Weber et al. 2001a, 2001b). A suitable 3′ PCR primer was used to introduce the T233R mutation; two overlapping primers were used to construct the G128A mutant. All cDNA constructs were verified by sequencing and subcloned into pcDNA3 (Invitrogen) for expression in Madin-Darby canine kidney (MDCK) cells. Constructs coding for the three PDZ domains of ZO-1, ZO-2, and ZO-3 in pcDNA3 were used for in vitro transcription and translation. Sequences of primers are available upon request.
Culture and Transfection of MDCK Cells
MDCK strain II cells were cultured and transfected as described elsewhere (Reichert et al. 2000). Clones stably expressing wild-type or mutant CLDN16 were selected for the presence of G418 and were screened for expression by immunofluorescence or western blot analysis, after induction with 5 mM butyrate for 12 h (Höning and Hunziker 1995). Polarized cell monolayers were obtained by growing cells on 0.4 μm Transwell polycarbonate filters (Costar).
Pull-Down Experiments
Peptides corresponding to the 11 C-terminal amino acids of wild-type or T233R mutant CLDN16 were custom synthesized and coupled to Dynabeads (Dynal) by Biogenes. Labeled PDZ domains of ZO-1, ZO-2, or ZO-3 were generated by in vitro transcription and translation (Quick Coupled T7 TNT; Promega) in the presence of radioactive methionine. Between 5 and 10 μl of the in vitro transcription and translation reaction were incubated with peptide-coupled Dynabeads (2–10 μg peptide) for 2 h at 4°C in binding buffer (25 mM Tris [pH 7.5], 50 mM NaCl, and 0.1% Tween-20; or Triton X-100, 20 mM MgCl2, and 1 mM DTT). Beads were then rinsed with washing buffer (25 mM Tris [pH 7.5], 150 mM NaCl, and 0.1% Tween-20; or Triton X-100, 20 mM MgCl2, and 1 mM DTT) and were resuspended in SDS sample buffer. Bound PDZ domains were analyzed by SDS-PAGE and autoradiography. For competition experiments, in vitro translated PDZ domains were preincubated with 100 mM uncoupled wild-type or T233R peptide before addition to the beads.
Coimmunoprecipitations
Control cells or MDCK cells stably expressing HA-tagged wild-type or mutant CLDN16 were washed with PBS and lysed on ice in lysis buffer (0.5% Triton X-100 in PBS supplemented with a protease inhibitor cocktail). The postnuclear supernatant was precleared and immunoprecipitated with anti–ZO-1 (Zymed) or anti-HA (Roche) antibodies and protein G sepharose (Pharmacia). Immunoprecipitates were washed with lysis buffer, fractionated by SDS-PAGE (10% acrylamide), blotted onto polyinylidene difluoride membranes, and probed with antibodies against HA or ZO-1, respectively, followed by incubation with suitable HRP-labeled secondary antibodies (Jackson Laboratories and Pierce) and chemiluminescence detection (Pierce).
Immunofluorescence Microscopy
MDCK cells grown on glass cover slips or Transwell filters (Costar) were fixed with 3.7% paraformaldehyde for 30 min and were permeabilized with 0.2% Triton X-100 in PBS. After blocking with 10% goat serum (GibcoBRL), cells were incubated with different primary antibodies (5μg/ml) followed by suitable fluorescent secondary antibodies (1:1,000). Images were acquired using a confocal laser scanning microscope (BioRad) and IMARIS software (Bitplane), essentially as described by Kausalya et al. (2001).
Results
Identification of a Novel Mutation in the CLDN16 Gene
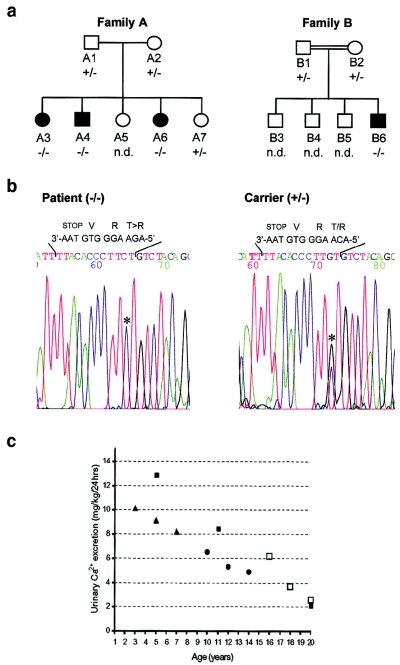
Screening for mutation in the CLDN16 gene was performed in 27 individuals from a cohort of 11 families with HC (Weber et al. 2001b); 18 members of 9 families (in each family, one affected and one nonaffected member were investigated) did not show a mutation in any of the sequenced alleles. However, in two families—one of Scandinavian (A) and the other of Spanish (B) origin—mutation screening revealed a novel single homozygous substitution of a cytosine for a guanine at position 697 of the ORF in patients A3, A4, A6, and B6 (fig. 1a and 1b). Individuals A1, A2, and A7, as well as B1 and B2, were heterozygous for the mutation, whereas A5, B3, B4, and B5 were not eligible for genotyping. At the protein level, the mutation results in the substitution of a conserved C-terminal threonine, at position 233, for an arginine (T233R; fig. 2). Sequencing the corresponding exon of the CLDN16 gene of 111 unaffected individuals, together with previous analysis of 160 control chromosomes (Simon et al. 1999), did not reveal polymorphisms or allelic variations.
Figure 1.
Clinical and molecular data regarding patients from two families with infantile familial HC with NC. a, Pedigrees of patient families. Blackened symbols indicate affected individuals; unblackened symbols indicate nonaffected individuals; squares indicate males; circles indicate females. Family B is consanguineous (see text for details). b, Sequencing data for the region in the CLDN16 gene carrying the T233R mutation. Homozygous (−/−) amino acid exchange (T→R) was detected in affected patients A3, A4, A6, and B6; heterozygous (+/−) mutations were detected in the nonaffected members A1, A2, A7, B1, and B2. Individuals B3, B4, and B5 were not available for analysis (“n.d.”). Sequences in the chromatogram read from 3′ to 5′, and the mutation is indicated with an asterisk (*). The coding and corresponding amino acid sequences are provided above the chromatogram. c, Urinary Ca2+ excretion decreases to levels within the normal range when patients reach adolescence. Ca2+ excretion was measure in affected members of families A and B during follow-up.
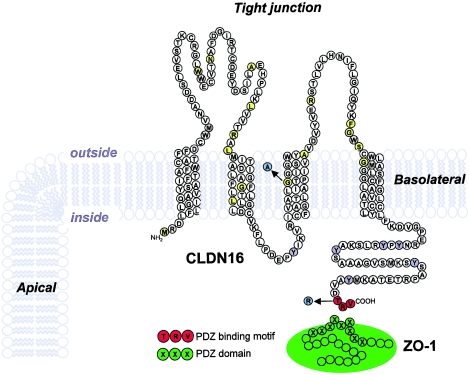
Figure 2.
Predicted topology of CLDN16 and location of the different mutations reported. Shown is the amino acid sequence from the second translation-initiation site, which is used in vivo (Ohba et al. 2000; Weber et al. 2001a, 2001b). Mutated amino acids thus far reported in the literature that are associated with classical FHHNC are shown (Simon et al. 1999; Weber et al. 2000; Weber et al. 2001b) (yellow), with the G128A mutation characterized in this study highlighted (blue). The C-terminal PDZ binding motif (red) and the novel T233R mutation encountered in the patients of families A and B (blue) are also shown. Tyrosine residues in the C-terminal cytosolic domain of CLDN16 that may be part of endocytosis and/or lysosomal sorting signals are indicated (gray).
Clinical Characterization of Patients Carrying the T233R Mutation
The second youngest child of family A (A6; fig. 1a) was admitted to the clinic for nocturnal enuresis. Diagnostic work-up revealed marked HC (10.1 mg/kg body weight per 24 h; fig. 1c) and renal medullary NC. Because the mother reported nocturnal enuresis and urinary tract infections in two of the other siblings, biochemical and ultrasound investigations were performed for all children, revealing HC and NC in patients A3 and A4, but the other children (A5 and A7) were unremarkable. During the 6-year follow-up period, urinary Ca2+ excretion decreased in each consecutive year in the affected children (fig. 1c), and, despite their age differences, there was a clear linear decrease over time in all patients. Although NC was still detectable on renal ultrasound examination, the oldest affected sister already displayed normal values for Ca2+ excretion at the time of the last examination (fig. 1c and 1d). Glomerular filtration rate remained normal in all children affected (112–122 ml/min/1.73 m2), with no evidence of a further progression of medullary NC (data not shown, ultrasound images available on request). In all affected members, Mg2+ levels were within the low normal ranges (1.8, 1.7, and 1.6 mg/dl), although they were consistently lower than in the nonaffected members (2.2 and 2.1 mg/dl), and urinary Mg2+ excretion was slightly elevated in the patients (0.13–.02 mmol/kg body weight per 24 h vs. 0.03–0.07 mmol/kg body weight per 24 h). 1,25(OH)2D3 (42–59 pg/ml), as well as intact parathyroid hormone (iPTH) levels, were within the normal ranges in all cases as were urinary sodium and potassium levels and their fractional excretion (patients A3, A4, and A6). During a 6-wk Ca2+ restriction test on the patients of family A, urinary Ca2+ remained constant and 1,25(OH)2D3 and iPTH levels increased. This effect was reversible when the patients were returned to a normal Ca2+-containing diet (1000 mg/d). In all affected individuals, a daily dose of 20 μg intranasally administered desmopressin resolved nocturnal enuresis without affecting urinary Ca2+ excretion.
The parents of family B (B1 and B2; fig. 1b) were second-degree relatives. The patient (B6) had three healthy brothers (B3–B5) and was first admitted to the hospital at the age of 5 years for recurring urinary tract infections and nephrolithiasis. He showed marked HC (12.9 mg/kg body weight per 24 h). Renal ultrasound showed medullary NC. Elevated levels of plasma renin and aldosterone and of urinary prostaglandin E2 were also observed. During the years of follow-up, the patient was admitted several times for nephrolithiasis. As for classical FHNNC, administration of hydrochlorothiazide did not reduce the amount of urinary Ca2+ excretion, indicating that the anticalciuric capabilities of thiazides are surpassed by the unreabsorbed Ca2+ load. Glomerular filtration rates always remained within normal range (last value 102.4 ml/min/1.73 m2). During follow-up, urinary Ca2+ excretion decreased (8.5 mg/kg body weight per 24 h at 11 years of age) and was within normal range at the last visit (at age 20 years) (fig. 1c). Asymptomatic hypomagnesemia (1.4 mg/dl) was encountered once at the age of 17 years. The most recent ultrasound of the kidneys showed that NC did not progress.
CLDN16, but Not the T233R Mutant, Binds to ZO-1
T233 is part of the C-terminal threonine-arginine-valine (TRV) sequence that resembles a putative type 1 PDZ-binding motif (Saras and Heldin 1996; Hung and Sheng 2002), and some claudins have been reported to interact with PDZ-domain proteins, in particular the TJ scaffold proteins ZO-1, ZO-2, and ZO-3 (Itoh et al. 1999; Gonzalez-Mariscal et al. 2000). We therefore determined whether CLDN16 interacts with ZO proteins and localizes to TJ and whether the interaction or localization is affected by the T233R mutation.
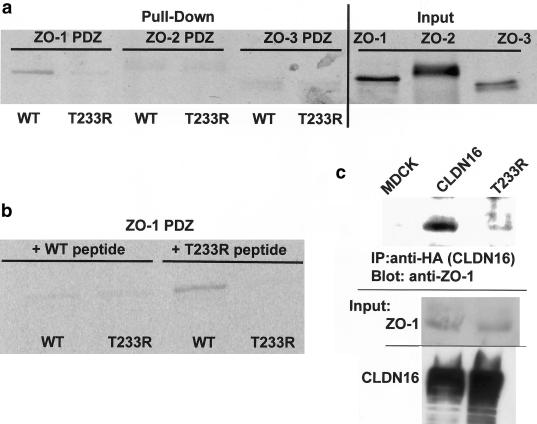
Immobilized peptides corresponding to the C-terminal 11 amino acids of wild-type CLDN16 bound in vitro translated PDZ domains of ZO-1, showing that the TRV sequence codes for a functional PDZ-binding motif. No specific interaction with the PDZ domains of ZO-2 or ZO-3 was observed (fig. 3a). The interaction between the T233R mutant peptide and the ZO-1 PDZ domains was strongly impaired. Binding to the immobilized wild-type CLDN16 peptide was abolished if the in vitro translated ZO-1 PDZ domains were preincubated with soluble wild-type CLDN16 peptide, whereas the T233R peptide had no effect (fig. 3b), confirming the specificity of the interaction. Furthermore, in renal distal tubule MDCK cells stably expressing wild-type or mutant CLDN16 carrying an N-terminal hemagglutinin-epitope tag, wild-type CLDN16, but not the T233R mutant, coprecipitated with ZO-1 (fig. 3c), consistent with the in vitro binding data. In conclusion, CLDN16 specifically binds to ZO-1, and this interaction is abolished by the T233R mutation.
Figure 3.
Binding of wild-type and mutant CLDN16 to ZO-1. a, Wild-type CLDN16, but not the T233R mutant, binds to the ZO-1 but not the ZO-2 and ZO-3 PDZ domains. In vitro translated and radioactively labeled ZO-1, ZO-2, or ZO-3 PDZ domains were incubated with peptides corresponding to the C-terminus of wild-type or T233R mutant CLDN16 coupled to beads. Protein bound to the beads (“Pull-Down”) was analyzed by SDS-PAGE and autoradiography, and an aliquot (5%) of the in vitro translated material was directly analyzed to confirm that similar amounts of the in vitro translated proteins were added to the binding reaction (“Input”). b, Preincubation with wild-type, but not T233R mutant, peptides competes with ZO-1 PDZ binding to beads. In vitro translated and radioactively labeled ZO-1 PDZ domains were pre-incubated with soluble wild-type or mutant CLDN16 peptides prior to the incubation with the peptide-coupled beads. c, Wild-type CLDN16, but not the T233R mutant, coprecipitates with ZO-1. Control cells (MDCK) or cells stably expressing wild-type (CLDN16) or mutant (T233R) CLDN16 were lysed, and equal amounts of total protein were used to immunoprecipitate CLDN16. Precipitates were blotted with anti–ZO-1 antibody to detect ZO-1 bound to CLDN16. In the reverse experiment, wild-type, but not mutant CLDN16, which is also associated with anti–ZO-1, precipitates (data not shown). Aliquots of the cell lysate were blotted directly to confirm that the cells expressed similar amounts of ZO-1, as well as wild-type and mutant CLDN16 (“Input”). WT = wild type.
The T233R Mutation Results in Lysosomal Mislocalization of CLDN16
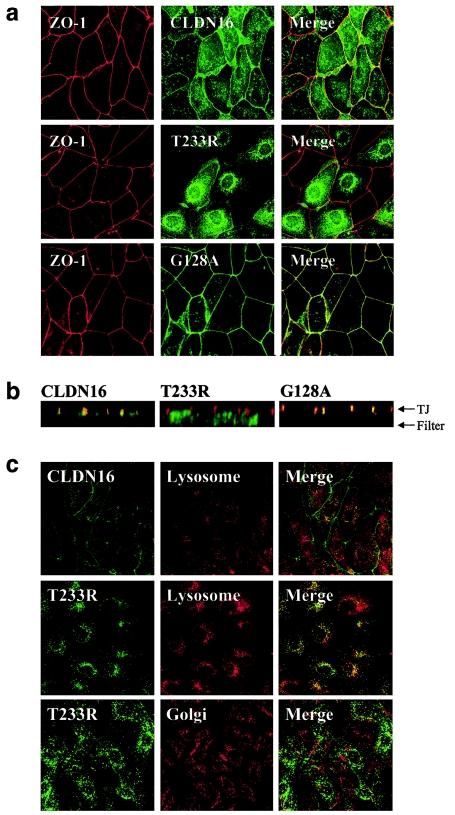
In agreement with the TJ localization in kidney cells in human tissue (Simon et al. 1999), wild-type CLDN16 was present at the height of TJ of transfected polarized MDCK cell monolayers, in which confocal microscopy showed that it colocalized with ZO-1 (fig. 4a). Intriguingly, however, the bulk of the T233R mutant showed an intracellular vesicular distribution and was no longer present at the plasma membrane. Vertical confocal sections along the apicobasal axis of the cell monolayer confirmed the colocalization of CLDN16 and ZO-1 to the TJ and the mislocalization of the T233R mutant (fig. 4b). ZO-1 localization was not affected in cells expressing T233R, a result expected because of the lack of interaction between the two proteins. Thus, the T233R mutation affects the association of CLDN16 with ZO-1 as well as its correct TJ targeting. In contrast to T233R, a previously described CLDN16 mutation associated with FHNNC, G128A (see fig. 2), was still correctly targeted to the cell surface, where it colocalized with ZO-1 at TJ (fig. 4a and 4b). Therefore, at least some of the FHNNC-linked mutations affecting the transmembrane or luminal domains of CLDN16 do not interfere with intracellular transport and/or correct localization of CLDN16 to TJ.
Figure 4.
Subcellular localization of wild-type and mutant CLDN16. a, Colocalization of CLDN16 and ZO-1 is abolished by the T233R mutation. MDCK cells stably expressing wild-type (CLDN16) or mutant (T233R or G128A) CLDN16 grown as polarized cell monolayers on polycarbonate filters were stained for ZO-1 (red) and CLDN16 (green), and confocal images corresponding to horizontal sections at the height of TJ were acquired. The merged image shows regions where CLDN16 and ZO-1 colocalize (yellow). b, TJ localization of CLDN16 is abolished by the T233R mutation. Vertical confocal sections along the apico-basal axis of the cell monolayers expressing wild-type (CLDN16) or mutant (T233R or G128A) CLDN16 labeled for CLDN16 (green) or ZO-1 (red). The positions of the filter supporting the cells and the TJ are indicated. c, T233R localized to lysosomes. MDCK cells transfected with wild-type (CLDN16) or mutant (T233R) CLDN16 were stained to visualize lysosomes (anti–lamp-2) or the Golgi complex (anti-GM130) (red) and CLDN16 (green), and confocal images were acquired. The T223R mutant shows extensive localization to lyososmes (merged image; yellow), but not the Golgi complex.
To determine the nature of the intracellular compartment in which the T223R mutant accumulated, CLDN16 and different organelles were labeled in cells expressing either wild-type or mutant CLDN16. Mutant, but not wild-type, CLDN16 extensively colocalized with lamp-2, a marker for late endosomes and lysosomes (Nabi et al. 1991; Hunziker and Geuze 1996) (fig. 4c). In contrast, no colocalization with the Golgi marker GM130 was observed, a result indicative of normal transport of T233R in the secretory pathway. Thus, the T233R mutant CLDN16 is mistargeted to lysosomes.
Discussion
Different members of the claudin family are involved in the establishment of the epithelial TJ barrier, in which they form paracellular ion channels with distinct substrate selectivity (Heiskala et al. 2001). The crucial role of claudins in ion homeostasis has been highlighted by the association of mutations in CLDN14 with deafness (Wilcox et al. 2001) and CLDN16 with FHHNC (Simon et al. 1999). Despite minor variations in the clinical phenotype in classical FHHNC, patients generally exhibit renal Mg2+ and Ca2+ wasting, impaired renal function, and, in most cases, chronic renal failure at the time of diagnosis (Weber et al. 2000; Blanchard et al. 2001). About one-third of patients develop end stage renal disease in the 1st to 2nd decade of life, resulting in the need for renal replacement therapy (Weber et al. 2000).
In the patients described in the present article, an identical and novel mutation in the PDZ-binding motif of CLDN16 correlated with similar clinical manifestations that differ in several aspects from the reported cases of classical FHHNC (Simon et al. 1999; Weber et al. 2000, 2001b; Blanchard et al. 2001). First, HC was clearly reduced from childhood to adolescence, returning to normal values in adulthood. NC was either reversible or did not progress. Second, hypomagnesemia was either not encountered (family A) or was only borderline during a single examination (family B), indicating that, although Mg2+ loss was slightly greater than normal, the loss was small enough to be compensated, probably by increased uptake via the gut. Third, renal function, as assessed by glomerular filtration rates, was unaffected. These clinical differences indicate that these patients only partially fulfill the criteria of FHHNC. Thus, if the present results are confirmed by larger cohort screenings, mutations affecting the PDZ-binding motif of CLDN16 may help to define a group of HC-associated disorders in which self-limiting infantile renal Ca2+ wasting is the most obvious symptom (Alon and Berenbom 2000; the present study). Mg2+ wastage, although consistently different between affected and nonaffected family members, was not of pathological significance. No similar cases of self-limiting HC could be clearly documented in the other nine families in the cohort that did not present mutations in the CLDN16 gene (Weber et al. 2001b).
The extent of HC in infancy may reflect a higher turnover of Ca2+ in childhood, indicating that CLDN16 may play a more important role in Ca2+ metabolism during growth than during adolescence and adulthood. On the other hand, since levels of PTH and 1,25(OH)2D3 increased at the end of a Ca2+-restriction diet, the reduced renal Ca2+ absorption is probably compensated by increased production of PTH and increased Ca2+ resorption from the bone. Thus, physiological bone mass loss in senescence might deteriorate by additional renal Ca2+ loss, and the patients carrying the T233R mutant may be at a higher risk for osteoporosis later in life, when renal Ca2+ conservation mechanisms are required. This particular importance of CLDN16 in Ca2+-homeostasis is supported by the high incidence of calcium-related kidney stone disease in individuals carrying heterozygous CLDN16 mutations (Weber et al. 2001b), although we did not observe HC or kidney stones in the heterozygous family members of our patients. The fact that desmopressin relieved nocturnal enuresis in all affected patients contradicts the hypothesis of a close correlation of urinary Ca2+ excretion and nocturnal enuresis (Alon et al. 1990; Valenti et al. 2000), indicating that its therapeutic benefit in this disorder may rather be due to a nonrenal effect (Muller et al. 2002b).
The CLDN16 mutations reported so far are all associated with classical FHHNC and located within the extracellular loops or the transmembrane domains of the molecule (Simon et al. 1999; Weber et al. 2000; Blanchard et al. 2001; Weber et al. 2001b) (fig. 2). To date, there are no functional expression studies of either wild-type or mutant CLDN16, but it was hypothesized that mutations in the extracellular loops alter the charge selectivity in the pore-forming region of the protein, hence interfering with the diffusion of divalent ions. The present study characterizes the mutation that, to our knowledge, is the first to be described in the C-terminal cytosolic tail of CLDN16 and the first to affect the PDZ-binding motif. Inactivation of the PDZ-binding motif in T233R abolishes the interaction of CLDN16 with the PDZ domains of the scaffolding protein ZO-1, one of whose functions is to link transmembrane TJ proteins to the actin cytoskeleton (Gonzalez-Mariscal et al. 2000). It is interesting that, instead of localizing to the TJ, the T233R mutant is misdirected to lysosomes. Thus, ZO-1 may tether CLDN16 to the TJ and prevent its internalization and subsequent lysosomal delivery. Because the T233R substitution does not create a known type of lysosomal sorting signal (Hunziker and Geuze 1996), several putative endocytosis and/or lysosomal sorting determinants present in the cytosolic domain of CLDN16 (fig. 2) may be responsible for lysosomal targeting once tethering to ZO-1 is disrupted. The detection of low amounts of T233R at the plasma membrane indeed suggests that T233R may be delivered to the TJ but not be retained there. Thus, the mutation may not affect the formation of a functional paracellular ion channel per se but rather shorten the residence time of CLDN16 at the TJ, thus reducing the number of CLDN16 molecules present at the TJ at any given time. Such a molecular mechanism—possibly combined with higher CLDN16 expression levels in adults and a general decrease, which occurs as individuals mature, in body weight and body surface that is related to Ca2+ excretion (Kruse et al. 1984)—may provide an explanation for the mild phenotype of FHHNC observed in the patients described here. Lysosomal mistargeting provides—for the first time, to our knowledge—insight into a molecular mechanism for a CLDN16 mutation associated with a clinical phenotype. Furthermore, because a mutant with an intact PDZ-binding motif but a single amino acid substitution in the luminal/transmembrane domain (G128A) correctly localizes to TJ, it is likely that, depending on the particular mutation in CLDN16, different molecular mechanisms can lead to FHHNC.
In conclusion, the T233R mutant will be useful to further dissect the role of the interaction with ZO proteins in TJ localization, intracellular transport, and turnover of CLDN16. The mutation may also provide a differential diagnostic and prognostic tool for a less severe form of FHHNC, with self-limiting childhood HC being the leading symptom.
Acknowledgments
We thank the family members for their support and cooperation. Andre Le Bivic and Wanjin Hong kindly provided antibodies. This work was supported by the Agency for Science, Technology and Research, Singapore (support to W.H.) and Deutsche Forschungsgemeinschaft grant MU 1497 2–1 (to D.M.).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CLDN16 sequence [accession number AF152101] and oligonucleotides [accession number NT005883])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FHHNC) [PubMed]
References
- Alon U, Warady BA, Hellerstein S (1990) Hypercalciuria in the frequency-dysuria syndrome of childhood. J Pediatr 116:103–105 [DOI] [PubMed] [Google Scholar]
- Alon US, Berenbom A (2000) Idiopathic hypercalciuria of childhood: 4- to 11-year outcome. Pediatr Nephrol 14:1011–1015 10.1007/s004670050064 [DOI] [PubMed] [Google Scholar]
- Asplin JR, Favus MJ, Coe FL (1996) In: Brenner BM (ed). The kidney, ed. 5. Philadelphia, W. B. Saunders, pp 1893–1935 [Google Scholar]
- Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P (2001) Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59:2206–2215 10.1046/j.1523-1755.2001.0590062206.x [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A (2000) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11:315–324 10.1006/scdb.2000.0178 [DOI] [PubMed] [Google Scholar]
- Heiskala M, Peterson PA, Yang Y (2001) The roles of claudin superfamily proteins in paracellular transport. Traffic 2:93–98 [DOI] [PubMed] [Google Scholar]
- Höning S, Hunziker W (1995) Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J Cell Biol 128:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Sheng M (2002) PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702 10.1074/jbc.R100065200 [DOI] [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ (1996) Intracellular trafficking of lysosomal membrane proteins. Bioessays 18:379–389 [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147:1351–1363 10.1083/jcb.147.6.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Reichert M, Hunziker W (2001) Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett 505:92–96 10.1016/S0014-5793(01)02786-7 [DOI] [PubMed] [Google Scholar]
- Kruse K, Kracht U, Kruse U (1984) Reference values for urinary calcium excretion and screening for hypercalciuria in children and adolescents. Eur J Pediatr 143:25–31 [DOI] [PubMed] [Google Scholar]
- Meij IC, Van Den Heuvel LP, Hemmes S, Van Der Vliet WA, Willems JL, Monnens LA, Knoers NV (2003) Exclusion of mutations in FXYD2, CLDN16 and SLC12A3 in two families with primary renal Mg2+ loss. Nephrol Dial Transplant 18:512–516 10.1093/ndt/18.3.512 [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96:511–516 10.1073/pnas.96.2.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Hoenderop JG, Vennekens R, Eggert P, Harangi F, Mehes K, Garcia-Nieto V, Claverie-Martin F, Os CH, Nilius B, Bindels RJM (2002a) Epithelial Ca2+ channel (ECAC1) in autosomal dominant idiopathic hypercalciuria. Nephrol Dial Transplant 17:1614–1620 10.1093/ndt/17.9.1614 [DOI] [PubMed] [Google Scholar]
- Muller D, Marr N, Ankermann T, Eggert P, Deen PM (2002b) Desmopressin for nocturnal enuresis in nephrogenic diabetes insipidus. Lancet 359:495–497 10.1016/S0140-6736(02)07667-5 [DOI] [PubMed] [Google Scholar]
- Nabi IR, Le Bivic A, Fambrough D, Rodriguez-Boulan E (1991) An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol 115:1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G (1997) The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89:445–455 [DOI] [PubMed] [Google Scholar]
- Ohba Y, Kitagawa H, Kitoh K, Sasaki Y, Takami M, Shinkai Y, Kunieda T (2000) A deletion of the paracellin-1 gene is responsible for renal tubular dysplasia in cattle. Genomics 68:229–236 10.1006/geno.2000.6298 [DOI] [PubMed] [Google Scholar]
- Reichert M, Muller T, Hunziker W (2000) The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells: evidence for a role of β-catenin/Tcf/Lef signaling. J Biol Chem 275:9492–9500 10.1074/jbc.275.13.9492 [DOI] [PubMed] [Google Scholar]
- Saras J, Heldin CH (1996) PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem Sci 21:455–458 10.1016/S0968-0004(96)30044-3 [DOI] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106 10.1126/science.285.5424.103 [DOI] [PubMed] [Google Scholar]
- Stapleton FB, Roy S 3rd, Noe HN, Jerkins G (1984) Hypercalciuria in children with hematuria. N Engl J Med 310:1345–1358 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- Valenti G, Laera A, Pace G, Aceto G, Lospalluti ML, Penza R, Selvaggi FP, Chiozza ML, Svelto M (2000) Urinary aquaporin 2 and calciuria correlate with the severity of enuresis in children. J Am Soc Nephrol 11:1873–1881 [DOI] [PubMed] [Google Scholar]
- Weber S, Hoffmann K, Jeck N, Saar K, Boeswald M, Kuwertz-Broeking E, Meij II, Knoers NV, Cochat P, Sulakova T, Bonzel KE, Soergel M, Manz F, Schaerer K, Seyberth HW, Reis A, Konrad M (2000) Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet 8:414–422 10.1038/sj.ejhg.5200475 [DOI] [PubMed] [Google Scholar]
- Weber S, Schlingmann KP, Peters M, Nejsum LN, Nielsen S, Engel H, Grzeschik KH, Seyberth HW, Grone HJ, Nusing R, Konrad M (2001a) Primary gene structure and expression studies of rodent paracellin-1. J Am Soc Nephrol 12:2664–2672 [DOI] [PubMed] [Google Scholar]
- Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M (2001b) Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 12:1872–1881 [DOI] [PubMed] [Google Scholar]
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Friedman TB (2001) Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104:165–172 [DOI] [PubMed] [Google Scholar]