Abstract
Background
Estimate glucose disposal rate (eGDR), Chinese visceral adiposity index (CVAI), triglyceride-glucose (TyG), TyG-body mass index (TyG-BMI), metabolic score for insulin resistance (METS-IR), and atherogenic index of plasma (AIP) are considered surrogate indexes of insulin resistance (IR). There is a lack of studies comparing the predictive values of different IR surrogate indexes for stroke risk among individuals with abnormal glucose metabolism. This study aimed to investigate the relationships between six IR surrogate indexes and stroke risk in individuals with abnormal glucose metabolism, evaluate their predictive abilities for stroke risk.
Methods
Data from the China Health and Retirement Longitudinal Study (CHARLS) were analysed in this study. Multivariate logistic regression models were applied to analyse the relationships of IR surrogate indexes with stroke risk. The dose-response relationships between IR surrogate indexes and stroke risk were explored using restricted cubic splines. The areas under the curve (AUCs) of IR surrogate indexes were calculated by receiver operating characteristic (ROC) analysis.
Results
After adjusting for potential confounders, we observed that each standard deviation (SD) increase in eGDR was associated with a reduced risk of stroke, with an adjusted odds ratio (OR) of 0.746 [95% confidence interval (CI): 0.661–0.842]. In contrast, each SD increase in CVAI, TyG, TyG-BMI, METS-IR, and AIP were associated with an increased risk of stroke, with adjusted ORs (95% CIs) of 1.232 (1.106–1.373), 1.246 (1.050–1.479), 1.186 (1.022–1.376), 1.222 (1.069–1.396), and 1.193 (1.050–1.355), respectively. Dose-response analyses showed that eGDR, CVAI, TyG-BMI and METS-IR were linearly associated with stroke risk (Pnonlinear ≥ 0.05), whereas TyG and AIP were nonlinearly associated with stroke risk (Pnonlinear < 0.05). According to ROC analysis, The AUC of eGDR for predicting stroke risk in the overall population with abnormal glucose metabolism (AUC: 0.612, 95% CI: 0.584–0.640) was significantly higher than that of other indexes.
Conclusion
The six IR surrogate indexes were closely associated with high risk of stroke in individuals with abnormal glucose metabolism. The eGDR showed promising potential in predicting stroke risk in Chinese middle-aged and elderly populations with abnormal glucose metabolism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02618-7.
Keywords: Insulin resistance surrogate index, Stroke, Abnormal glucose metabolism, CHARLS
Introduction
Stroke represents a global public health challenge, with its high incidence and mortality rates imposing substantial burdens on societies and healthcare systems. According to Global Burden of Disease studies, stroke has become a major contributor to the global health burden [1, 2]. The prevalence and incidence of stroke remain notably high in China [3]. IR is widely recognised as an important risk factor for stroke [4, 5]. The risk of stroke is significantly increased in individuals with abnormal glucose metabolism. Studies have reported that prediabetes increases the risk of stroke [6], and individuals with diabetes have a two to four times higher risk of stroke compared to those without diabetes [7]. This heightened risk is likely related to the multiple metabolic abnormalities commonly seen in diabetes, including IR, hyperglycaemia, dyslipidaemia, and hypertension [8]. Therefore, effective preventive measures and management strategies, such as optimal glycaemic control, lipid and blood pressure management, for patients with abnormal glucose metabolism, especially those with diabetes, are essential to reduce stroke risk.
Although the hyperinsulinemic–euglycemic clamp is considered the gold standard for assessing IR [9], it is not suitable for clinical and epidemiological studies due to its complexity, invasiveness and high cost. As a result, various surrogate indexes of IR, such as eGDR, CVAI, TyG, TyG-BMI, METS-IR, and AIP, have received increasing attention. These indexes have been shown to be valuable in assessing IR and are strongly associated with the risk and prognosis of cardiovascular diseases [10–14].
Previous studies have explored the relationships between IR surrogate indexes and stroke risk. However, the utility of these indexes in predicting stroke risk remains controversial, influenced by factors such as race, region, sex and age. In individuals with abnormal glucose metabolism, the associations of eGDR, CVAI, TyG, TyG-BMI, METS-IR, and AIP with stroke risk remain unclear. Furthermore, there is a lack of studies comparing the predictive values of these IR surrogate indexes for stroke risk in individuals with abnormal glucose metabolism. Therefore, the aim of this study was to evaluate the associations between IR surrogate indexes and stroke risk, as well as the predictive values of these indexes for stroke risk in a Chinese middle-aged and elderly population with abnormal glucose metabolism, by analysing data from the CHARLS database.
Methods
Study design and population
The China Health and Retirement Longitudinal Study (CHARLS) is an ongoing nationally representative longitudinal survey designed to reflect the social, economic, and health status of middle-aged and elderly individuals aged 45 and older in China. The CHARLS cohort was established using a multistage probability sampling method, selecting participants from 150 counties (districts) and 450 villages (communities) across 28 provinces. The first survey of CHARLS was conducted in 2011, followed by four subsequent surveys in 2013, 2015, 2018, and 2020. The CHARLS project was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015), and all participants signed an informed consent form before participating in the study.
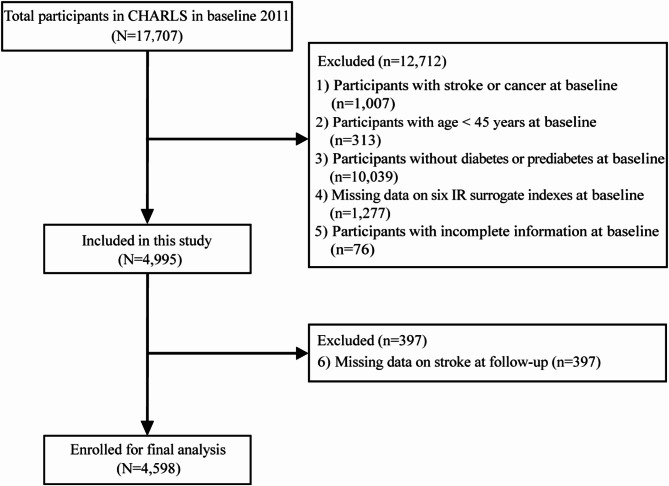
Our cohort study used data from the CHARLS surveys conducted in 2011, 2013, 2015, 2018, and 2020, with the 2011 survey serving as the baseline. A total of 17,707 participants from the 2011 baseline survey were included in this study. To refine the study, several exclusion criteria were applied. Participants were sequentially excluded based on the following steps: (1) participants with stroke or cancer at baseline; (2) participants aged under 45 years at baseline; (3) individuals without diabetes or prediabetes at baseline; (4) missing data on one of the six IR surrogate indexes at baseline; (5) incomplete information on socio-demographic, health-related, anthropometric, and other biomarkers at baseline; (6) missing stroke data at follow-up. Finally, a total of 4,598 participants were included in this study. The exclusion process is shown in Fig. 1.
Fig. 1.
Flowchart of participant selection
Data collection and measurement
During the baseline survey, interviewers collected socio-demographic (sex, age, education and marital status), health-related behaviours (smoking and alcohol consumption) and medical history (diabetes, hypertension and heart disease) through questionnaires. Educational level was categorised as no formal education, primary school, middle school, high school or above. Marital status was categorised as married and other marital statuses (separated, divorced, widowed and never married). Smoking more than 100 cigarettes in a lifetime was defined as smoking. Smoking status was categorised into three groups: never smoker, former smoker and current smoker. Alcohol consumption was classified into three categories: never drinkers (those who never or rarely drink alcohol, consuming less than once a month), former drinkers (those who drank more than once a month but stopped in the past year), and current drinkers (those who drink more than once a month). Anthropometric data such as height, weight, waist circumference (WC), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by trained professionals. All participants provided venous blood samples after fasting for at least 8 h. Blood sample information included fasting plasma glucose (FPG), glycosylated hemoglobin A1c (HbA1c), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum creatinine (Scr), serum uric acid (SUA), blood urea nitrogen (BUN) and C-reactive protein (CRP).
eGDR, CVAI, TyG, TyG-BMI, METS-IR, and AIP were calculated using the following equations:
BMI = weightkg /heightm2
eGDR = 21.158 − 0.09 × WCcm − 3.407 × hypertension(yes = 1/no = 0) − 0.551 × HbA1c%.
CVAI (male) = − 267.93 + 0.68 × ageyears + 0.03 ×  + 4.00 × WCcm + 22.00 × log10(TGmmol/L) − 16.32 × HDL-Cmmol/L.
+ 4.00 × WCcm + 22.00 × log10(TGmmol/L) − 16.32 × HDL-Cmmol/L.
CVAI (female) = − 187.32 + 1.71 × ageyears + 4.23 ×  + 1.12 × WCcm + 39.76 × log10(TGmmol/L) − 11.66 × HDL-Cmmol/L.
+ 1.12 × WCcm + 39.76 × log10(TGmmol/L) − 11.66 × HDL-Cmmol/L.
TyG = ln(TGmg/dl × FPGmg/dl /2).
TyG-BMI = TyG × 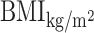 .
.
METS-IR = ln(2 × FPGmg/dl + TGmg/dl) × 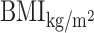 /ln(HDL-Cmg/dl).
/ln(HDL-Cmg/dl).
AIP = log(TGmg/dl /HDL-Cmg/dl).
Definition
Prediabetes was defined as FPG levels between 100 and 125 mg/dL or HbA1c levels between 5.7% and 6.4%. Diabetes was defined as self-reported physician diagnosis, use of glucose-lowering medications, FPG ≥ 126 mg/dL or HbA1c ≥ 6.5% [15]. Prediabetes or diabetes was categorised as glucose metabolism disorders. Hypertension was defined as self-reported physician diagnosis, use of antihypertensive medications, or average SBP/DBP ≥ 140/90 mmHg [16].
The outcome of this study was stroke events. Participants who had no history of stroke at baseline but reported a stroke during follow-up were recorded as incident cases. Data on stroke events were obtained from the survey questionnaires conducted during each follow-up wave from 2013 to 2020. In accordance with previous studies [17, 18], stroke events were assessed using the following standardised questions: “Have you been diagnosed with a stroke by a doctor?” or “Are you currently receiving any treatment (traditional Chinese medicine/Western medicine/physical therapy/acupuncture and moxibustion/occupational therapy) to control your stroke?”
Missing data processing
In our study, participants with incomplete IR surrogate index information (1,277, 20.12%), missing stroke follow-up data (397, 6.25%), or missing covariate data (e.g., baseline socio-demographic and health-related information) (76, 1.20%) were excluded. To assess potential selection bias, we compared the baseline characteristics of the excluded participants with those retained in the study (Additional file 1: Table S1).
Statistical analysis
Continuous variables were presented as means and standard deviations, and categorical variables were expressed as numbers and percentages. The independent t-test, Mann-Whitney U test, or chi-square test were employed for comparisons between groups. Correlations between surrogate indexes of IR and stroke risk were assessed using logistic regression analysis. To allow direct comparisons of OR values, the six IR surrogate indexes were converted into Z-scores. Three logistic models were used in this study. Model 1 was not adjusted for any variables. Model 2 was adjusted for sex, age, education level, marital status, smoking status, alcohol consumption, BMI, and WC. Model 3 was adjusted for sex, age, education level, marital status, smoking status, alcohol consumption, BMI, WC, TC, HDL-C, LDL-C, Scr, SUA, BUN, CRP, hypertension, and heart diseases. For all IR surrogate indexes, variables already included in the equations were not adjusted for in the regression models. We assessed potential multicollinearity among variables in each model using the variance inflation factor (VIF). The VIF values for all variables in each model were below 10 and no significant multicollinearity problems were detected. In addition, we explored the dose-response relationships of the IR surrogate indexes with stroke risk using restricted cubic splines. The abilities of these indexes to predict stroke risk were evaluated using ROC curves. The AUC, optimal cutoff value, sensitivity, specificity, and Youden’s index (sensitivity + specificity-1) were calculated for each index to predict stroke risk. We also used DeLong’s test to detect differences in the AUCs of different IR surrogate indexes. P < 0.05 indicated statistical significance. All statistical analyses in this study were performed using EmpowerStats (version 4.2) and R (version 4.4.1).
Results
Baseline characteristics of study participants
A total of 4,598 participants were enrolled in the study, including 2,069 males and 2,529 females, with mean ages of 59.90 and 59.06 years, respectively. General clinical and biochemical characteristics of stroke and non-stroke participants were described according to sex (Table 1). 199 males (9.62%) and 230 females (9.09%) were diagnosed with stroke, with a significantly higher proportion of males suffering from stroke. Compared to non-stroke males, stroke-affected males were older and had higher proportions of hypertension and heart disease. Stroke-affected males exhibited higher levels of SBP, DBP, BMI, WC, TC, TG, LDL-C, Scr, SUA, CRP, HbA1c, CVAI, TyG, TyG-BMI, METS-IR, and AIP, and lower levels of eGDR (P < 0.05). Similarly, compared to non-stroke females, stroke-affected females were older and had higher proportions of hypertension, heart disease and alcohol consumption. Stroke-affected females also showed higher levels of SBP, DBP, WC, TG, Scr, CRP, FPG, HbA1c, CVAI, TyG, TyG-BMI, METS-IR, and AIP, and lower levels of HDL-C and eGDR (P < 0.05). Additionally, we compared the baseline characteristics of participants excluded due to missing data (e.g., incomplete IR surrogate index information, missing stroke follow-up data, and missing covariate data) with those retained in the study (Additional file 1: Table S1). The results revealed some differences between the excluded and retained groups in terms of SUA and CRP levels, while the differences in most other baseline characteristics were minimal. Therefore, we believe that the exclusion of participants due to missing data is unlikely to result in significant selection bias overall.
Table 1.
Baseline characteristics of the study participants with and without stroke by sex
| Variable | Male | Female | ||||
|---|---|---|---|---|---|---|
| Non-Stroke | Stroke | P value | Non-Stroke | Stroke | P value | |
| Number | 1870 | 199 | 2299 | 230 | ||
| Age (years) | 59.71 ± 8.90 | 61.74 ± 8.45 | 0.001 | 58.87 ± 9.20 | 60.96 ± 8.43 | < 0.001 |
| Education level, n (%) | 0.169 | 0.498 | ||||
| Below primary school | 256 (13.69%) | 33 (16.58%) | 1032 (44.89%) | 107 (46.52%) | ||
| Primary school | 880 (47.06%) | 96 (48.24%) | 800 (34.80%) | 81 (35.22%) | ||
| Middle school | 528 (28.24%) | 43 (21.61%) | 343 (14.92%) | 35 (15.22%) | ||
| High school or above | 206 (11.02%) | 27 (13.57%) | 124 (5.39%) | 7 (3.04%) | ||
| Marital status, n (%) | 0.194 | 0.911 | ||||
| Married | 189 (10.11%) | 26 (13.07%) | 413 (17.96%) | 42 (18.26%) | ||
| Others | 1681 (89.89%) | 173 (86.93%) | 1886 (82.04%) | 188 (81.74%) | ||
| Smoking status, n (%) | 0.181 | 0.528 | ||||
| Never | 490 (26.20%) | 43 (21.61%) | 2123 (92.34%) | 209 (90.87%) | ||
| Former | 314 (16.79%) | 42 (21.11%) | 53 (2.31%) | 8 (3.48%) | ||
| Current | 1066 (57.01%) | 114 (57.29%) | 123 (5.35%) | 13 (5.65%) | ||
| Alcohol consumption, n (%) | 0.277 | 0.044 | ||||
| Never | 563 (30.11%) | 56 (28.14%) | 1923 (83.65%) | 178 (77.39%) | ||
| Former | 219 (11.71%) | 31 (15.58%) | 108 (4.70%) | 17 (7.39%) | ||
| Current | 1088 (58.18%) | 112 (56.28%) | 268 (11.66%) | 35 (15.22%) | ||
| Hypertension, n (%) | 763 (40.80%) | 122 (61.31%) | < 0.001 | 1011 (43.98%) | 134 (58.26%) | < 0.001 |
| Heart diseases, n (%) | 244 (13.05%) | 38 (19.10%) | 0.018 | 382 (16.62%) | 64 (27.83%) | < 0.001 |
| SBP (mmHg) | 130.63 ± 19.46 | 141.07 ± 23.24 | < 0.001 | 131.57 ± 21.67 | 138.02 ± 24.53 | < 0.001 |
| DBP (mmHg) | 76.31 ± 11.90 | 80.72 ± 13.22 | < 0.001 | 75.91 ± 11.44 | 77.63 ± 11.50 | 0.024 |
| BMI (kg/m2) | 23.25 ± 3.49 | 24.27 ± 3.91 | < 0.001 | 24.42 ± 3.92 | 24.93 ± 4.20 | 0.065 |
| WC (cm) | 85.72 ± 9.74 | 88.52 ± 9.60 | < 0.001 | 85.75 ± 12.67 | 87.61 ± 12.55 | 0.011 |
| TC (mg/dl) | 192.90 ± 38.59 | 200.99 ± 40.68 | 0.005 | 203.69 ± 39.62 | 206.45 ± 38.27 | 0.312 |
| TG (mg/dl) | 138.16 ± 116.63 | 149.71 ± 101.88 | < 0.001 | 149.73 ± 108.51 | 166.65 ± 113.90 | 0.007 |
| HDL-C (mg/dl) | 50.83 ± 17.14 | 48.66 ± 16.07 | 0.088 | 50.54 ± 14.47 | 48.40 ± 13.17 | 0.032 |
| LDL-C (mg/dl) | 114.17 ± 35.63 | 121.21 ± 39.06 | 0.009 | 123.81 ± 37.18 | 125.00 ± 37.05 | 0.645 |
| Scr (mg/dl) | 0.88 ± 0.20 | 0.91 ± 0.17 | 0.022 | 0.69 ± 0.15 | 0.71 ± 0.14 | 0.030 |
| SUA (mg/dl) | 4.99 ± 1.27 | 5.20 ± 1.36 | 0.019 | 4.09 ± 1.07 | 4.03 ± 1.05 | 0.398 |
| BUN (mg/dl) | 16.69 ± 4.59 | 16.92 ± 4.41 | 0.500 | 15.19 ± 4.31 | 14.95 ± 4.03 | 0.411 |
| CRP (mg/L) | 2.87 ± 8.35 | 3.78 ± 10.96 | 0.031 | 2.66 ± 6.68 | 3.31 ± 9.69 | 0.012 |
| FPG (mg/dl) | 121.49 ± 39.54 | 125.58 ± 51.83 | 0.179 | 120.37 ± 38.27 | 130.51 ± 56.08 | < 0.001 |
| HbA1c (%) | 5.40 ± 0.87 | 5.55 ± 1.02 | 0.031 | 5.48 ± 0.96 | 5.70 ± 1.25 | < 0.001 |
| eGDR | 9.08 ± 2.17 | 8.05 ± 2.21 | < 0.001 | 8.93 ± 2.34 | 8.15 ± 2.32 | < 0.001 |
| CVAI | 97.02 ± 44.50 | 111.74 ± 43.34 | < 0.001 | 103.62 ± 35.61 | 113.89 ± 35.93 | < 0.001 |
| TyG | 8.79 ± 0.69 | 8.93 ± 0.70 | 0.003 | 8.91 ± 0.64 | 9.07 ± 0.71 | < 0.001 |
| TyG-BMI | 205.11 ± 39.26 | 217.38 ± 42.10 | < 0.001 | 218.08 ± 41.24 | 226.52 ± 44.36 | 0.003 |
| METS-IR | 35.91 ± 8.26 | 38.20 ± 9.03 | < 0.001 | 37.73 ± 8.32 | 39.41 ± 9.10 | 0.004 |
| AIP | 0.37 ± 0.36 | 0.44 ± 0.34 | 0.003 | 0.42 ± 0.34 | 0.48 ± 0.33 | 0.006 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Scr, serum creatinine; SUA, serum uric acid; BUN, blood urea nitrogen; CRP, C-reactive protein; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin A1c; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma
Data are presented as mean ± standard deviation or number (%)
Associations and dose-response relationships between IR surrogate indexes and stroke risk
The associations between IR surrogate indexes and stroke risk in participants with abnormal glucose metabolism are shown in Table 2. The results indicated that in both Model 1 and Model 2, eGDR was negatively associated with stroke risk, whereas other IR surrogate indexes (CVAI, TyG, TyG-BMI, METS-IR, and AIP) were positively associated with stroke risk. This associations between the IR surrogate indexes and stroke risk remained significant after adjusting for all confounders (Model 3). A per-SD increase in eGDR corresponded to adjusted OR (95% CI) of 0.746 (0.661–0.842). A per-SD increase in CVAI, TyG, TyG-BMI, METS-IR, and AIP corresponded to adjusted ORs (95% CIs) of 1.232 (1.106–1.373), 1.246 (1.050–1.479), 1.186 (1.022–1.376), 1.222 (1.069–1.396), and 1.193 (1.050–1.355), respectively.
Table 2.
Multivariate regression analysis of the associations between six IR surrogate indexes and stroke risk
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| eGDR | 0.677 (0.612, 0.748) | < 0.001 | 0.716 (0.636, 0.807) | < 0.001 | 0.746 (0.661, 0.842) | < 0.001 |
| CVAI | 1.353 (1.226, 1.494) | < 0.001 | 1.356 (1.227, 1.499) | < 0.001 | 1.232 (1.106, 1.373) | < 0.001 |
| TyG | 1.236 (1.125, 1.357) | < 0.001 | 1.207 (1.093, 1.334) | < 0.001 | 1.246 (1.050, 1.479) | 0.012 |
| TyG-BMI | 1.256 (1.144, 1.379) | < 0.001 | 1.295 (1.141, 1.469) | < 0.001 | 1.186 (1.022, 1.376) | 0.025 |
| METS-IR | 1.238 (1.128, 1.359) | < 0.001 | 1.252 (1.106, 1.417) | < 0.001 | 1.222 (1.069, 1.396) | 0.003 |
| AIP | 1.204 (1.094, 1.325) | < 0.001 | 1.172 (1.057, 1.300) | 0.003 | 1.193 (1.050, 1.355) | 0.007 |
Model 1 was unadjusted.
Model 2 was adjusted for sex, age, education level, marital status, smoking status, alcohol consumption, BMI, and WC.
Model 3 was adjusted for Model 2 + TC, HDL-C, LDL-C, Scr, SUA, BUN, CRP, hypertension, and heart diseases.
OR, odds ratio; CI, confidence interval; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma; WC, waist circumference; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Scr, serum creatinine; SUA, serum uric acid; BUN, blood urea nitrogen; CRP, C-reactive protein.
ORs are presented as per 1 SD increase in the IR surrogate indexes for stroke risk.
We analysed the dose-response relationships between six IR surrogate indexes and stroke risk using the RCS. As shown in Fig. 2, after adjusting for confounding factors, eGDR was linearly and negatively associated with stroke risk (Pnonlinear > 0.05, Fig. 2A). CVAI, TyG-BMI, and METS-IR were linearly and positively associated with stroke risk (Pnonlinear > 0.05, Fig. 2B, D, and E). TyG and AIP were nonlinearly and positively associated with stroke risk (Pnonlinear < 0.05, Fig. 2C, F). The results from the RCS analysis were generally consistent with those from the multivariate regression analysis.
Fig. 2.
Dose-response relationships between IR surrogate indexes and stroke risk. We adjusted the model fully for sex, age, education level, marital status, smoking status, alcohol consumption, BMI, WC, TC, HDL-C, LDL-C, Scr, SUA, BUN, CRP, hypertension, and heart diseases. CI, confidence interval; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma; WC, waist circumference; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Scr, serum creatinine; SUA, serum uric acid; BUN, blood urea nitrogen; CRP, C-reactive protein.
Subgroup analyses to determine the associations between IR surrogate indexes and stroke risk
To further explore the relationships between six IR surrogate indexes and stroke risk in participants with abnormal glucose metabolism, subgroup analyses were performed by sex and age. As shown in Fig. 3, we found that the associations between IR surrogate indexes and stroke risk varied by sex and age. eGDR was significantly associated with stroke risk across both sex and age groups, with stronger associations observed in males and middle-aged participants (under 60 years). The significant associations of other IR surrogate indexes with stroke risk were predominantly observed in females and older participants (over 60 years). Additionally, significant interactions were found between eGDR (P for interaction = 0.0191) and CVAI (P for interaction = 0.0334) with age.
Fig. 3.
Subgroup analyses of the associations between IR surrogate indexes and stroke risk. Each subgroup was adjusted for sex, age, education level, marital status, smoking status, alcohol consumption, BMI, WC, TC, HDL-C, LDL-C, Scr, SUA, BUN, CRP, hypertension, and heart diseases, except for stratification variables. ORs are presented as per 1 SD increase in the IR surrogate indexes for stroke risk. OR, odds ratio; CI, confidence interval; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma; WC, waist circumference; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Scr, serum creatinine; SUA, serum uric acid; BUN, blood urea nitrogen; CRP, C-reactive protein.
Predictive performance of IR surrogate indexes for stroke risk in the overall population and in different sex groups
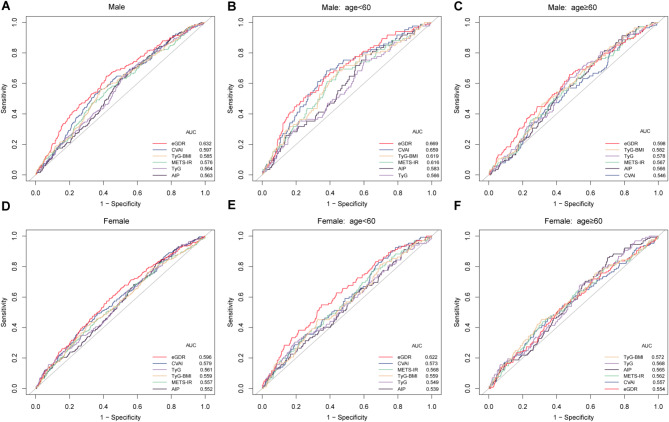
Table 3; Fig. 4 present the predictive abilities of IR surrogate indexes for stroke risk in the overall population, as well as in male and female subgroups. Among the overall population with abnormal glucose metabolism, eGDR demonstrated superior predictive ability for stroke risk compared to other IR surrogate indexes, with the highest AUC value (AUC: 0.612, 95% CI: 0.584–0.640), followed by CVAI (AUC: 0.588, 95% CI: 0.560–0.616). The predictive performances of different IR surrogate indexes for stroke risk were further compared in different sex groups. In males, eGDR showed significantly better predictive ability for stroke risk compared to other IR surrogate indexes (P < 0.05). In females, eGDR outperformed TyG-BMI and METS-IR in predicting stroke risk (P < 0.05). For other indexes, no significant differences were observed in their predictive performance for stroke risk between sex groups.
Table 3.
ROC curves of IR surrogate indexes and stroke risk in different sex groups
| Group | Variable | AUC (95% CI) | P value | P for comparison | Optimal cutoff value | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|---|---|
| All | eGDR | 0.612(0.584, 0.640) | < 0.001 | Reference | 8.759 | 0.627 | 0.558 | 0.185 |
| CVAI | 0.588(0.560, 0.616) | < 0.001 | 0.040 | 116.312 | 0.490 | 0.658 | 0.148 | |
| TyG | 0.560(0.533, 0.588) | < 0.001 | 0.002 | 8.577 | 0.737 | 0.375 | 0.112 | |
| TyG-BMI | 0.568(0.539, 0.596) | < 0.001 | < 0.001 | 198.203 | 0.711 | 0.406 | 0.117 | |
| METS-IR | 0.564(0.536, 0.592) | < 0.001 | < 0.001 | 35.777 | 0.611 | 0.503 | 0.114 | |
| AIP | 0.556(0.529, 0.583) | < 0.001 | < 0.001 | 0.325 | 0.660 | 0.455 | 0.115 | |
| Male | eGDR | 0.632(0.591, 0.673) | < 0.001 | Reference | 8.669 | 0.648 | 0.582 | 0.230 |
| CVAI | 0.597(0.556, 0.637) | < 0.001 | 0.036 | 113.652 | 0.528 | 0.651 | 0.178 | |
| TyG | 0.564(0.524, 0.604) | 0.003 | 0.007 | 8.616 | 0.678 | 0.456 | 0.135 | |
| TyG-BMI | 0.585(0.544, 0.626) | < 0.001 | 0.011 | 211.450 | 0.558 | 0.618 | 0.176 | |
| METS-IR | 0.576(0.535, 0.617) | < 0.001 | 0.004 | 38.062 | 0.472 | 0.666 | 0.139 | |
| AIP | 0.563(0.524, 0.602) | 0.003 | 0.004 | 0.324 | 0.633 | 0.498 | 0.131 | |
| Female | eGDR | 0.596(0.558, 0.634) | < 0.001 | Reference | 8.430 | 0.591 | 0.565 | 0.156 |
| CVAI | 0.579(0.541, 0.617) | < 0.001 | 0.320 | 114.503 | 0.491 | 0.636 | 0.127 | |
| TyG | 0.561(0.522, 0.599) | 0.002 | 0.125 | 8.786 | 0.635 | 0.466 | 0.101 | |
| TyG-BMI | 0.559(0.519, 0.598) | 0.003 | 0.040 | 236.113 | 0.409 | 0.700 | 0.109 | |
| METS-IR | 0.557(0.518, 0.596) | 0.004 | 0.036 | 35.777 | 0.648 | 0.452 | 0.100 | |
| AIP | 0.552(0.515, 0.589) | 0.010 | 0.057 | 0.213 | 0.817 | 0.293 | 0.110 |
ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma.
Fig. 4.
The ROC curves of IR surrogates and stroke risk in different sex and age groups. ROC, receiver operating characteristic; AUC, area under the curve; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma.
Predictive performance of IR surrogate indexes for stroke risk in different sex and age groups
Table 4; Fig. 4 show the predictive abilities of IR surrogate indexes for stroke risk at different ages for males and females. We found that eGDR had the highest AUC values in middle-aged males, older males, and middle-aged females, with AUCs of 0.669 (95% CI: 0.610–0.729), 0.598 (95% CI: 0.542–0.654), and 0.622 (95% CI: 0.566–0.677), respectively. We compared the predictive abilities of different IR surrogate indexes for stroke risk in different sex and age groups. Among middle-aged males and females, eGDR demonstrated better predictive ability for stroke risk compared to TyG and AIP (P < 0.05). In middle-aged males, CVAI showed superior predictive performance for stroke risk compared to TyG, TyG-BMI, METS-IR, and AIP (P < 0.05). In different sex and age groups, the majority of IR surrogate indexes did not show significant statistical differences in their predictive abilities for stroke risk. Across different sex and age groups, the AUC values of all six IR surrogate indexes for predicting stroke risk exceeded 0.5, indicating their predictive values for stroke risk in populations with abnormal glucose metabolism.
Table 4.
ROC curves of IR surrogates and stroke risk at different ages for males and females
| Group | Variable | AUC (95% CI) | P value | P for comparison | Optimal cutoff value | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|---|---|
| Male | ||||||||
| < 60 | eGDR | 0.669(0.610, 0.729) | < 0.001 | Reference | 8.638 | 0.635 | 0.639 | 0.274 |
| CVAI | 0.659(0.600, 0.719) | < 0.001 | 0.678 | 108.804 | 0.682 | 0.621 | 0.303 | |
| TyG | 0.566(0.503, 0.630) | 0.043 | 0.011 | 8.590 | 0.753 | 0.391 | 0.144 | |
| TyG-BMI | 0.619(0.556, 0.682) | < 0.001 | 0.081 | 211.450 | 0.682 | 0.557 | 0.240 | |
| METS-IR | 0.616(0.553, 0.678) | < 0.001 | 0.071 | 36.632 | 0.694 | 0.544 | 0.238 | |
| AIP | 0.583(0.522, 0.643) | 0.011 | 0.022 | 0.262 | 0.788 | 0.389 | 0.177 | |
| ≥ 60 | eGDR | 0.598(0.542, 0.654) | 0.001 | Reference | 8.674 | 0.658 | 0.525 | 0.183 |
| CVAI | 0.546(0.492, 0.601) | 0.107 | 0.018 | 112.803 | 0.465 | 0.644 | 0.108 | |
| TyG | 0.578(0.526, 0.630) | 0.007 | 0.530 | 8.628 | 0.640 | 0.521 | 0.162 | |
| TyG-BMI | 0.582(0.530, 0.635) | 0.004 | 0.505 | 211.680 | 0.465 | 0.686 | 0.151 | |
| METS-IR | 0.567(0.513, 0.620) | 0.020 | 0.207 | 31.768 | 0.702 | 0.429 | 0.131 | |
| AIP | 0.566(0.515, 0.617) | 0.021 | 0.303 | 0.143 | 0.807 | 0.330 | 0.137 | |
| Female | ||||||||
| < 60 | eGDR | 0.622(0.566, 0.677) | < 0.001 | Reference | 8.414 | 0.549 | 0.651 | 0.200 |
| CVAI | 0.573(0.518, 0.627) | 0.014 | 0.057 | 73.675 | 0.853 | 0.286 | 0.139 | |
| TyG | 0.549(0.489, 0.610) | 0.096 | 0.030 | 9.442 | 0.304 | 0.808 | 0.112 | |
| TyG-BMI | 0.559(0.501, 0.616) | 0.048 | 0.024 | 236.457 | 0.451 | 0.675 | 0.126 | |
| METS-IR | 0.568(0.512, 0.625) | 0.022 | 0.057 | 34.205 | 0.784 | 0.336 | 0.120 | |
| AIP | 0.539(0.481, 0.597) | 0.190 | 0.016 | 0.092 | 0.912 | 0.164 | 0.076 | |
| ≥ 60 | eGDR | 0.554(0.501, 0.607) | 0.047 | Reference | 7.640 | 0.555 | 0.553 | 0.108 |
| CVAI | 0.557(0.503, 0.611) | 0.036 | 0.891 | 129.977 | 0.461 | 0.653 | 0.114 | |
| TyG | 0.568(0.518, 0.619) | 0.012 | 0.648 | 8.786 | 0.680 | 0.462 | 0.142 | |
| TyG-BMI | 0.572(0.518, 0.626) | 0.008 | 0.442 | 229.412 | 0.453 | 0.690 | 0.143 | |
| METS-IR | 0.562(0.509, 0.616) | 0.021 | 0.721 | 35.219 | 0.641 | 0.479 | 0.120 | |
| AIP | 0.565(0.516, 0.614) | 0.016 | 0.721 | 0.206 | 0.859 | 0.298 | 0.157 | |
ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval; eGDR, estimated glucose disposal rate; CVAI, Chinese visceral adiposity index; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; METS-IR, metabolic score for insulin resistance; AIP, atherogenic index of plasma.
Discussion
Stroke is recognised as a major public health problem with serious consequences for individuals and society. IR is a critical risk factor for stroke. Developing a rapid and easy-to-use test for early identification and intervention of stroke is crucial. In this study, we evaluated the predictive abilities of six IR surrogate indexes for stroke risk in Chinese middle-aged and elderly populations with abnormal glucose metabolism. Our results revealed statistically significant relationships between the six IR surrogate indexes and stroke risk after adjusting for confounders. These indexes all provided predictive values for stroke risk in the Chinese middle-aged and elderly population with abnormal glucose metabolism. eGDR demonstrated significant potential in predicting stroke risk in populations with abnormal glucose metabolism.
IR is typically characterised by hyperglycaemia, dyslipidaemia, hypertension and obesity [19]. Surrogate indexes of IR (eGDR, CVAI, TyG, TyG-BMI, METS-IR and AIP) derived from different combinations of these characteristics may better reflect the degree of IR in different ways. The eGDR, calculated based on WC, hypertension, and HbA1c, was first developed by Williams et al. for patients with type 1 diabetes [20]. The index is validated by the hyperinsulinemic-euglycemic clamp technique and provides a good reflection of insulin sensitivity. eGDR has shown good potential for application in epidemiological studies of patients with type 1 diabetes [21–23]. In addition, the value of eGDR in type 2 diabetes is gaining attention. A review and meta-analysis showed that among patients with type 1 diabetes mellitus with a median follow-up period of 10 years, each 1-unit increase in the eGDR index was associated with a 17% reduction in the hazard ratio for cardiovascular disease [24]. A study conducted in populations with diabetes demonstrated that lower eGDR levels were associated with an increased risk of stroke and mortality [25]. Our study found a significant negative correlation between eGDR and the occurrence of stroke in individuals with abnormal glucose metabolism. CVAI, a reliable index of visceral fat distribution in the Chinese population, has been widely used to assess the risk of cardiovascular and metabolic diseases [11, 26, 27]. Studies have shown that CVAI outperforms traditional obesity indexes in predicting stroke risk and is considered a valuable index of abdominal obesity for identifying individuals at high risk of stroke [28, 29]. This enhanced predictive ability may be attributed to its comprehensive nature, integrating factors such as sex, age, BMI, WC, and lipid levels. In this study, we found that CVAI performed well in predicting stroke risk in the overall population with abnormal glucose metabolism, second only to eGDR. The TyG index, based on TG and FPG, has been widely used to predict diabetes and cardiovascular disease [30–32]. The association between obesity and IR has been well established [33, 34]. Some studies suggest that TyG-BMI is superior to TyG in predicting IR [35]. By integrating the TyG index and BMI, TyG-BMI likely provides a more comprehensive perspective for assessing IR. METS-IR and AIP have also been identified as strong independent predictors of adverse cardiovascular and cerebrovascular events, closely associated with stroke risk [36–39].
We explored the dose-response relationships between the IR surrogate indexes and stroke risk using RCS. Previous studies have shown a linear negative correlation between eGDR and stroke risk [40]. Our study, based on individuals with diabetes and prediabetes, also revealed a similar negative linear correlation. This was attributed to the fact that the eGDR calculation formula utilised negative WC, hypertension and HbA1c values. Most other IR surrogate indexes demonstrated linear positive correlations with stroke risk, which is consistent with previous research [41, 42]. For TyG, our study was similar to a study based on Chinese populations, which reported a nonlinear positive correlation between TyG and stroke risk [43]. A study assessing stroke incidence in the general population of rural China, found a linear positive correlation between AIP and stroke risk [44]. In contrast, our study observed a nonlinear positive correlation between AIP and stroke risk. This differences may be attributed to the heterogeneity of the study population, the complexity of the disease, the settings of the RCS curve parameters, and the models used for analysis. Considering the nonlinear dose-response relationships between these IR surrogates and stroke risk, it is recommended that clinical stroke risk assessment should be based on the characteristics of different populations and the specific thresholds of IR surrogates for more precise risk assessment.
In the subgroup analysis, we found significant associations between eGDR and stroke risk across different sex and age groups. Additionally, a significant interaction between eGDR and age on risk of stroke was observed. Specifically, the association between eGDR and stroke risk was more pronounced in the middle-aged population. This finding is consistent with a study that evaluated the relationship between eGDR and cardiovascular disease risk in individuals with diabetes and prediabetes in the United States, which found a more significant association between eGDR and cardiovascular disease in those under 60 years old [45]. Another study assessing the relationship between eGDR and atherosclerotic cardiovascular disease risk in the general population also observed a significant interaction between eGDR and age on stroke risk [10]. However, studies by Kong et al. and Le et al. did not find a significant impact of age on the association between eGDR and stroke risk [46, 47]. We also observed a significant interaction between CVAI and age on stroke risk, which aligns with previous research findings [48–50]. Furthermore, significant associations between other IR surrogate indexes and stroke risk were primarily observed in female and elderly participants. The heterogeneity of the study population and differences in data partitioning may partially explain the discrepancies in results. Therefore, future studies should consider these factors to further explore the role of IR surrogate indexes in different populations and to gain deeper insights into the potential impact of age on stroke risk.
In this study, we found that, among the overall population with abnormal glucose metabolism and within different sex groups, eGDR exhibited the highest AUC value compared to other IR surrogate indexes, demonstrating better performance in predicting stroke risk. After stratifying by sex and age, we observed that in middle-aged males and females, the predictive ability of eGDR for stroke risk was not significantly different from that of other IR surrogate indexes, such as CVAI and METS-IR. However, in elderly male and female groups, the predictive abilities of the six IR surrogate indexes for stroke risk were generally similar. Additionally, we found that eGDR appeared to predict stroke risk more effectively in middle-aged males than in other populations. Differences in fat distribution, hormone levels, and metabolic changes between males and females, as well as variations in metabolic function between middle-aged and elderly individuals, may influence the performances of IR surrogate indexes in predicting stroke risk. Therefore, when conducting stroke risk assessments, it is crucial not only to consider the overall effectiveness of these indexes but also to account for their performance differences across different sex and age groups, in order to facilitate personalised health management and risk prediction.
Our study utilised data from the nationally representative CHARLS dataset, and the prospective cohort design enhanced the causal inference of our findings. Additionally, this study evaluated the predictive values of six IR surrogate indexes for stroke risk in middle-aged and elderly individuals with abnormal glucose metabolism in China, providing new insights for early identification and prevention of stroke risk. Furthermore, we controlled potential confounders as much as possible during the analysis and conducted subgroup analyses to ensure the reliability and robustness of our results.
However, there are several limitations in this study. First, a major limitation is the lack of time-to-event analysis. Without considering time as a factor, we were unable to assess its impact on the relationships between IR surrogate indexes and stroke risk. Therefore, future studies should consider incorporating time-to-event analysis to more comprehensively evaluate the impact of IR surrogate indexes on stroke risk. Second, the stroke data relied on self-reporting from participants, which may lead to an underestimation of actual prevalence. Medical histories such as diabetes mellitus and hypertension also partially relied on self-reported data. Moreover, although we adjusted for and controlled the known major confounders, the influence of unknown factors (such as genetics, diet, physical activity, and environmental changes) on the results cannot be excluded. Finally, this study focused on middle-aged and elderly individuals with abnormal glucose metabolism in China, and whether the findings can be generalised to populations in other countries requires further investigation and validation.
Conclusion
In conclusion, our findings indicated that the six IR surrogate indexes were strongly associated with stroke occurrence in Chinese middle-aged and elderly populations with abnormal glucose metabolism. Among these indexes, eGDR showed considerable potential in assessing stroke risk in populations with abnormal glucose metabolism. However, its application should be tailored to account for the specific characteristics of different sex and age groups. Furthermore, our study has several limitations, such as the reliance on self-reported data. Therefore, future studies should further explore and validate these findings to provide a basis for early stroke prediction and personalised intervention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the CHARLS project members and every respondent for the time and effort that they have devoted to the CHARLS project.
Abbreviations
- eGDR
Estimated glucose disposal rate
- CVAI
Chinese visceral adiposity index
- TyG
Triglyceride-glucose
- TyG-BMI
TyG-body mass index
- METS-IR
Metabolic score for insulin resistance
- AIP
Atherogenic index of plasma
- IR
Insulin resistance
- CHARLS
China Health and Retirement Longitudinal Study
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- SD
Standard deviation
- OR
Odds ratio
- CI
Confidence interval
- WC
Waist circumference
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- FPG
Fasting plasma glucose
- HbA1c
Glycosylated hemoglobin A1c
- TC
Total cholesterol
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- Scr
Serum creatinine
- SUA
Serum uric acid
- BUN
Blood urea nitrogen
- CRP
C-reactive protein
- VIF
Variance inflation factor
Author contributions
LJ contributed to the study design, statistical analysis, and drafting of the manuscript. TZ, WS, YZ, and YT participated in the study design and data collection. FR, ZX, LL, and XF performed the statistical analysis. DL, AC, and QW contributed to the editing and review of the manuscript. All authors made critical revision of the manuscript for important intellectual content and approved the final manuscript.
Funding
This study was supported by the Youth Teacher Training Project of Education Department of Anhui Province (No. JWFX2024028) and Anhui Province Key Clinical Specialist Construction Programs.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by the Ethical Review Committee of Peking University (IRB00001052-11015), and all participants provided written informed consent at the time of participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aidong Chen, Email: aidongchen@njmu.edu.cn.
Qiwen Wu, Email: 20141283@wnmc.edu.cn.
References
- 1.GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2021: a systematic analysis for the global burden of Disease Study 2021. Lancet Neurol. 2024;23(10):973–1003. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2024;403(10440):2133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a Nationwide Population-based survey of 480 687 adults. Circulation. 2017;135(8):759–71. [DOI] [PubMed] [Google Scholar]
- 4.Rehni AK, Cho S, Dave KR. Ischemic brain injury in diabetes and endoplasmic reticulum stress. Neurochem Int. 2022;152:105219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y, Kim CK, Kim MK, Seo WK, Oh K. Insulin resistance is associated with poor functional outcome after acute ischemic stroke in non-diabetic patients. Sci Rep. 2021;11(1):1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344:e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DM, Atkinson M, Evans M. Stroke prevention and treatment in people with type 2 diabetes: is there a role for GLP-1 (Glucagon-Like Peptide-1. Analogues? Stroke. 2023;54(5):1441–51. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 10.Yi J, Qu C, Li X, Gao H. Insulin resistance assessed by estimated glucose disposal rate and risk of atherosclerotic cardiovascular diseases incidence: the multi-ethnic study of atherosclerosis. Cardiovasc Diabetol. 2024;23(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, et al. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui C, Qi Y, Song J, Shang X, Han T, Han N, et al. Comparison of triglyceride glucose index and modified triglyceride glucose indices in prediction of cardiovascular diseases in middle aged and older Chinese adults. Cardiovasc Diabetol. 2024;23(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44. [DOI] [PubMed] [Google Scholar]
- 14.Yin B, Wu Z, Xia Y, Xiao S, Chen L, Li Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2023;22(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–33. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. [DOI] [PubMed] [Google Scholar]
- 17.Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, et al. Association between Sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. 2022;44:101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X, et al. Association of depressive symptoms with Incident Cardiovascular diseases in Middle-aged and older Chinese adults. JAMA Netw Open. 2019;2(12):e1916591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uehara K, Santoleri D, Whitlock AEG, Titchenell PM. Insulin regulation of hepatic lipid homeostasis. Compr Physiol. 2023;13(3):4785–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–32. [DOI] [PubMed] [Google Scholar]
- 21.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes complications Study. Diabetes Care. 2003;26(5):1374–9. [DOI] [PubMed] [Google Scholar]
- 22.Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36(8):2280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmink MAG, de Vries M, Visseren FLJ, de Ranitz WL, de Valk HW, Westerink J. Insulin resistance and risk of vascular events, interventions and mortality in type 1 diabetes. Eur J Endocrinol. 2021;185(6):831–40. [DOI] [PubMed] [Google Scholar]
- 24.Sun R, Wang J, Li M, Li J, Pan Y, Liu B, et al. Association of insulin resistance with cardiovascular disease and all-cause mortality in type 1 diabetes: systematic review and Meta-analysis. Diabetes Care. 2024;47(12):2266–74. [DOI] [PubMed] [Google Scholar]
- 25.Zabala A, Darsalia V, Lind M, Svensson AM, Franzén S, Eliasson B, et al. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2021;20(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao D, Sun H, Chen L, Li X, Huo H, Zhou G, et al. Assessment of six surrogate insulin resistance indexes for predicting cardiometabolic multimorbidity incidence in Chinese middle-aged and older populations: insights from the China health and retirement longitudinal study. Diabetes Metab Res Rev. 2024;40(1):e3764. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Zhang J, Chen C, Qin P, Zhang M, Shi X, et al. Comparison of six surrogate insulin resistance indexes for predicting the risk of incident stroke: the rural Chinese cohort study. Diabetes Metab Res Rev. 2022;38(7):e3567. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Huo W, Chen W, Li X, Qin P, Zhang M, et al. Association of traditional and novel obesity indicators with stroke risk: findings from the rural Chinese cohort study. Nutr Metab Cardiovasc Dis. 2024;34(9):2065–74. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc Diabetol. 2017;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X, Zuo Y, Chen S, Meng X, Chen P, Wang Y, et al. Distinct triglyceride-glucose trajectories are associated with different risks of incident cardiovascular disease in normal-weight adults. Am Heart J. 2022;248:63–71. [DOI] [PubMed] [Google Scholar]
- 32.Xu F, Feng Y, Zhong X. Higher triglycerideglucose index is associated with increased risk of stroke among middle-aged and elderly Chinese: a national longitudinal study. Sci Rep. 2024;14(1):19054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–27. [DOI] [PubMed] [Google Scholar]
- 34.Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am. 2018;102(1):13–33. [DOI] [PubMed] [Google Scholar]
- 35.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS ONE. 2019;14(3):e0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W, Cai X, Hu J, Wen W, Mulalibieke H, Yao X, et al. The metabolic score for insulin resistance (METS-IR) predicts Cardiovascular Disease and its subtypes in patients with hypertension and obstructive sleep apnea. Clin Epidemiol. 2023;15:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Cui H, Zhang Y, Liu L, Zhang W, Xiong W, et al. The impact of the metabolic score for insulin resistance on cardiovascular disease: a 10-year follow-up cohort study. J Endocrinol Invest. 2023;46(3):523–33. [DOI] [PubMed] [Google Scholar]
- 38.Zheng H, Wu K, Wu W, Chen G, Chen Z, Cai Z, et al. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: a retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu L, Fang S, Lan Z, Xu S, Jiang J, Pan Y, et al. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: insights from CHARLS. Cardiovasc Diabetol. 2024;23(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Zhao L, Lu Y, Xiao Y, Zhou X. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: findings from a nationwide, population based, prospective cohort study. Cardiovasc Diabetol. 2024;23(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Zhao L, Lu Y, Meng X, Zhou X. Association between Chinese visceral adiposity index and risk of stroke incidence in middle-aged and elderly Chinese population: evidence from a large national cohort study. J Transl Med. 2023;21(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng R, Dong X, Wang T, Zhang H, Zhou Y, Wang D. Linear positive association of metabolic score for insulin resistance with stroke risk among American adults: a cross-sectional analysis of National Health and Nutrition Examination Survey datasets. J Stroke Cerebrovasc Dis. 2024;33(11):107994. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, Li C, Yu Y, Zeng L, Qiu Y, Liu J, et al. Association between the triglyceride-glucose (TyG) index and stroke risk in Chinese normal-weight adults: a population-based study. Diabetol Metab Syndr. 2024;16(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Du Z, Ye N, Liu S, Geng D, Wang P, et al. Using the atherogenic index of plasma to estimate the prevalence of ischemic stroke within a general population in a rural area of China. Biomed Res Int. 2020;2020:7197054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao J, Wang L, Duan L, Gong F, Zhu H, Pan H, et al. Association between estimated glucose disposal rate and cardiovascular diseases in patients with diabetes or prediabetes: a cross-sectional study. Cardiovasc Diabetol. 2025;24(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong X, Wang W. Estimated glucose disposal rate and risk of cardiovascular disease and mortality in U.S. adults with prediabetes: a nationwide cross-sectional and prospective cohort study. Acta Diabetol. 2024;61(11):1413–21. [DOI] [PubMed] [Google Scholar]
- 47.Le C, Qin Y, Wang Z, Wang D, Zhong F, Yang S, et al. Association of estimated glucose disposal rate with incident cardiovascular disease under different metabolic and circadian rhythm states: findings from a national population-based prospective cohort study. Diabetol Metab Syndr. 2024;16(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y, Xu W, Guo L, Li W, Zhang L, Gao L, et al. Association of the time course of Chinese visceral adiposity index accumulation with cardiovascular events in patients with hypertension. Lipids Health Dis. 2023;22(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv Z, Ji Y, Xu S, Li C, Cai W. Chinese visceral adiposity index and its transition patterns: impact on cardiovascular and cerebrovascular diseases in a national cohort study. Lipids Health Dis. 2024;23(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Zhan Q, Dong F, Gao X, Zeng F, Yao J, et al. Associations of Chinese visceral adiposity index and new-onset stroke in middle-aged and older Chinese adults: an observational study. Lipids Health Dis. 2023;22(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.