Abstract
Aim
HIV remains one of the major epidemics and public health concerns within low and middle-income countries such as Tanzania. This study aimed to assess the prevalence and the factors associated with HIV testing-seeking behaviors among women of childbearing age in Tanzania.
Methods
This study used the 2022 Tanzania Demographic and Health Survey dataset. The study utilized individual recodes (IR) files where data was collected using the Women’s Questionnaire to analyze factors influencing HIV testing behavior among women, Descriptive analysis, and bivariate and multivariate logistic regressions were performed and all the data were processed and analyzed using STATA version 17 at 95% CI and significance level P < 0.05.
Results
This study included 2531 women with 90.0% having ever tested for HIV while 7.0% had never tested for HIV. Not employed [AOR:0.35, CI (0.20–0.61)] has lower odds of HIV testing than All-year employed status. Rural residents have reduced odds of HIV testing [AOR:0.43, CI (0.21–0.88)] compared to women living in urban areas. Those able to ask their partner to use a condom are more likely to have been tested with increased odds [AOR: 3.52, CI (2.31–5.37)]. Participants with a history of genital discharge [AOR:4.30, CI (1.28–14.46)] and those who don’t know their genital discharge history have [AOR: 0.20, CI (0.07–0.55)] are significant for HIV testing. Women who have heard about PrEP but are not uncertain about its approval [AOR: 36.07, CI (3.33–390.25)], respondents who have tested before with HIV testing kits [AOR:35.99, CI (4.00–324.13)] and women who are aware of HIV testing kids but never tested with them before [AOR: 2.80, CI (1.19–6.58)] are predictors of HIV testing seeking behaviors.
Conclusion
The government and other concerned agencies should introduce mobile or community-based testing units and subsidize testing costs to reach economically disadvantaged or rural populations. Promote Open Communication on Sexual Health: Public health campaigns should encourage open discussions about sexual health within relationships, emphasizing condom negotiation and mutual health checks as preventive measures. Raise Awareness and Accessibility of HIV Prevention Tools: Expand education on PrEP and HIV self-test kits to improve familiarity and acceptance, which may empower individuals to proactively seek testing. Integrate Sexual Health Screening into Routine Healthcare: Health facilities should incorporate HIV testing when individuals present with symptoms like genital discharge to improve early detection and intervention.
Keywords: HIV, HIV self-testing, Health-seeking behaviors, HIV prevalence, Youth, Reproductive health, Tanzania, 2022 TDHS
Introduction
Sub-Saharan Africa remains the epicenter of the global HIV epidemic, with approximately 67% of all people living with HIV residing in the region [1]. Tanzania, in particular, has made significant strides in combating the HIV crisis, achieving the UNAIDS 95–95-95 targets by 2030, with 82.7% of HIV-positive adults aware of their status, 97.9% on ART (antiretroviral therapy), and 94.3% having achieved viral load suppression [2]. Despite this progress, the country continues to grapple with the burden of HIV, particularly among women, who have a higher prevalence compared to men [1]. A 2022–2023 survey revealed that while HIV incidence among adults aged 15 and older stood at 0.18%, women had a higher rate of infection at 5.6%. One critical aspect of HIV prevention and care is early detection through HIV testing, which serves as the first step towards prevention, treatment, and reducing mother-to-child transmission [3]. However, HIV testing uptake among women in Tanzania is influenced by a variety of individual, social, and structural barriers [4]. These include factors such as fear of stigma, cultural norms, lack of awareness, limited access to healthcare, and economic constraints. Understanding these factors is essential for improving testing rates and, by extension, HIV prevention and treatment outcomes.
Previous studies have identified several factors influencing HIV testing behavior, particularly among women in sub-Saharan Africa. Socioeconomic factors, such as education and employment, have been shown to play a significant role in testing uptake. For instance, women with higher educational levels or stable employment are more likely to get tested due to better access to information and resources. In Tanzania, a study showed that 85.5% of women had ever tested for HIV, yet younger women had lower testing odds compared to older women [5, 29]. Furthermore, the importance of community-based factors, including marital status, cultural norms, and partner support, cannot be overlooked. Women who have partners who are supportive of HIV testing or who actively participate in the decision-making process are more likely to seek testing [6]. However, cultural barriers such as stigma and misconceptions surrounding HIV continue to hinder testing behaviors, particularly in rural areas [7, 30].
Tanzania has implemented multiple HIV testing strategies, including voluntary counseling and testing (VCT), provider-initiated testing and counseling (PITC), and self-testing, all aimed at improving testing uptake, particularly in rural and hard-to-reach areas [8, 31]. Despite these efforts, certain groups, such as women of reproductive age, remain underserved. These women often face compounded barriers due to gender inequality, cultural expectations, and limited healthcare infrastructure in rural settings [9, 35]. Additionally, while HIV testing services have expanded in antenatal care settings, many women still do not have access to these services outside of pregnancy, suggesting a need for broader, more inclusive testing programs.
Despite a wealth of research on HIV testing behaviors in sub-Saharan Africa, there remains a significant gap in understanding the complex interplay of individual, social, and structural factors influencing testing uptake among women in Tanzania, particularly in rural and underserved populations [10, 32]. Existing studies have largely focused on isolated factors such as education, stigma, or partner influence, often without exploring their combined effect in a comprehensive multivariate analysis [11, 12, 34]. Moreover, while some studies have examined the role of healthcare infrastructure and service accessibility, few have integrated these factors with more nuanced variables, such as sexual autonomy, partner support, and knowledge of self-testing kits. This study seeks to fill these gaps by providing a detailed multivariate analysis of the factors associated with HIV testing uptake, using data from the 2022 Demographic and Health Survey [17, 33]. By doing so, it offers a more holistic view of the barriers and facilitators of HIV testing among women of reproductive age in Tanzania, with a focus on improving testing rates in both rural and urban settings.
The primary aim of this study is to identify and analyze the factors influencing HIV testing behavior among women of reproductive age in Tanzania. By exploring the relationships between socio-demographic, behavioral, and knowledge-based variables, this study aims to provide actionable insights that can guide future interventions and policies aimed at increasing HIV testing uptake in the country.
Methods and materials
Study setting
The study is set in Tanzania, East Africa, which includes the mainland and the Zanzibar Archipelago. Tanzania is bordered by Kenya and Uganda to the north, Rwanda and Burundi to the northwest, and Zambia, Malawi, and Mozambique to the south. The geographic scope of the survey encompasses nine zones within Tanzania Mainland and five zones in Zanzibar, allowing for comprehensive estimates of child mortality and other health indicators [25].
Study design and study population
This was a cross-sectional study that used data from the 2022 Tanzania Demographic and Health Survey (2022 TDHS) [17]. The survey was implemented by the National Bureau of Statistics (NBS) and the Office of the Chief Government Statistician Zanzibar (OCGS), with collaboration from the Ministries of Health (MoH) in Tanzania Mainland and Zanzibar. The Tanzania Food and Nutrition Centre (TFNC) contributed to aspects related to biomarkers. Data collection occurred from February to July 2022 across all 31 regions of Tanzania, including both urban and rural areas. The survey employed a stratified two-stage sampling design to ensure national representativeness, including both urban and rural areas in Tanzania [17]. The design allows for the estimation of various health and demographic indicators, including HIV testing-seeking behavior. The study population comprises reproductive-age women aged 15–49 years residing in Tanzania. This population was selected from the 2022 TDHSMIS, which included all women in this age group who were either usual residents or visitors in the selected households the night before the survey interview.
Questionnaires used and data collection
The 2022 TDHS-MIS employed several types of questionnaires to gather comprehensive data. The Household Questionnaire collected basic household characteristics and demographic information. The Women’s Questionnaire, administered to women aged 15–49, included detailed questions on reproductive health, fertility preferences, family planning, maternal health, child mortality, domestic violence, women’s empowerment, and awareness of HIV and other STIs.
Description of variables
Dependent variable
The dependent variable for this analysis was whether the respondent had Ever been tested for HIV. This variable indicates whether the respondent has undergone HIV testing before or not which was coded as a binary outcome (1 = Yes, 0 = No).
Independent variables
The independent variables included age, Highest educational level, Husband/partner's education level, Employment all year/seasonal, Type of earnings, Marital Status, Type of place of residence, Respondent can ask their partner to use a condom, had any STI in their last 12 months, Had genital sore/ulcer in last 12 months, Had genital discharge in last 12 months, Knowledge and attitude to PrEP to prevent getting HIV, Knowledge and use of HIV test kits.
Data analysis and management
Data analysis was performed using Stata version 16. The data were weighted to account for non-responsiveness and potential biases. The weighting variable was identified and divided by 1,000,000. Following this adjustment, population estimates for frequency with decimal places were rounded to the nearest whole number to reflect that individuals cannot be represented in fractions. Descriptive analysis was conducted to obtain frequency tables, presenting the distribution of individuals who had or had not tested for HIV across various independent variables in percentages.
Bivariate analysis was then conducted using the logistic regression test to explore the association between the independent variables and the dependent variable (Ever been tested for HIV). The analysis produced adjusted odds ratios (AORs), confidence intervals (CIs), and corresponding p-values. A significance level of p < 0.05 and a confidence interval that did not include 1, were used to determine statistical significance. Variables with p-values greater than 0.05 and a confidence interval that included 1, were considered non-significant and excluded from further analysis.
Significant variables from the bivariate analysis were included in the multivariate analysis, which was conducted using logistic regression. The analysis produced adjusted odds ratios (AORs), confidence intervals (CIs), and corresponding p-values, with statistical significance set at p < 0.05.
Results
Descriptive statistics
The data revealed several critical insights regarding socio-demographic factors, sexual health, and HIV awareness among 2,531 individuals. A significant portion had only primary education (59.7%), with 19.5% having no formal education. Most respondents (73.2%) were married, and a large proportion (71.8%) lived in rural areas, where access to healthcare was limited. Employment was primarily seasonal (49.2%), with 40% not being paid. Regarding sexual health, 94.59% reported no STIs in the past year, but 5.1% had experienced one, suggesting the need for better sexual health education. Awareness of HIV prevention tools like PrEP and test kits was low (90.4% and 84.2%, respectively). Despite some empowerment in condom use (59.6%), 38% still could not negotiate condom use, indicating a gap in sexual decision-making.
The data in Table 1 highlighted the need for interventions to improve education, healthcare access, sexual empowerment, and HIV prevention awareness, especially in rural and less educated populations.
Table 1.
Descriptive analysis of characteristics of women of reproductive age (15–49) years in Tanzania
| Characteristics of women | Frequency 2531 | Percentage 100% |
|---|---|---|
| Highest educational level | ||
| No education | 496 | 19.5 |
| Primary | 1519 | 59.7 |
| Secondary | 504 | 19.8 |
| Higher | 24 | 0.9 |
| Husband/partner's education level | ||
| No education, preschool/early childhood | 302 | 11.9 |
| Primary | 1643 | 64.6 |
| Secondary | 522 | 20.5 |
| Higher | 75 | 3.0 |
| Employment all year/seasonal | ||
| All year | 765 | 45.6 |
| Seasonal | 826 | 49.2 |
| Occasional | 88 | 5.3 |
| Type of earnings | ||
| Not paid | 672 | 40.0 |
| Cash only | 714 | 42.5 |
| Cash and in-kind | 279 | 16.6 |
| In-kind only | 15 | 0.9 |
| Marital status | ||
| Married | 1861 | 73.2 |
| Living with partner | 682 | 26.8 |
| Type of place of residence | ||
| Urban | 718 | 28.2 |
| Rural | 1824 | 71.8 |
| Respondent can ask partner to use a condom | ||
| No | 966 | 38 |
| Yes | 1514 | 59.6 |
| Don't know/not sure/depends | 61 | 2.4 |
| Had any STI in last 12 months | ||
| No | 2405 | 94.59 |
| Yes | 130 | 5.1 |
| Don't know | 8 | 0.31 |
| Had genital sore/ulcer in last 12 months | ||
| No | 2436 | 95.8 |
| Yes | 100 | 4.0 |
| Don't know | 6 | 0.2 |
| Had genital discharge in last 12 months | ||
| No | 2321 | 91 |
| Yes | 210 | 8.3 |
| Don't know | 12 | 0.5 |
| Knowledge and attitude to PrEP to prevent getting HIV | ||
| Haven't heard | 2298 | 90.4 |
| Heard and approved to take it every day | 133 | 5.2 |
| Heard, but don't approve of taking it eve | 60 | 2.3 |
| Heard, but not sure about approving its | 52 | 2.0 |
| Knowledge and use of HIV test kits | ||
| Never heard of HIV test kits | 2139 | 84.2 |
| Has tested with HIV test kits | 62 | 2.4 |
| Knows test kits but never tested with them | 341 | 13.4 |
| Age (Mean ± SD) years | 29.85 ± 7.54 | |
Figure 1 illustrates the proportions of women who have ever tested for HIV compared to those who have never tested. Of the participants, 93.0% have undergone HIV testing, while 7.0% have never tested. This breakdown highlights the significant proportion of women who have accessed HIV testing services, suggesting a higher awareness or engagement with health practices, while also pointing to a notable percentage who may lack access or awareness about testing opportunities.
Fig. 1.
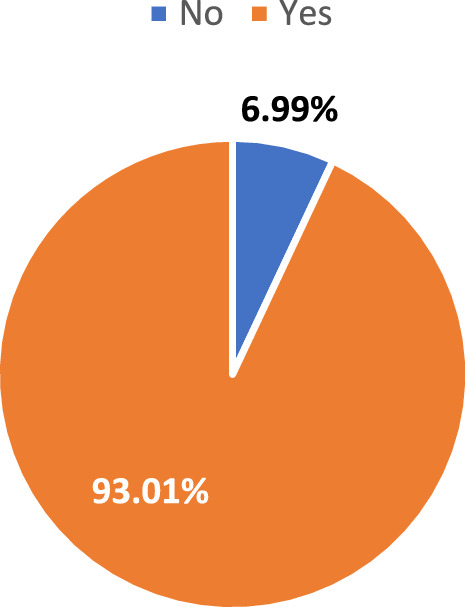
Proportions of women who have ever tested versus never tested for HIV
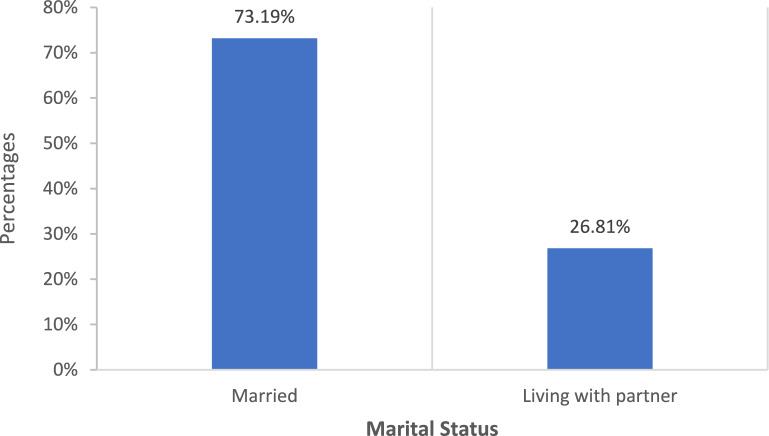
Figure 2 illustrates the distribution of marital status among the study population. Of the participants, 73.2% were married while 26.8% were living with a partner. This highlights the predominant marital status within the sample, with the majority being married compared to those living with a partner, offering a comprehensive view of the relationship status within the study population.
Fig. 2.
Distribution of marital status among the study population
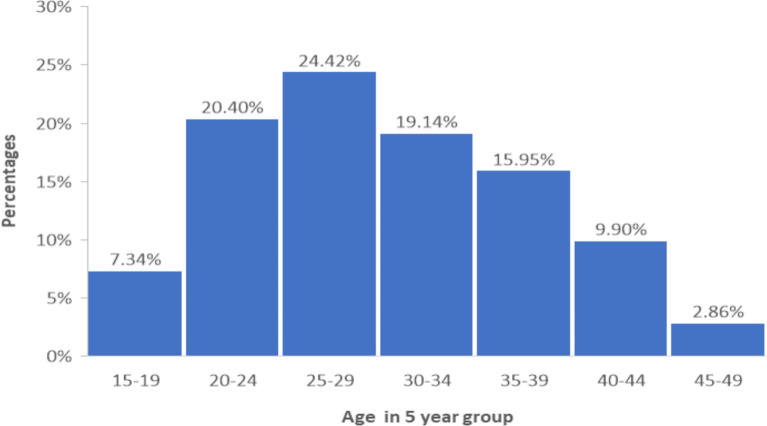
Figure 3 presents a histogram depicting the age distribution of the study participants. The chart reveals that the majority of participants fall within the 25–29 age range, followed by a smaller proportion in the 35–34 range. Fewer participants are present in the younger (15–19) and older age groups (45–49). This distribution provides a clear overview of the age demographics, indicating that the study predominantly involves individuals in early to mid-adulthood.
Fig. 3.
Histogram of age distribution of the study participants
Table 2 shows the results of binary logistic regression analysis of factors associated with HIV testing among women in Tanzania revealing several significant predictors. Higher educational levels were strongly associated with increased odds of HIV testing, with individuals who had completed primary (cOR = 2.19, CI 1.47–3.25) and secondary education (cOR = 3.14, CI 1.71–5.76) having notably higher odds compared to those with no education. Partner education also influenced testing, with higher odds seen in women whose partners had secondary or higher education (cOR = 3.62, CI 1.62–8.10). Women who could request condom use had significantly higher odds of testing (cOR = 4.82, CI 3.19–7.30). Rural residence (cOR = 0.32, CI 0.17–0.60) and not being employed (cOR = 0.29, CI 0.17–0.52) were associated with lower testing odds. Knowledge of HIV test kits and PrEP also showed strong associations, particularly for those who had used HIV test kits (cOR = 52.77, CI 6.80–409.46). Age, Type of earnings, had any STI in the last 12 months were found not significant in the bivariate logistic model.
Table 2.
Binary logistic regression of factors associated with HIV testing among women in Tanzania
| Characteristics of women | cOR | P-value | [95% Conf.Interval] | |
|---|---|---|---|---|
| Highest educational level | ||||
| No education | 1 | |||
| Primary | 2.19 | 0.000 | 1.47 | 3.25 |
| Secondary | 3.14 | 0.000 | 1.71 | 5.76 |
| Husband/partner’s education level | ||||
| No education, preschool/early childhood | 1 | |||
| Primary | 1.95 | 0.002 | 1.28 | 2.98 |
| Secondary | 3.62 | 0.002 | 1.62 | 8.10 |
| Higher | 4.26 | 0.061 | 0.94 | 19.40 |
| Employment all year/seasonal | ||||
| All year | 1 | |||
| Seasonal | 0.73 | 0.274 | 0.42 | 1.28 |
| Occasional | 0.38 | 0.115 | 0.11 | 1.27 |
| Not employed | 0.29 | 0.000 | 0.17 | 0.52 |
| Marital Status | ||||
| Married | 1 | |||
| Living with partner | 1.70 | 0.026 | 1.06 | 2.70 |
| Type of place of residence | ||||
| Urban | 1 | |||
| Rural | 0.32 | 0.000 | 0.17 | 0.60 |
| Respondent can ask partner to use a condom | ||||
| No | 1 | |||
| Yes | 4.82 | 0.000 | 3.19 | 7.30 |
| Don't know/not sure/depends | 0.63 | 0.176 | 0.33 | 1.23 |
| Had genital sore/ulcer in last 12 months | ||||
| No | 1 | |||
| Yes | 1.77 | 0.353 | 0.53 | 5.87 |
| Don't know | 0.04 | 0.000 | 0.01 | 0.21 |
| Had genital discharge in last 12 months | ||||
| No | 1 | |||
| Yes | 3.64 | 0.014 | 1.29 | 10.22 |
| Don't know | 0.12 | 0.003 | 0.03 | 0.49 |
| Knowledge and attitude to PrEP to prevent getting HIV | ||||
| Haven't heard | 1 | |||
| Heard and approved to take it every day | 1.97 | 0.192 | 0.71 | 5.47 |
| Heard, but don't approve of taking it every day | 2.30 | 0.273 | 0.52 | 10.19 |
| Heard, but not sure about approving its use | 40.65 | 0.000 | 5.49 | 301.21 |
| Knowledge and use of HIV test kits | ||||
| Never heard of HIV test kits | 1 | |||
| Has tested with HIV test kits | 52.77 | 0.000 | 6.80 | 409.46 |
| Knows test kits but never tested with them | 4.84 | 0.000 | 2.05 | 11.42 |
Figure 4 presents HIV testing status stratified by marital status. Among the married participants, 5.7% have not been tested, while 67.5% have, making up 73.2% of the total. For those living with a partner, 1.3% have not tested, and 25.5% have, contributing 26.8%. This indicates a higher proportion of HIV testing among married individuals compared to those living with a partner.
Fig. 4.
HIV testing status (tested/not tested) stratified by marital status
Figure 5 compares the age distribution between women who have tested and those who have not tested for HIV. The data suggests that women who have tested for HIV are more likely to be in the 25–34 age range, with a notable peak in this group. In contrast, women who have not tested tend to be younger or older, with fewer participants in the middle age brackets. This highlight age-related differences in HIV testing behavior among women.
Fig. 5.
Age comparison between women who have tested and those who have not tested for HIV
The multivariate logistic regression analysis Table 3 identified several key factors influencing HIV testing among women in Tanzania. Employment status played a critical role, with women who were not employed having significantly lower odds of HIV testing (aOR = 0.35, CI 0.20–0.61), compared to those employed year-round. Rural residence was also associated with lower odds of testing (aOR = 0.43, CI 0.21–0.87), indicating geographical barriers to healthcare access. Women who could request condom use had much higher odds of testing (aOR = 3.52, CI 2.31–5.37), highlighting the importance of sexual autonomy in health decision-making. Additionally, women who reported genital discharge in the last 12 months were significantly more likely to have been tested (aOR = 4.30, CI 1.28–14.46), suggesting a link between symptom awareness and testing behavior. Knowledge of HIV test kits was strongly associated with higher odds of testing, with those who had tested using HIV kits having an odds ratio of (aOR = 35.99, CI 4.00–324.13). Highest educational level, Husband/partner's education level, Marital Status, had genital sore/ulcer in the last 12 months were found not significant.
Table 3.
Multivariate Logistic regression of factors associated with HIV testing among women in Tanzania
| Characteristics of women | aOR | P-value | [95% Conf.Interval] | |
|---|---|---|---|---|
| Employment all year/seasonal | ||||
| All year | 1 | |||
| Seasonal | 0.85 | 0.570 | 0.49 | 1.48 |
| Occasional | 0.33 | 0.065 | 0.10 | 1.08 |
| Not employed | 0.35 | 0.000 | 0.20 | 0.61 |
| Type of place of residence | ||||
| Urban | 1 | |||
| Rural | 0.43 | 0.020 | 0.21 | 0.88 |
| Respondent can ask a partner to use a condom | ||||
| No | 1 | |||
| Yes | 3.52 | 0.000 | 2.31 | 5.37 |
| Don't know/not sure/depends | 0.58 | 0.097 | 0.30 | 1.11 |
| Had genital discharge in last 12 months | ||||
| No | 1 | |||
| Yes | 4.30 | 0.019 | 1.28 | 14.46 |
| Don't know | 0.20 | 0.002 | 0.07 | 0.55 |
| Knowledge and attitude to PrEP to prevent getting HIV | ||||
| Haven't heard | 1 | |||
| Heard and approved to take it every day | 1.11 | 0.854 | 0.37 | 3.31 |
| Heard, but don’t approve of taking it every day | 1.17 | 0.852 | 0.22 | 6.15 |
| Heard, but not sure about approving its use | 36.07 | 0.003 | 3.33 | 390.25 |
| Knowledge and use of HIV test kits | ||||
| Never heard of HIV test kits | 1 | |||
| Has tested with HIV test kits | 35.99 | 0.001 | 4.00 | 324.13 |
| Knows test kits but never tested with them | 2.80 | 0.019 | 1.19 | 6.58 |
Discussion
This study revealed several significant factors influencing HIV testing among women in Tanzania. Key determinants included employment status, place of residence, sexual autonomy, awareness of genital symptoms, and knowledge of HIV test kits. Women who were not employed had significantly lower odds of ever testing for HIV, which suggests that economic barriers and limited access to healthcare may hinder testing uptake. Additionally, women residing in rural areas were less likely to test for HIV, pointing to geographical disparities in healthcare access. Women who reported being able to request condom use had significantly higher odds of testing for HIV, highlighting the role of sexual autonomy in HIV-related health decisions.
Women who experienced genital discharge in the last 12 months had higher odds of testing for HIV, suggesting that symptom awareness might drive testing behavior. Furthermore, knowledge of HIV test kits was strongly associated with increased testing, with women who had tested using HIV test kits exhibiting higher odds. This underscores the importance of awareness and access to HIV testing tools in promoting HIV testing [27, 28].
The findings align with prior studies that have emphasized the role of education, sexual autonomy, and access to healthcare in HIV testing behaviors. For instance, studies have shown that women with higher educational attainment or those with more autonomy in sexual decision-making are more likely to engage in HIV testing [13, 18]. The finding that rural residence is associated with lower odds of testing corroborates previous research highlighting the geographical barriers to healthcare in sub-Saharan Africa, where rural areas often have fewer healthcare facilities and lower health service utilization [14, 21].
The association between genital symptoms and HIV testing behavior is consistent with studies suggesting that women who experience symptoms like genital discharge are more likely to seek medical care and get tested for HIV [15, 22–26]. However, the relationship between employment status and HIV testing remains understudied, and this study contributes to the literature by identifying economic inactivity as a significant barrier to HIV testing among women [16, 17, 20].
These findings have several implications for public health policy and HIV prevention programs. First, the low odds of testing among non-employed women suggest the need for targeted interventions that provide financial and logistical support for HIV testing, particularly for those in informal or seasonal employment. Programs that address economic vulnerabilities could improve testing uptake by reducing the barriers posed by lack of income [36].
The geographical divide in testing rates between rural and urban women highlights the need for interventions that enhance healthcare accessibility in rural areas, such as mobile testing units, telemedicine, or community-based testing initiatives. Increasing awareness and education about HIV test kits, as well as their availability, could also play a significant role in improving testing rates [19, 37]. Public health campaigns should focus on raising awareness about symptoms like genital discharge and encouraging women to seek HIV testing proactively.
The strong association between condom use negotiation and HIV testing underscores the importance of sexual autonomy in HIV prevention strategies. Empowering women to negotiate safe sexual practices could improve both HIV testing rates and overall sexual health.
Future research should focus on longitudinal studies to explore causal relationships between the identified factors and HIV testing behavior. Additionally, exploring the role of stigma and cultural beliefs in HIV testing, particularly in rural areas, could provide deeper insights into why some women may avoid testing despite experiencing symptoms. Further research on economic interventions that facilitate access to healthcare and HIV testing for economically vulnerable women would also be valuable [38].
Given the role of sexual autonomy in HIV testing behavior, future studies could investigate how empowerment programs that focus on sexual rights and negotiation skills affect HIV testing rates and sexual health outcomes.
This research contributes to the theoretical understanding of health decision-making, particularly in the context of HIV testing in sub-Saharan Africa. It emphasizes the intersection of socio-demographic factors, sexual autonomy, and health knowledge in shaping health behavior. Practically, the findings suggest targeted interventions aimed at improving education, sexual rights, and access to healthcare could significantly increase HIV testing rates among women.
While this study provides valuable insights, alternative interpretations of the results must be considered. For instance, the lack of significance in marital status and educational level might suggest that factors beyond formal education and marital ties, such as community support or access to peer networks, could also influence HIV testing. Further qualitative research exploring women’s lived experiences and motivations for HIV testing could offer alternative perspectives that complement the quantitative findings.
Strengths and limitations
A key strength of this study lies in its large sample size and the use of multivariate logistic regression, which allowed for the control of multiple confounding factors and provided a robust analysis of the factors influencing HIV testing. This approach provides valuable insights into the specific socio-demographic, behavioral, and knowledge-related barriers to HIV testing among women in Tanzania.
However, the study also has limitations. First, the reliance on self-reported data may introduce recall or social desirability biases, particularly regarding sensitive behaviors such as HIV testing and condom use. Second, while the sample is large, it may not be fully representative of all women in Tanzania, particularly those in very remote or marginalized communities. Lastly, the cross-sectional nature of the study prevents any causal inferences, as it only provides a snapshot of factors associated with HIV testing rather than a comprehensive understanding of how these factors evolve.
Conclusion
This study highlights several key factors influencing HIV testing among women in Tanzania, including employment status, place of residence, sexual autonomy, symptom awareness, and knowledge of HIV test kits. The findings suggest that improving access to HIV testing, particularly in rural areas and among economically vulnerable women, is essential for increasing testing uptake. Empowering women to make informed sexual health decisions and promoting awareness of HIV prevention tools, such as test kits, could further enhance testing rates. These results contribute to the growing body of literature on HIV testing behavior and offer valuable insights for future public health interventions.
Acknowledgements
The authors acknowledge and thank the National Bureau of Statistics (NBS), Ministry of Health (MOH)-Tanzania mainland, the Ministry of Health (MoH)-Zanzibar and the office of the Chief Government Statistician (OCGS) for implementing the 2022 Tanzania Demographic and Health Survey (2022 TDHS). The authors extend great thanks to the Institutional Review Board (IRB) of the Tanzania National Institute for Medical Research (NIMR) and the ICF International IRB who examined and approved the survey protocol before the commencement of data collection. The authors also the United States Agency for International Development for Funding the 7th TDHS of 2022 and the DHS program for granting us access to the data.
Abbreviations
- HIV
Human Immunodeficiency Virus
- AIDS
Acquired Immune Deficiency Syndrome
- TDHS
Tanzania Demographic and Health Survey
- MoH
Ministries of Health
- OCGS
Office of the Chief Government Statistician Zanzibar
- NBS
National Bureau of Statistics
- TDHS-MIS
Tanzania Demographic and Health Survey-Malaria Indicator survey
- STI
Sexually Transmitted Infections
- PrEP
Pre-exposure Prophylaxis
- UNAIDS
The Joint United Nations Programme on HIV/AIDS
- HIVST
HIV self-testing
- NIMR
Tanzania National Institute for Medical Research
Author contributions
JMA Conceptualized the research idea; JMA, II, and KFR analyzed the data, II, JMA, MJPI, and LNO wrote the methodology and presented and interpreted the results; II, HO, and AM wrote the introduction, EAI and ELA wrote the discussion; II JMA, BOA, AAB, AEI and WYK reviewed the manuscript, and II compiled the final draft of the manuscript. All the authors reviewed and approved the final draft of the manuscript.
Funding
No funding for this study.
Availability of data and materials
The Tanzania DHS 2022 secondary dataset used for this study can be accessed from the website upon request. https://dhsprogram.com/data/dataset_admin/index.cfm,specifically.
Declarations
Ethics approval and consent to participate
Ethical approval was not required for this study. Data from the Tanzania Demographic and Health Survey (DHS) for 2022 were used in this study and after permission, the dataset was obtained via the DHS program website for the secondary data analysis. The DHS program makes sure that all surveys follow strict ethical guidelines to safeguard participants' rights and welfare. The Institutional Review Board (IRB) of the Tanzania National Institute for Medical Research (NIMR) and the ICF International IRB examined and approved the survey protocol with approval IDs of NIMY/HQ/R.8a/Vol.IX/3834 and ICF IRB FWA00002349 Exp. 07/12/2023 respectively. Before every participant was included in the survey, their informed consent was sought.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amuche NJ, Emmanuel EI, Innocent NE. HIV/AIDS in sub-Saharan Africa: current status, challenges and prospects. 2017.
- 2.Lichtwarck HO. Pre-Exposure prophylaxis use among female sex workers in Dar es Salaam, Tanzania: a mixed-methods study. 2024.
- 3.Ghadrshenas A, Amor YB, Chang J, Dale H, Sherman G, Vojnov L, et al. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to-child transmission agenda. AIDS. 2013;27:S197–205. [DOI] [PubMed] [Google Scholar]
- 4.Layer EH, Kennedy CE, Beckham SW, Mbwambo JK, Likindikoki S, Davis WW, et al. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS ONE. 2014;9(8): e104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonner VA, Mbwambo JK, Kennedy CE, Sweat MD. The gendered experience of HIV testing: factors associated with prior testing differ among men and women in rural Tanzania. Int J STD AIDS. 2019;30(9):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musheke M, Ntalasha H, Gari S, Mckenzie O, Bond V, Martin-Hilber A, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in sub-Saharan Africa. BMC Public Health. 2013;13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen S, Nguyen HD, Kim SY, Aguilar J. HIV knowledge, risk behavior, stigma, and their impact on HIV testing among Asian American and Pacific Islanders: a review of literature. Soc Work Public Health. 2017;32(1):11–29. [DOI] [PubMed] [Google Scholar]
- 8.Lembuka MH. Perceptions and practices of youths towards voluntary counselling and testing (VCT) uptake services: the case of Kinondoni municipality: The Open University of Tanzania; 2017.
- 9.Ali M, Cordero JP, Khan F, Folz R. ‘Leaving no one behind’: a scoping review on the provision of sexual and reproductive health care to nomadic populations. BMC Womens Health. 2019;19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwansa E. Factors influencing the use of HIV self-testing kits among adolescents: a case of Kalingalinga compound. Lusaka: The University of Zambia; 2024. [Google Scholar]
- 11.Turan JM, Elafros MA, Logie CH, Banik S, Turan B, Crockett KB, et al. Challenges and opportunities in examining and addressing intersectional stigma and health. BMC Med. 2019;17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbona SV, Chifurira R, Ndlovu BD, Ananth A. Prevalence and determinants of pregnancy termination for childbearing women using the modified Poisson regression model: a cross-sectional study of the Tanzania Demographic and Health Survey (TDHS) 2022. BMC Public Health. 2025;25(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mekonnen MM, Hoekstra AY. Water footprint benchmarks for crop production: a first global assessment. Ecol Ind. 2014;46:214–23. [Google Scholar]
- 14.Rutherford ME, Mulholland K, Hill PC. How access to health care relates to under-five mortality in sub-Saharan Africa: systematic review. Trop Med Int Health. 2010;15(5):508–19. [DOI] [PubMed] [Google Scholar]
- 15.Restar AJ, Tocco JU, Mantell JE, Lafort Y, Gichangi P, Masvawure TB, et al. Perspectives on HIV pre-and post-exposure prophylaxes (PrEP and PEP) among female and male sex workers in Mombasa, Kenya: implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29(2):141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergamaschi A, d’Arripe-Longueville F, Gray LL, Colson SS, Goujard C, Ferez S, et al. Perceived HIV-related physical fatigue, sociodemographic characteristics and physical activity: a cross-sectional study. J Clin Nurs. 2019;28(11–12):2147–56. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health (MoH) [Tanzania Mainland], Ministry of Health (MoH) [Zanzibar], National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF. 2022. Tanzania Demographic and Health Survey and Malaria Indicator Survey 2022 Final Report. Dodoma, Tanzania, and Rockville,Maryland, USA: MoH, NBS, OCGS, and ICF.
- 18.Kifle D, Azale T, Gelaw YA, Melsew YA. Maternal health care service seeking behaviors and associated factors among women in rural Haramaya District, Eastern Ethiopia: a triangulated community-based cross-sectional study. Reprod Health. 2017;14(1):6. 10.1186/s12978-016-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musekiwa A, Silinda P, Bamogo A, Twabi HS, Mohammed M, Batidzirai JM, et al. Prevalence and factors associated with self-reported HIV testing among adolescent girls and young women in Rwanda: evidence from 2019/20 Rwanda Demographic and Health Survey. BMC Public Health. 2022;22(1):1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teklehaimanot HD, Teklehaimanot A, Yohannes M, Biratu D. Factors influencing the uptake of voluntary HIV counseling and testing in rural Ethiopia: a cross sectional study. BMC Public Health. 2016;16(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pebody R. Economic and social inequality. Large HIV testing gap between rich and poor living in African countries. 2020.
- 22.Faust L, Yaya S, Ekholuenetale M. Wealth inequality as a predictor of HIV-related knowledge in Nigeria. BMJ Glob Heal. 2017;2(4): e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabo KG, Seifu BL, Kase BF, Asebe HA, Asmare ZA, Asgedom YS, et al. Factors influencing HIV testing uptake in Sub-Saharan Africa: a comprehensive multi-level analysis using demographic and health survey data (2015–2022). BMC Infect Dis. 2024;24(1):821. 10.1186/s12879-024-09695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashemera DR, Nhembo MJ, Benedict G. The role of women’s empowerment in influencing HIV testing. DHS Working Papers No. 101. Calverton: ICF International; 2013. http://dhsprogram.com/pubs/pdf/WP101/WP101.pdf.
- 25.Iddrisu AK, Opoku-Ameyaw K, Bukari FK, Mahama B, Akooti JJA. HIV testing decision and determining factors in Ghana. World J AIDS. 2019;9(2):85–104. [Google Scholar]
- 26.Swann M. Economic strengthening for HIV prevention and risk reduction: a review of the evidence. AIDS Care. 2018;30(sup3):37–84. 10.1080/09540121.2018.1479029. [DOI] [PubMed] [Google Scholar]
- 27.Spasojevic N, Vasilj I, Hrabac B, Celik D. Rural–urban differences in health care quality assessment. Mater Soc Med. 2015;27(6):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dambach P, Mahenge B, Mashasi I, Muya A, Barnhart DA, Bärnighausen TW, et al. Socio-demographic characteristics and risk factors for HIV transmission in female bar workers in sub-Saharan Africa: a systematic literature review. BMC Public Health. 2020;20(1):697. 10.1186/s12889-020-08838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath N, Eaton JW, Newell ML, Hosegood V. Migration, sexual behaviour, and HIV risk: a general population cohort in rural South Africa. Lancet HIV. 2015;2(6):e252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandiwa C, Namondwe B. Uptake and correlates of HIV testing among men in Malawi: evidence from a national population–based household survey. BMC Health Serv Res. 2019;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saaka SA, Pienaah CKA, Stampp Z, Antabe R. Safe sex negotiation and HIV risk reduction among women: a cross-sectional analysis of Burkina Faso 2021 demographic and health survey. PLOS Glob Public Health. 2024;4(4): e0003134. 10.1371/journal.pgph.0003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitpitan EV, Kalichman SC, Cain D, Eaton LA, Carey KB, Carey MP, et al. Condom negotiation, HIV testing, and HIV risks among women from alcohol serving venues in Cape town, South Africa. PLoS ONE. 2012;7(10): e45631. 10.1371/journal.pone.0045631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piper KN, Fuller TJ, Ayers AA, Lambert DN, Sales JM, Wingood GM. A qualitative exploration of religion, gender norms, and sexual decision-making within African American faith-based communities. Sex Roles. 2020;82:189–205. [Google Scholar]
- 34.Feyisetan B, Oyediran KA. Can married or cohabiting women negotiate protective sex? Findings from demographic and health surveys of two West African countries. J Biosoc Sci. 2020;52(6):785–808. [DOI] [PubMed] [Google Scholar]
- 35.UNAIDS. HIV and AIDS: basic facts. Jt United Nations Program HIV/ AIDS. 2021;3–4. Available from: https://www.unaids.org/en/frequently-asked-questions-about-hiv-and-aids.
- 36.Foessleitner P, Petricevic L, Boerger I, Steiner I, Kiss H, Rieger A, et al. HIV infection as a risk factor for vaginal dysbiosis, bacterial vaginosis, and candidosis in pregnancy: a matched case-control study. Birth. 2021;48(1):139–46. 10.1111/birt.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. Bacterial vaginosis key facts. 2023.
- 38.WHO Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Tanzania DHS 2022 secondary dataset used for this study can be accessed from the website upon request. https://dhsprogram.com/data/dataset_admin/index.cfm,specifically.