Abstract
A newly recognized syndrome, characterized by sudden death of farmed deer that are in good to excellent nutritional condition, with lesions of small intestinal mucosal hemorrhage and splenomegaly, is described. Other frequently observed lesions were small intestinal mucosal necrosis, abomasal hemorrhage, random hepatic necrosis, and multifocal hepatic congestion. Clostridium perfringens type A was isolated in high numbers from the intestines of many of the deer affected by the syndrome; however, the numbers were not significantly different from those of control deer. The syndrome did not appear to be related to dietary or any other specific management factors and a definitive cause of the syndrome was not identified.
Résumé
Enquête sur un syndrome de mort subite, de splénomégalie et d’hémorragie de l’intestin grêle chez le wapiti d’élevage. Cette étude décrit un nouveau syndrome affectant le wapiti d’élevage et caractérisé par la mort subite d’individus en bon ou en excellent état nutritionnel, présentant des lésions hémorragiques de la muqueuse de l’intestin grêle et de la splénomégalie. Les autres lésions les plus fréquentes comprenaient une nécrose de la muqueuse de l’intestin grêle, des hémorragies de l’abomasum, de la nécrose hépatique erratique et de la congestion hépatique multifocale. Du Clostridium perfringens de type A a été isolé en grande quantité des intestins de plusieurs wapitis atteints par le syndrome; cependant, la quantité ne différait pas significativement de celle retrouvée chez les wapitis témoins. Le syndrome ne semble pas relié à l’alimentation ou à aucun autre facteur spécifique de gestion et la cause véritable du syndrome n’a pas été identifiée.
(Traduit par Docteur André Blouin)
Introduction
During 2001, a newly recognized syndrome in farmed deer of acute death associated with small intestinal hemorrhage and splenomegaly was diagnosed in 17 animals submitted for necropsy to Prairie Diagnostic Services (PDS) at the Western College of Veterinary Medicine (WCVM), University of Saskatchewan. These cases accounted for 12% (17/141) of the total deer submissions for the year 2001. Animals with clinical signs similar to those of this syndrome accounted for only 0.8% (7/853) of the total deer submissions from 1994 to 2000 and were not observed prior to 1994. Based on this information, it appeared that the frequency of the syndrome was increasing. Little is known about this syndrome in deer and no information is available in the veterinary literature. High numbers of Clostridium perfringens were cultured from many of these cases, with some of the isolates being identified by polymerase chain reaction (PCR) as type A; however, the significance of the intestinal presence of this organism was unclear.
Clostridium perfringens is considered to be an important cause of enteric disease in domestic animals and humans (1,2). It is divided into 5 types (types A to E), based on production of 4 major toxins. Type A strains produce alpha toxin; type B, alpha, beta, and epsilon toxins; type C, alpha and beta toxins; type D, alpha and epsilon toxins; and type E, alpha and iota toxins (3). Clostridium perfringens type A is a common postmortem invader; it is found frequently in the intestines of healthy animals and is ubiquitous in the environment (4). Nonpathogenic isolates of type A do not seem to play a major role in enteric disease (2), and some strains may be referred to as nontoxigenic, because they do not produce sufficient alpha toxin to kill mice under test conditions (1).
Despite their widespread presence in healthy animals and the environment, C. perfringens type A organisms have been known to cause disease in both domestic animals and humans. The role that C. perfringens type A plays in the pathogenesis of diseases, such as gas gangrene (2), avian necrotic enteritis (5,6), and enterotoxemia in lambs (7), is well known. However, there are several other diseases in which, although the presence of C. perfringens type A is associated with clinical disease or pathological gastrointestinal disease, or both, the pathogenic role of the organism remains to be elucidated. Examples include enterocolitis in horses (8), necrotizing enteritis in piglets (9), hemorrhagic canine gastroenteritis (10), and jejunal hemorrhage syndrome in cattle (11).
The purpose of this study was to establish a case definition for this newly recognized syndrome in deer, to determine the frequency of the syndrome in farmed deer in Saskatchewan, to determine whether any management risk factors could be identified, and to investigate the etiology of this syndrome. Information was acquired from the retrospective study of necropsy reports and histological examination of tissues, as well as from a survey distributed to deer producers. The possible role of C. perfringens in this syndrome was investigated by using quantitative bacterial culture and testing for clostridial toxins.
Materials and methods
Selection of cases
The case histories of all farmed deer submitted for necropsy to the diagnostic laboratory of the Department of Veterinary Pathology and PDS at the WCVM between 1986 and 2001 were reviewed. Necropsy records for deer submitted to the University of Minnesota Veterinary Diagnostic Laboratory, the North Dakota State University Veterinary Diagnostic Laboratory, and the South Dakota State University Animal Disease Research and Diagnostic Laboratory from 1994 to 2001 were reviewed, as were necropsy records from the Alberta provincial laboratory database from 1990 to 2000 and from the University of Guelph Animal Health Laboratory.
Cases were selected for further study based on the presence of at least 3 of the following criteria: history of sudden death (no clinical signs noted prior to death), small intestinal hemorrhage, splenomegaly, severe splenic congestion, and hepatic necrosis. Deer with at least 3 of the above criteria were considered to be affected by the syndrome (hereafter referred to as “affected deer”). A total of 32 cases met the selection criteria. Of these, 26 were from Saskatchewan, 1 was from Minnesota, and 5 were from South Dakota. No cases from Alberta, Ontario, or North Dakota met the selection criteria.
Necropsy reports for the remaining deer of the 232 deer submitted to the diagnostic laboratory at the WCVM between 1995 and 2001 were also reviewed. The cause of death in these deer was not consistent with the syndrome and thus these deer are referred to as “unaffected deer” in the remainder of this paper. Case history and signalment data for both the affected and unaffected deer were compared. The difference between the average ages of the affected and unaffected deer was assessed by using a 2-sample t-test on log10-transformed data (SYSTAT, version 9; Systat Software, Point Richmond, California, USA).
Histological evaluation
Histologic sections of all available tissues, stained with hematoxylin and eosin (H&E), were examined microscopically in 32 cases. Significant lesions were found in small intestine, liver, abomasum, and spleen. These tissues were reexamined histologically and the lesions categorized. Hemorrhage in the small intestine and abomasum was classified as mild (< 30% of the histological section affected), moderate (30% to 60% of the histological section affected), or severe (> 60% of the histological section affected). In cases where different sections of intestine or abomasum had dissimilar degrees of hemorrhage, the case was classified on the greatest degree of severity observed for that case. A Gram’s stain was applied to intestinal sections from 8 cases by using a standard technique (12).
Bacteriology
Results from bacteriologic studies were reported semi-quantitatively as 1+ to 4+, according to a previously reported procedure (13). In addition, quantitative clostridial culture was performed for 3 affected and 6 unaffected (control) animals. Briefly, 1 gram of homogenized mucosa of each duodenum, jejunum, ileum, and spiral colon was diluted in 9 mL of prereduced thioglycolate broth. This broth was then serially diluted in 10-fold dilutions with thioglycolate broth, and 100 μL of each dilution was plated in duplicate on blood agar, as well as blood agar with neomycin. Plates were incubated anaerobically for 48 h; then, colonies were counted and multiplied by the dilution factor to obtain a result, which was reported as colony forming units per gram (CFU/g) of intestinal tissue. Numbers of C. perfringens cultured from affected and unaffected deer were compared by using a 2-sample t-test on log10 transformed data (SYSTAT, version 9). Isolates of C. perfringens from intestinal samples were tested for production of type A (alpha) toxin by using the Nagler reaction (14).
Isolates of C. perfringens from unaffected and affected deer were tested for the presence of genes for the major toxins, including genes cpa (alpha toxin), cpb (beta toxin), etx (epsilon toxin), Ia (iota toxin), cpe (enterotoxin), and cpb2 (beta-2 toxin) by using a multiplex PCR assay, slightly modified from Meer and Songer (15). Modification involved the performance of 2 multiplex assays of 3 primer sets each (cpa, cpb, cpb2 and etx, Ia, cpe) rather than a multiplex assay with all 6 primer sets. Samples of intestinal contents (jejunal, duodenal, ileal, and colonic) were tested for the presence of alpha toxin by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Batch ACP01l11; Cypress Diagnostics, Langdorp, Belgium).
Survey
Questionnaires were submitted to 98 Saskatchewan deer producers to obtain information on losses occurring as a result of the above-described sudden death, splenomegaly, and intestinal hemorrhage syndrome, as well as information on dietary and other management characteristics, such as increased feeding of grain, change in diet, adverse weather, and medical treatments that might be risk factors for the development of the syndrome.
Results
Signalment
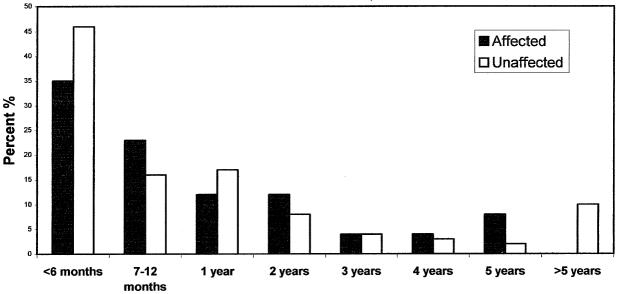
Of the 32 cases examined, 31 were white-tailed deer (Odocoileus virginianus) and 1 was a mule deer (Odocoileus hemionus). Affected deer were 50% (16/32) female, 41% (13/32) male, and 9% (3/32) unspecified sex. Vaccination with a 7-way or 8-way clostridial vaccine was reported for 34% (11/32) of affected deer. Age of affected deer ranged from 3 mo to 6 y, with a mean age of 20 mo. The mean age of unaffected deer was 18 mo, which was not significantly different (P = 0.66) from the mean age of affected deer (Figure 1). Among both affected and unaffected deer, the greatest proportion of deaths occurred during the 1st y of life (58% and 62%, respectively). The diets of affected and unaffected groups of deer were similar and included varying combinations of alfalfa hay, pelleted deer ration (16% to 18% protein), oats, and corn.
Figure 1.
Percentage of total deaths of affected and unaffected deer occurring within each age category
Pathologic examination
Sudden death with no previous clinical signs had occurred in all 32 cases and all deer were reported to be in adequate to excellent body condition. The most frequently observed lesions in affected deer were intestinal hemorrhage (32/32), followed by splenomegaly and congestion (30/32), hepatic congestion (17/32), hepatic necrosis (17/32), small intestinal mucosal necrosis (11/32), and abomasal hemorrhage (13/32). Observed lesions are summarized in Table 1.
Table 1.
Occurrence of lesions and microbiological findings in 32 deer submitted with a history of sudden death
| Case number | Splenomegaly | SI hemorrhagea | Hepatic congestionb | Hepatic necrosisb | Abomasal hemorragec | SI necrosisb | Clostridium perfringensd | Toxin typee |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | X | X | X | A | 1+ | NT |
| 2 | + | + | − | − | + | A | 4+ | NT |
| 3 | + | + | − | − | + | − | 3+ | NT |
| 4 | + | + | − | − | − | + | 4+ | NT |
| 5 | + | + | + | + | + | A | 4+ | α |
| 6 | + | + | + | − | X | A | 4+ | NT |
| 7 | − | + | + | + | X | A | 4+ | NT |
| 8 | + | + | + | + | − | − | 4+ | α, β-2, CPE |
| 9 | + | + | + | + | X | A | 4+ | α, β-2 |
| 10 | + | + | − | + | − | A | 4+ | α |
| 11 | + | + | + | + | + | + | 3+ | NT |
| 12 | + | + | + | + | − | A | 4+ | α |
| 13 | + | + | A | A | + | A | 1+ | NT |
| 14 | + | + | + | − | + | + | 3+ | α |
| 15 | + | + | + | + | − | A | 4+ | α |
| 16 | + | + | − | + | + | − | 4+ | α, ɛ |
| 17 | − | + | + | + | X | + | 4− | NT |
| 18 | + | + | + | + | − | A | 4+ | NT |
| 19 | + | + | A | A | − | − | 4+ | NT |
| 20 | + | + | + | + | + | + | 3+ | No toxinf |
| 21 | + | + | + | + | + | + | 4+ | No toxinf |
| 22 | + | + | − | + | X | + | 1+ | NT |
| 23 | + | + | + | + | + | + | 3+ | No toxinf |
| 24 | + | + | + | − | + | + | 4+ | α |
| 25 | + | + | + | + | + | X | 4+ | NT |
| 26 | + | + | X | X | X | + | 4+ | NT |
| 27 | + | + | − | − | − | + | 4+ | α |
| 28 | + | + | A | A | X | A | 4+ | α, CPE |
| 29 | + | + | − | − | − | A | 4+ | α, CPE |
| 30 | + | + | + | + | + | − | 4+ | α |
| 31 | + | + | X | X | X | − | 3+ | α |
| 32 | + | + | A | A | A | A | 4+ | α |
X — tissue not submitted; A — too autolyzed to interpret; NT — not tested; SI — small intestine; − — negative; + — positive; CPE — enterotoxin
Small intestinal hemorrhage determined by gross or histological examination
Determined by histological examination
Determined by gross or histological examination
Determined by anaerobic culture of intestine
Clostridium perfringens toxin type determined by polymerase chain reaction (PCR) except where specified
No toxin detected using mouse assay
Grossly enlarged spleens were described as engorged with blood. All spleens examined histologically were moderately to severely congested. Hemorrhage within the lamina propria of the small intestine ranged from multifocal to diffuse (Figure 2). Of 31 small intestines examined histologically, 2 (7%) had mild hemorrhage, 9 (29%) had moderate hemorrhage, 6 (19%) had severe hemorrhage, and 14 (45%) had intestines that were too autolyzed to interpret. Moderate to abundant numbers of large rod-shaped bacteria were observed in the lumen and throughout the mucosa of all small intestines examined. These bacteria were determined to be Gram positive in the 8 cases for which Gram staining was performed. In 37% (12/32) of cases, there was evidence of small intestinal mucosal necrosis. Only 1 case had evidence of small intestinal mucosal vascular thrombosis. On histological examination of the abomasum of the 15 deer for which fixed abomasal tissue was submitted, 5 (33%) had no evidence of hemorrhage, 2 (13%) had mild hemorrhage, 6 (40%) had moderate hemorrhage, and 2 (13%) had severe hemorrhage.
Figure 2.
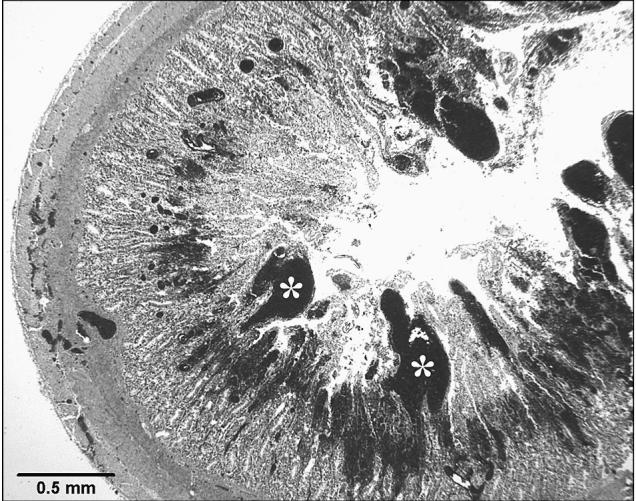
Severe small intestinal mucosal hemorrhage (*). Hematoxylin and eosin (H&E) stain.
Gross lesions in the liver were observed less frequently than were histological lesions. Gross lesions described in the liver included congestion in 25% (8/32) (Figure 3) and necrosis in 19% (6/32) of the cases. A prominent histologic feature seen in 68% (17/25) of those livers that were well preserved, was the presence of multifocal congestion, which frequently affected only specific acini within a hepatic lobule (Figure 4). Random, multifocal hepatic necrosis occurred in 68% (17/25) of livers. In 52% (13/25) of livers, there was multifocal dilation of sinusoids containing variably empty space, granular eosinophilic material, or red blood cells (Figure 4).
Figure 3.
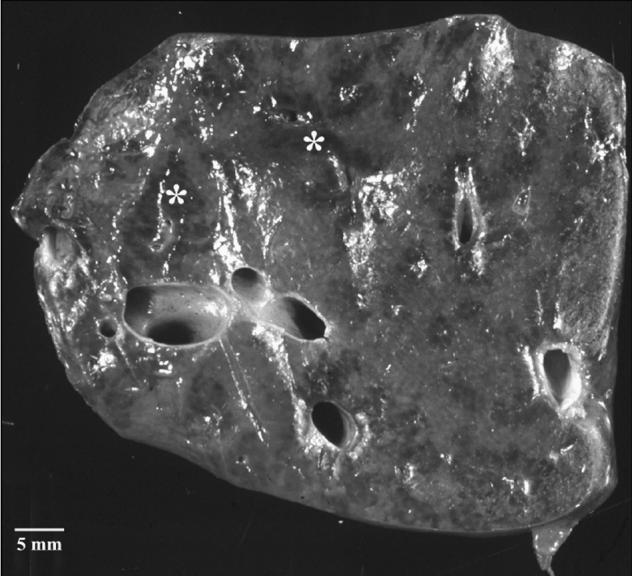
Multifocal dark areas (*) of hepatic congestion were observed grossly in 24% of cases.
Figure 4.
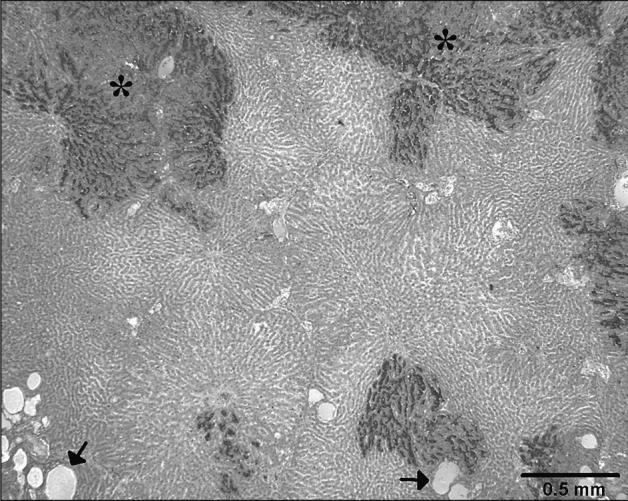
Multifocal clearly demarcated dark areas of hepatic congestion (*). Numerous sinusoids are dilated and filled with a pale eosinophilic material (arrows). Hematoxylin and eosin (H&E) stain.
Bacteriologic studies
Results of routine bacteriologic culture and PCR toxin typing are shown in Table 1. No significant bacteria, other than C. perfringens, were cultured under aerobic and anaerobic conditions from any of the affected deer. All C. perfringens isolates had the alpha toxin gene, as determined by PCR. In addition, 1 isolate from an unaffected deer tested positive for both alpha and beta-2 toxin genes. All C. perfringens isolates tested positive for the production of alpha toxin by using the Nagler reaction test.
Quantitative culture for C. perfringens was performed on small intestinal samples from 3 cases of the described syndrome (affected deer) and from 6 unaffected deer that died of causes not involving the gastrointestinal system (Table 2). There was no significant difference between the number of C. perfringens organisms cultured in the duodenum (P = 0.26), jejunum (P = 0.07), ileum (P = 0.46), or colon (P = 0.80) of affected deer compared with unaffected deer.
Table 2.
Number ofClostridium perfringens bacteria (mean, s) cultured from intestinal samples of affected and control deer reported in colony forming units (CFU) per milliliter of sample
| Duodenum
|
Jejunum
|
Ileum
|
Colon
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | s | n | Mean | s | n | Mean | s | n | Mean | s | |
| Affected | 2 | 2.2 × 106 | 3.1 × 106 | 3 | 5.6 × 106 | 6.0 × 106 | 2 | 2.6 × 105 | 3.5 × 105 | 2 | 6.1 × 104 | 8.4 × 104 |
| Unaffected | 6 | 6.6 × 103 | 1.2 × 104 | 6 | 8.7 × 103 | 4.1 × 104 | 6 | 3.1 × 107 | 2.9 × 107 | 6 | 6.1 × 104 | 3.1 × 106 |
n — Number of samples; s — Standard deviation
Positive ELISA results for the presence of alpha toxin in intestinal contents were observed in at least 1 sample for all 4 sections of intestine tested (duodenum, jejunum, ileum, colon) in both unaffected and affected deer (Table 3).
Table 3.
Presence of alpha toxin in intestinal contents, as detected by using an enzyme-linked immunosorbent assay (ELISA)
| Duodenum
|
Jejunum
|
Ileum
|
Ileum
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | + | − | n | + | − | n | + | − | n | + | − | |
| Affected | 2 | 2 | 0 | 3 | 3 | 0 | 2 | 1 | 1 | 2 | 0 | 2 |
| Unaffected | 5 | 3 | 2 | 6 | 4 | 2 | 5 | 4 | 1 | 5 | 3 | 2 |
n — Number of samples, + — Number of samples that tested positive for the presence of alpha toxin; − — Number of samples that tested negative for the presence of alpha toxin
Of 98 surveys distributed, 28 (28.6%) were returned. During the period January 2001 to September 2002, producers reported 161 deaths among a total of 2055 deer, representing a crude mortality rate of 7.8%. Out of 63 reported “sudden deaths,” 9 were attributed to the splenomegaly and intestinal hemorrhage syndrome, based on fulfillment of the case definition. Twenty-two of 63 reported sudden deaths were attributed to another known cause (trauma, pneumonia, etc.) and for 32 cases, the cause of death was unknown (no necropsy was performed). According to producers, sudden deaths were not associated with increased feeding of grain, change of diet, movement of animals, adverse weather, or medical treatments of animals.
Discussion
A syndrome of sudden death, splenomegaly, and intestinal hemorrhage in farmed deer is characterized by sudden death of deer that are in good to excellent nutritional condition, in association with lesions of small intestinal mucosal hemorrhage and splenomegaly. Other frequent findings were small intestinal mucosal necrosis, abomasal hemorrhage, random hepatic necrosis, and multifocal hepatic congestion.
The presence of large numbers of C. perfringens type A organisms in association with significant intestinal lesions provides some support for a possible etiologic role for C. perfringens. However, the roles of C. perfringens type A and alpha toxin expression are uncertain in this disease. The organism is presumed to be part of the normal intestinal flora of livestock (16) and free-ranging reindeer (Rangifer tarandus) (17); it proliferates quickly after death, making it difficult to interpret results of bacterial culture (18). Large numbers of C. perfringens cultured from the intestine are potentially significant, and it has been speculated that enteropathogenicity might result from high levels of alpha toxin production in cases of hemorrhagic bowel syndrome in dairy cattle (19), enterotoxemia in neonatal calves and adult cattle (20,21), and necrotizing enteritis in piglets (9).
The largest numbers of C. perfringens type A were observed in the jejunum. Bacterial numbers in the affected deer ranged from 105 to 107 CFU/g compared with 102 to 104 CFU/g in unaffected deer. Although the difference between jejunal bacterial numbers from unaffected and affected deer was not statistically significant (P = 0.07), this may be the result of the small sample size and high variability of the data. There were no significant differences between numbers of bacteria from unaffected and affected deer from the duodenum, ileum, or colon. The number of organisms cultured from the jejunum of affected deer was similar to the numbers found in cases of bovine enterotoxemia (21) and enterotoxemia in reindeer (22). The observed gross and histological lesions, in conjunction with the high bacterial numbers, support a diagnosis of enterotoxemia. The most severe lesions occurred in the superficial mucosa of the small intestine, where large numbers of gram-positive, rod-shaped bacteria were observed, suggesting the presence of a luminal toxin. Acute liver necrosis and splenomegaly may be associated with absorption of toxins from areas of intestinal damage.
The presence of beta2 toxin in 2 of the C. perfringens type A isolates and C. perfringens enterotoxin (CPE) in 3 of the isolates is also of interest, because both of these toxins in association with alpha toxin have been implicated in intestinal disorders in other animals (23). Beta2 toxin has only recently been identified (24) and is produced by an unassigned C. perfringens that also produces alpha toxin. Recent studies suggest that this toxin is associated with necrotic enteritis in piglets and enterocolitis in horses (23), as well as bovine enterotoxemia (25). Alpha and beta2 toxigenic C. perfringens has also been associated with intestinal hemorrhage and necrosis in a ligated loop assay in a calf (25). Despite some supporting evidence, a clear role for the pathogenicity of beta2 toxin has not been established, since the beta2 toxin gene has also been detected from intestines of non-diseased foals, lambs, and calves (23). Clostridium perfringens enterotoxin has been implicated in enteric disease in foals, piglets, and dogs (1). However, since disease caused by CPE most commonly presents as diarrhea (26), it does not fit the clinical presentation of the disease in deer. This does not exclude the possibility that CPE may act synergistically with alpha or beta2 toxin.
Identification of beta2 and CPE toxins in association with alpha toxin further complicates the possible role of C. perfringens type A in enteric disease. In many instances, these toxins are not tested for, so it is possible that C. perfringens organisms previously identified as type A actually belong to this unidentified type of C. perfringens that produces beta2 toxin as well as alpha toxin. The amount of toxin produced within the intestine also might be an important consideration when trying to determine the significance of specific toxins in causing disease. Many studies have used PCR to determine the type of C. perfringens involved in a particular disease; however, this only provides evidence that the gene for the toxin is present and does not determine whether the gene is being expressed and to what level. Although the production of toxin is likely related to the numbers of C. perfringens organisms in the intestine, there may be other unknown factors that influence the amount of toxin produced.
In 1 deer, alpha and epsilon toxins were detected, which identifies the organism as C. perfringens type D. Lesions seen in this case were similar to lesions observed in cases where only alpha toxin was present. Infection with C. perfringens type D (“overeating disease” or “pulpy kidney disease”) often results in sudden death and sometimes causes neurological signs (20); however, small intestinal lesions are rarely seen. Epsilon toxin is absorbed by the small intestine and causes increased capillary permeability in many tissues, leading to renal damage and edema, especially of the brain (27). Lesions consistent with type D enterotoxemia were not seen in this study.
The presence of alpha toxin within intestinal content was confirmed by using an ELISA. Alpha toxin was detected from intestines of both unaffected and affected deer. However, since C. perfringens type A was isolated from all of the deer, the presence of some level of alpha toxin was expected. A higher level of alpha toxin may have been present in intestines with higher numbers of C. perfringens organisms, but since this test was not quantitative, the significance of the presence of the toxin is uncertain.
There was no apparent sex predisposition for development of the syndrome. Most deer affected by the syndrome were less than 1 y old, but there were also many in the 2 to 5 y age categories. The age distribution of the affected deer was not different from that of unaffected deer, suggesting that deer are more likely to die in the 1st y of life, regardless of the cause of death.
Based on the survey results, the frequency of this syndrome in Saskatchewan appears to be low. In 2001, reported deaths due to this syndrome accounted for 14% of the sudden deaths and 5.6% of the total reported deaths. However, in the year 2002, only 3 cases fitting the case description for this syndrome were submitted to the WCVM for necropsy. In a survey of selected laboratories outside Saskatchewan, only 6 cases of this syndrome were recognized, with these cases occurring from 1997 to 2002. The reason for the large number of cases (n = 17) observed in 2001 is unknown.
We hypothesized that this syndrome might be related to increased grain in the diet or other dietary changes. Other clostridial organisms have been known to cause disease related to dietary factors. Such is the case with C. perfringens type D enterotoxemia or “overeating disease,” which occurs in sheep, goats, and calves (27). This organism proliferates profusely when conditions in the intestinal lumen become suitable, as when large quantities of feed rich in carbohydrates and proteins are ingested or when the diet is abruptly changed, disturbing the normal balance of the enteric bacteria (27). Increased soluble carbohydrate feeding has also been identified as a risk factor for the development of jejunal hemorrhage syndrome in dairy cattle (28); however, the role of C. perfringens in this syndrome is still uncertain. According to necropsy reports and survey data, the intestinal hemorrhage and splenomegaly syndrome was not associated with increased grain or a change of diet. However, overeating or mild indigestion may also cause the intestines to become static, which prevents the normal flushing of toxic substances out of the system (20), and it is possible that this may have occurred in affected deer without the knowledge of the producer. Any injury to the mucosa of the small intestine also may predispose to changes in the bacterial flora that result in clostridial overgrowth (20). No other management factors, such as movements of animals, treatment, or adverse weather conditions, were reported by the producers to be associated with the occurrence of this syndrome.
In conclusion, the syndrome of sudden death, intestinal hemorrhage, and splenomegaly occurs sporadically in well-conditioned farmed deer from 3 mo to 5 y of age. The overall prevalence of the syndrome appears to be low; however, in the year 2001, in Saskatchewan, it accounted for 5.6% of the total deaths reported by producers for that time period. The syndrome did not appear to be related to dietary or any other specific management factors and a definitive cause of the syndrome could not be identified. Based on the presence of large numbers of C. perfringens type A organisms cultured from the small intestine of deer with intestinal lesions, involvement of C. perfringens in this syndrome cannot be ruled out. However, large numbers of bacteria were also cultured from some control deer. The bacteriological data do not support a definitive etiologic role for C. perfringens type A in this syndrome. To prove that alpha toxin is involved in this syndrome, future studies must (i) compare bacterial numbers of control and affected deer using a larger sample size, (ii) quantify the amount of alpha toxin in intestinal contents using a quantitative ELISA, and (iii) reproduce the disease in susceptible animals.
As this syndrome did not appear to be related to any management or dietary factors, no specific recommendation can be made to deer producers at this time. There currently are no vaccines approved for use in cattle or cervids in North America that contain C. perfringens type A or C. perfringens type A with the beta2 toxin gene (19). Since several of the affected animals were vaccinated with 7-way or 8-way clostridial vaccines, these vaccines do not appear to be protective against the presently described syndrome.
Acknowledgments
The authors thank Dr. David Zeman and diagnosticians of the South Dakota State University Animal Disease Research and Diagnostic Laboratory for providing some of the case material, Dr. Josepha Delay and Dr. Ilze Matisse for conducting searches of necropsy records and providing additional case material, Dr. Musangu Ngeleka and Margaret Schwab for valuable advice and technical assistance in bacteriology, Brian Chelack for assistance in conducting ELISA, and Ian Shirley for photographic assistance. CVJ
Footnotes
This work was done in partial fulfillment of the requirements for a Master of Veterinary Science Degree at the University of Saskatchewan by the first author.
Dr. Embury-Hyatt was supported by an Interprovincial Graduate Student Fellowship.
Funding for this study was obtained from the WCVM Specialized Livestock Research and Development Program (Saskatchewan Agri-Food Innovation Fund).
References
- 1.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonel JL. Toxins of Clostridium perfringens types A, B, C, D and E. In: Dorner F, Drews J, eds. Pharmacology of Bacterial Toxins. Oxford, England: Pergamon Pr, 1986:477–502.
- 4.Timoney JF, Gillespie JH, Scott FW, Barlough JE. Hagan and Bruner’s Microbiology and Infectious Diseases of Domestic Animals. Ithaca, NY: Comstock Publishing Associates, 1988.
- 5.Long JR, Pettit JR, Barnum DA. Necrotic enteritis in chickens. II. Pathology and proposed pathogenesis. Can J Comp Med. 1974;38:467–474. [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Sheikhly F, Truscott RB. The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis of chickens. Avian Dis. 1977;21:256–263. [PubMed] [Google Scholar]
- 7.McGowan G, Moulton JE, Rood SE. Lamb losses associated with Clostridium perfringens type A. J Am Vet Med Assoc. 1958;133:219–221. [PubMed] [Google Scholar]
- 8.Weirup M, DiPetro JA. Bacteriologic examination of equine fecal flora as a diagnostic tool for equine intestinal clostridiosis Clostridium perfringens. Am J Vet Res. 1981;42:2167–2169. [PubMed] [Google Scholar]
- 9.Madsen DP. Managing management-induced Clostridium perfringens type A infection in suckling pigs: A case study. Swine Health Product. 1995;3:201–208. [Google Scholar]
- 10.Sasaki J, Goryo M, Asahina M, Makara M, Shishido S, Okada K. Hemorrhagic enteritis associated with Clostridium perfringens Type A in a dog. J Vet Med Sci. 1999;61:175–177. doi: 10.1292/jvms.61.175. [DOI] [PubMed] [Google Scholar]
- 11.Abutarbush SM, Carmalt JL, Wilson DG, O’Connor BP, Clark EG, Naylor JM. Jejunal hemorrhage syndrome in 2 Canadian beef cows. Can Vet J. 2004;45:48–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Murray P, Baron E, Pfaller M, Tenover F, Yolken K. Manual of Clinical Microbiology, 7th ed. Washington DC: Am Soc Microbiol, 1999:1678.
- 13.Barry AL. Clinical specimens for microbiologic examination. In: Haeprich PD, ed. A Guide to Understanding and Management of Infectious Processes. New York: Harper and Row, 1972:103–107.
- 14.Quinn PJ, Carter ME, Markey B, Carter GR. Clinical Veterinary Microbiology. Mosby-Year Book Europe Limited: Wolfe Publ, 1994:202–203.
- 15.Meer RR, Songer JG. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997;58:702–705. [PubMed] [Google Scholar]
- 16.Songer JG. Clostridium perfringens Type A infection in cattle. Proc 32nd Annu Conf Am Assoc Bovine Pract 1999:40–44.
- 17.Aschfalk A, Valentin-Weigand P, Muller W, Goethe R. Toxin types of Clostridium perfringens isolated from free-ranging, semi-domesticated reindeer in Norway. Vet Rec. 2002;151:210–213. doi: 10.1136/vr.151.7.210. [DOI] [PubMed] [Google Scholar]
- 18.Niilo L. Clostridium perfringens in animal disease: a review of current knowledge. Can Vet J. 1980;21:141–148. [PMC free article] [PubMed] [Google Scholar]
- 19.Dennison AC, VanMetre DC, Callan RJ, Dinsmore P, Mason GL, Ellis RP. Hemorrhagic bowel syndrome in dairy cattle: 22 cases (1997–2000) J Am Vet Med Assoc. 2002;221:686–689. doi: 10.2460/javma.2002.221.686. [DOI] [PubMed] [Google Scholar]
- 20.Fleming S. Enterotoxemia in neonatal calves. Vet Clin North Am Food Anim Pract. 1985;1:509–513. doi: 10.1016/s0749-0720(15)31299-8. [DOI] [PubMed] [Google Scholar]
- 21.Manteca C, Daube G, Pirson V, Limbourg B, Kaeckenbeeck A, Mainil JG. Bacterial intestinal flora associated with enterotoxaemia in Belgian Blue calves. Vet Microbiol. 2001;81:21–32. doi: 10.1016/s0378-1135(01)00329-7. [DOI] [PubMed] [Google Scholar]
- 22.Kummeneje K, Bakken G. Clostridium perfringens enterotoxaemia in reindeer. Nord Vet Med. 1973;25:196–202. [PubMed] [Google Scholar]
- 23.Garmory HS, Chanter N, French NP, Bueschel JG, Songer JG, Titball RW. Occurrence of Clostridium perfringens β2-toxin amongst animals determined using genotyping and subtyping PCR assays. Epidemiol Infect. 2000;124:61–67. doi: 10.1017/s0950268899003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert M, Jolivet-Renaud C, Popoff MR. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene. 1997;203:65–73. doi: 10.1016/s0378-1119(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 25.Manteca C, Daube G, Jauniaux T, et al. A role for the Clostridium perfringens β2 toxin in bovine enterotoxemia? Vet Microbiol. 2002;86:191–202. doi: 10.1016/S0378-1135(02)00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClane BA. An overview of Clostridium perfringens enterotoxin. Toxicon. 1996;34:1335–1343. doi: 10.1016/s0041-0101(96)00101-8. [DOI] [PubMed] [Google Scholar]
- 27.Uzal FA, Kelly WA. Experimental Clostridium perfringens Type D enterotoxemia in goats. Vet Pathol. 1998;35:132–140. doi: 10.1177/030098589803500207. [DOI] [PubMed] [Google Scholar]
- 28.Kirkpatrick MA, Timms LL, Kersting KW, Kinyon JM. Case report-jejunal hemorrhage syndrome of dairy cattle. Bovine Pract. 2001;35:104–11. [Google Scholar]